Abstract
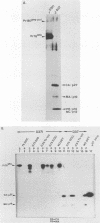
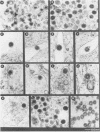
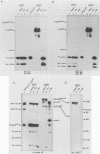
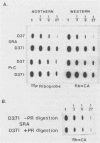
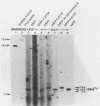
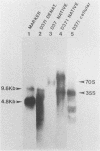
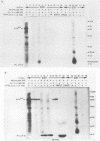
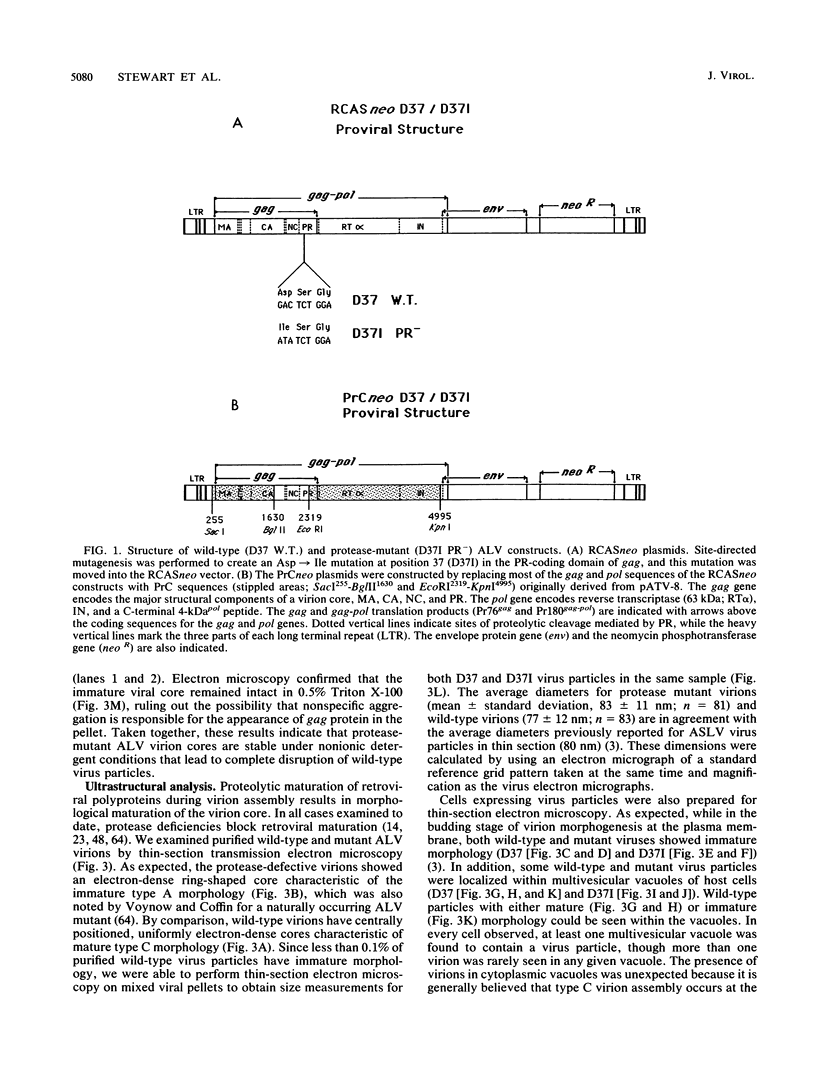
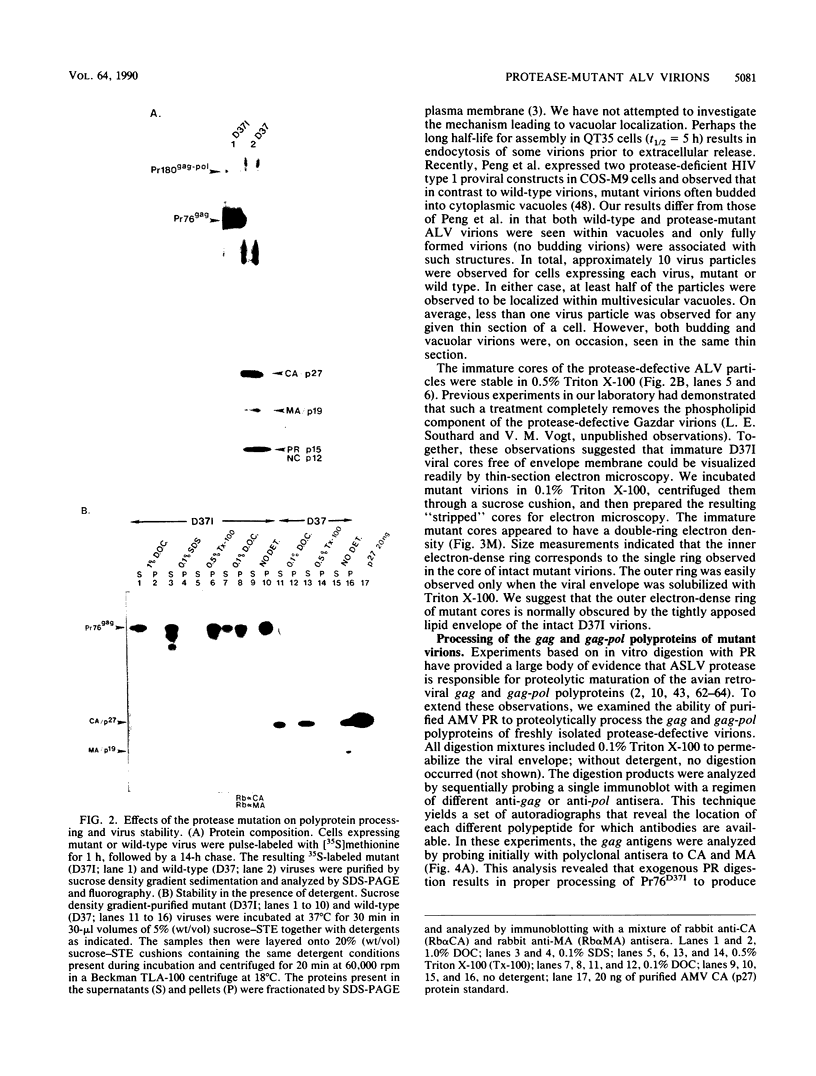
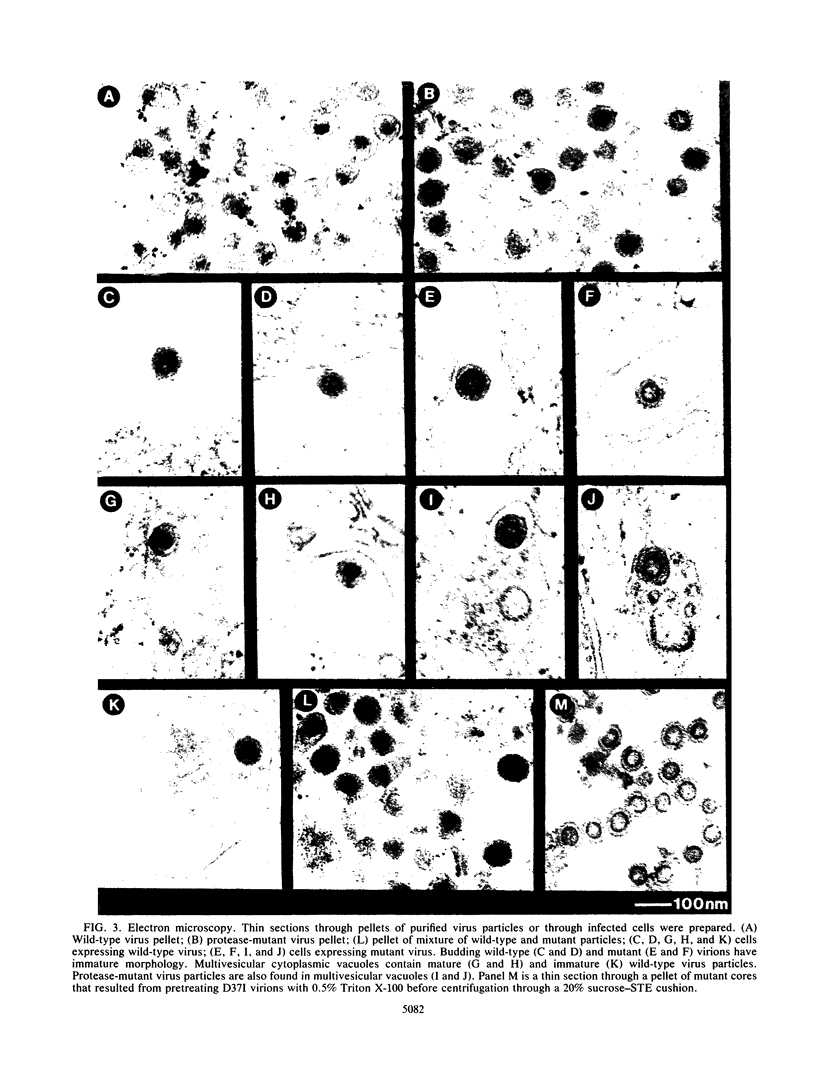
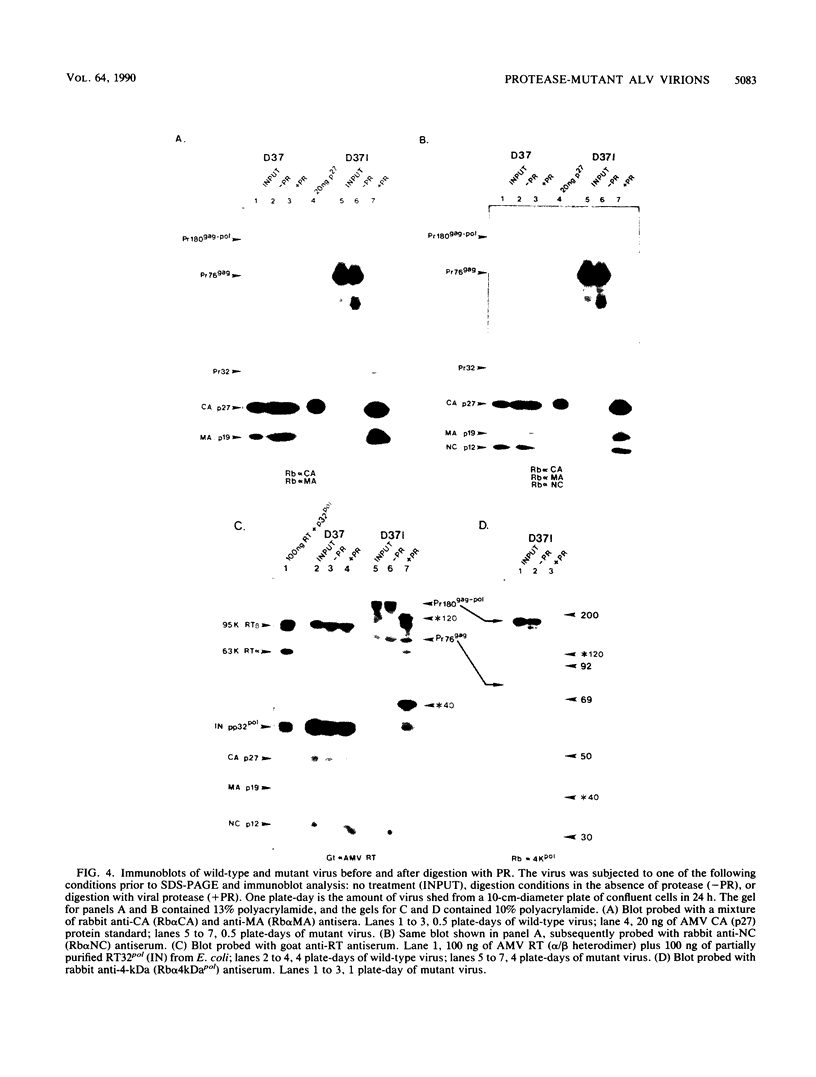
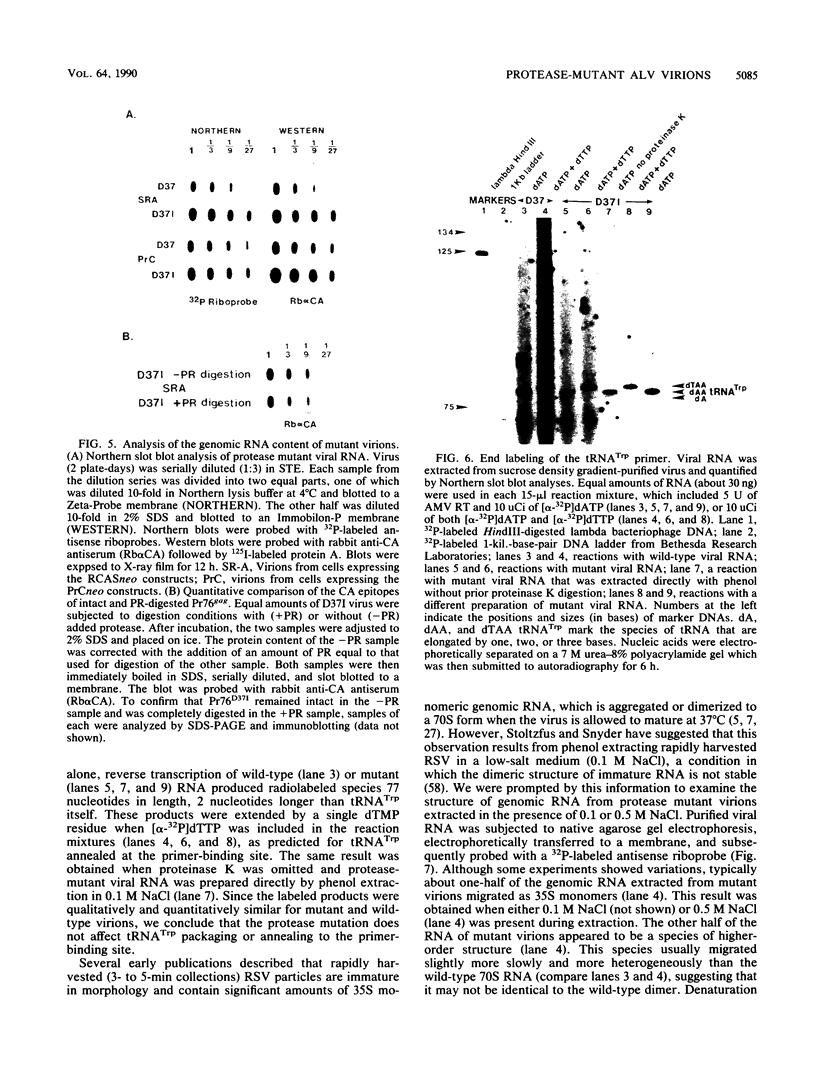
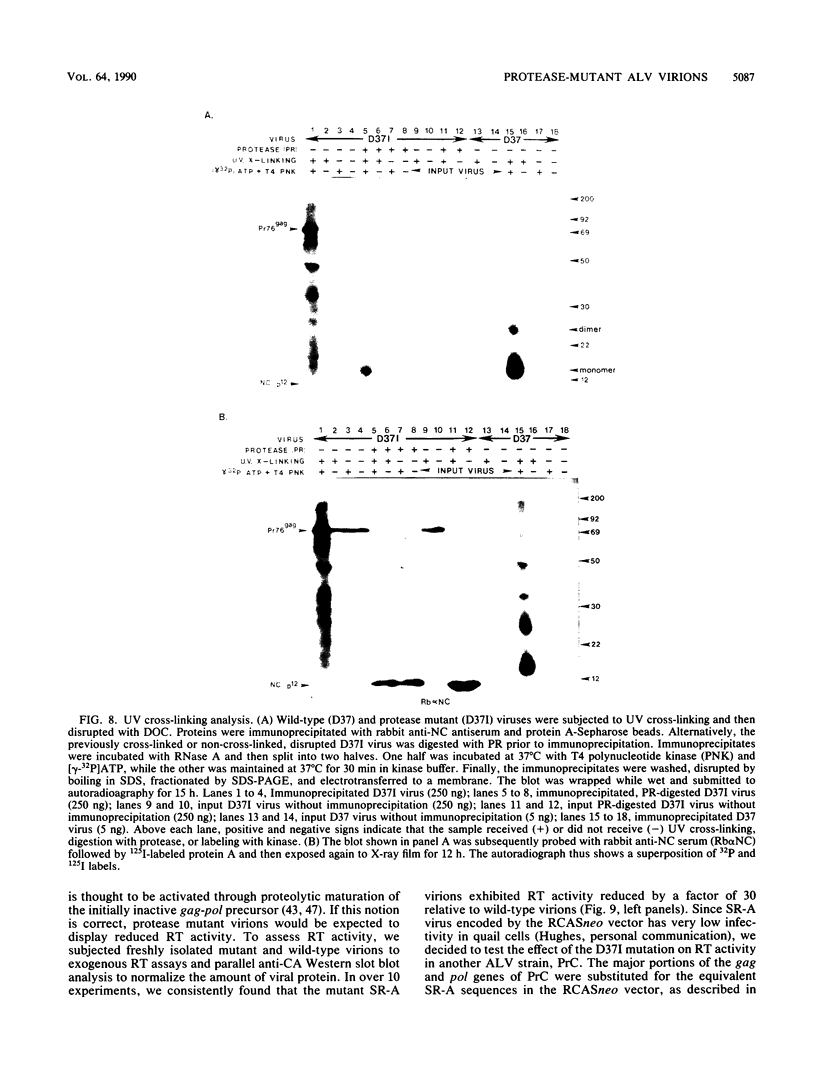
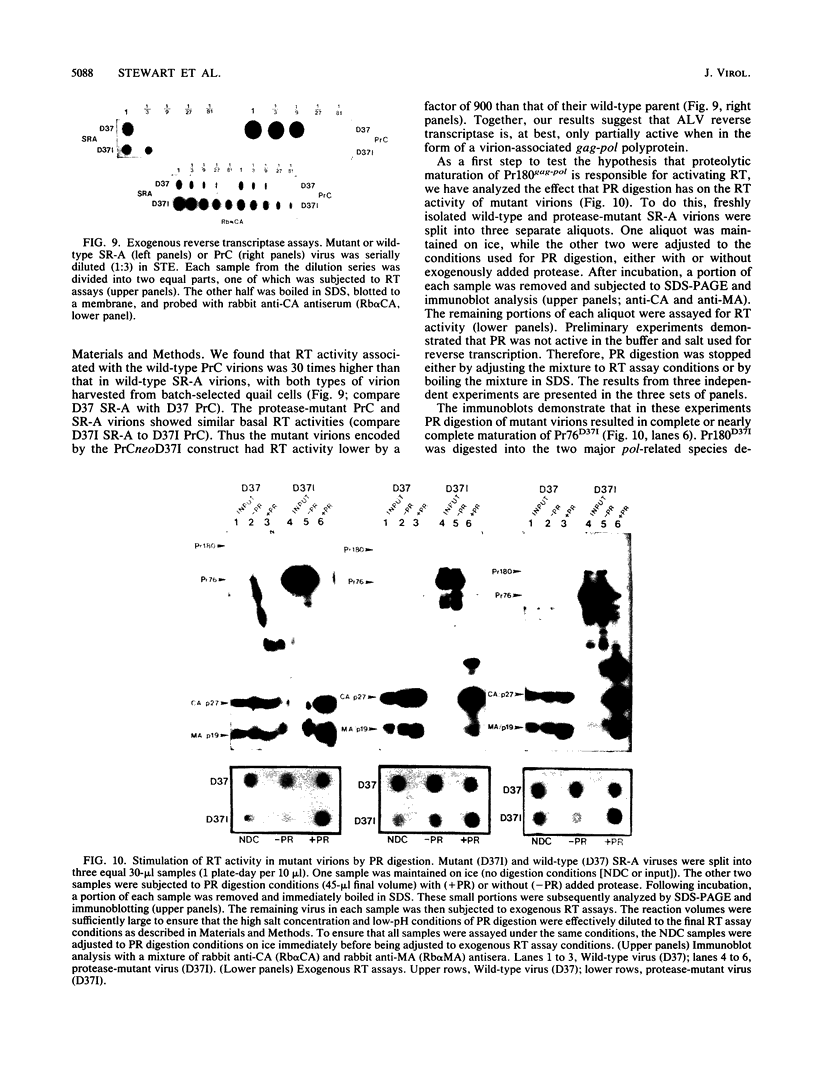
The structural and enzymatic components of retroviral cores are formed by proteolytic cleavage of precursor polypeptides, mediated by the viral protease (PR). We constructed an active-site mutation, D37I, in the PR of avian leukosis virus. The D37I mutation was introduced into an infectious DNA clone, and quail cell lines expressing the mutant virus were established. These cell lines produce normal amounts of virus particles, the major internal protein components of which are the uncleaved gag and gag-pol precursors. As in other retroviral systems, the protease-defective virions are noninfectious and retain the "immature" type A morphology as determined by thin-section transmission electron microscopy. The virion cores are stable at nonionic detergent concentrations that completely disrupt wild-type cores. Digestion of mutant virions with exogenous PR in the presence of detergent leads to complete and correct cleavage of the gag precursor but incomplete cleavage of the gag-pol precursor. The protease-defective virions encapsidate normal amounts of genomic RNA and tRNA(Trp) that is properly annealed to the primer-binding site, but some of the genomic RNA remains monomeric. Results from UV cross-linking experiments show that the gag polyprotein of mutant virions interacts with viral RNA and that this interaction occurs through the nucleocapsid (NC) domain. However, within mutant virions the interaction of the NC domain with RNA differs from that of mature NC with RNA in wild-type virions. Reverse transcriptase (RT) activity associated with mutant virions is diminished but still detectable. Digestion of the virions with PR leads to a fivefold increase in activity, but this PR-mediated activation of RT is incomplete. Since in vitro cleavage of the gag-pol precursor is also incomplete, we hypothesize that amino acid sequences N terminal to the reverse transcriptase domain inhibit RT activity.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. E., Mellor J., Gull K., Sim R. B., Tuite M. F., Kingsman S. M., Kingsman A. J. The functions and relationships of Ty-VLP proteins in yeast reflect those of mammalian retroviral proteins. Cell. 1987 Apr 10;49(1):111–119. doi: 10.1016/0092-8674(87)90761-6. [DOI] [PubMed] [Google Scholar]
- Alexander F., Leis J., Soltis D. A., Crowl R. M., Danho W., Poonian M. S., Pan Y. C., Skalka A. M. Proteolytic processing of avian sarcoma and leukosis viruses pol-endo recombinant proteins reveals another pol gene domain. J Virol. 1987 Feb;61(2):534–542. doi: 10.1128/jvi.61.2.534-542.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieth E., Gabus C., Darlix J. L. A study of the dimer formation of Rous sarcoma virus RNA and of its effect on viral protein synthesis in vitro. Nucleic Acids Res. 1990 Jan 11;18(1):119–127. doi: 10.1093/nar/18.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaani E., Helm K. V., Duesberg P. Evidence for 30-40S RNA as precursor of the 60-70S RNA of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1973 Feb;70(2):401–405. doi: 10.1073/pnas.70.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung K. S., Smith R. E., Stone M. P., Joklik W. K. Comparison of immature (rapid harvest) and mature Rous sarcoma virus particles. Virology. 1972 Dec;50(3):851–864. doi: 10.1016/0042-6822(72)90439-4. [DOI] [PubMed] [Google Scholar]
- Crawford S., Goff S. P. A deletion mutation in the 5' part of the pol gene of Moloney murine leukemia virus blocks proteolytic processing of the gag and pol polyproteins. J Virol. 1985 Mar;53(3):899–907. doi: 10.1128/jvi.53.3.899-907.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman R. N., Mason W. S., Linial M. Synthesis and processing of polymerase proteins of wild-type and mutant avian retroviruses. J Virol. 1980 Oct;36(1):62–78. doi: 10.1128/jvi.36.1.62-78.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmerie W. G., Loeb D. D., Casavant N. C., Hutchison C. A., 3rd, Edgell M. H., Swanstrom R. Expression and processing of the AIDS virus reverse transcriptase in Escherichia coli. Science. 1987 Apr 17;236(4799):305–308. doi: 10.1126/science.2436298. [DOI] [PubMed] [Google Scholar]
- Friis R. R., Ogura H., Gelderblom H., Halpern M. S. The defective maturation of viral progeny with a temperature-sensitive mutant of avian sarcoma virus. Virology. 1976 Aug;73(1):259–272. doi: 10.1016/0042-6822(76)90079-9. [DOI] [PubMed] [Google Scholar]
- Goff S., Traktman P., Baltimore D. Isolation and properties of Moloney murine leukemia virus mutants: use of a rapid assay for release of virion reverse transcriptase. J Virol. 1981 Apr;38(1):239–248. doi: 10.1128/jvi.38.1.239-248.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göttlinger H. G., Sodroski J. G., Haseltine W. A. Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5781–5785. doi: 10.1073/pnas.86.15.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J., Billich S., Schulze T., Sukrow S., Moelling K. Partial purification and substrate analysis of bacterially expressed HIV protease by means of monoclonal antibody. EMBO J. 1988 Jun;7(6):1785–1791. doi: 10.1002/j.1460-2075.1988.tb03009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms E., Rodhe W., Friis R. R., Bauer H. Influence of defective virion core proteins on RNA maturation with an avian sarcoma virus. J Virol. 1977 Jan;21(1):419–422. doi: 10.1128/jvi.21.1.419-422.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippenmeyer P. J., Grandgenett D. P. Requirement of the avian retrovirus pp32 DNA binding protein domain for replication. Virology. 1984 Sep;137(2):358–370. doi: 10.1016/0042-6822(84)90228-9. [DOI] [PubMed] [Google Scholar]
- Hughes S. H., Greenhouse J. J., Petropoulos C. J., Sutrave P. Adaptor plasmids simplify the insertion of foreign DNA into helper-independent retroviral vectors. J Virol. 1987 Oct;61(10):3004–3012. doi: 10.1128/jvi.61.10.3004-3012.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter E., Hayman M. J., Rongey R. W., Vogt P. K. An avian sarcoma virus mutant that is temperature sensitive for virion assembly. Virology. 1976 Jan;69(1):35–49. doi: 10.1016/0042-6822(76)90192-6. [DOI] [PubMed] [Google Scholar]
- Jacks T., Madhani H. D., Masiarz F. R., Varmus H. E. Signals for ribosomal frameshifting in the Rous sarcoma virus gag-pol region. Cell. 1988 Nov 4;55(3):447–458. doi: 10.1016/0092-8674(88)90031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S. P., Veigl M., Vanaman T., Leis J. Cyanogen bromide digestion of the avian myeloblastosis virus pp19 protein: isolation of an amino-terminal peptide that binds to viral RNA. J Virol. 1983 Feb;45(2):876–881. doi: 10.1128/jvi.45.2.876-881.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh I., Ikawa Y., Yoshinaka Y. Retrovirus protease characterized as a dimeric aspartic proteinase. J Virol. 1989 May;63(5):2226–2232. doi: 10.1128/jvi.63.5.2226-2232.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh I., Yoshinaka Y., Rein A., Shibuya M., Odaka T., Oroszlan S. Murine leukemia virus maturation: protease region required for conversion from "immature" to "mature" core form and for virus infectivity. Virology. 1985 Sep;145(2):280–292. doi: 10.1016/0042-6822(85)90161-8. [DOI] [PubMed] [Google Scholar]
- Katz R. A., Terry R. W., Skalka A. M. A conserved cis-acting sequence in the 5' leader of avian sarcoma virus RNA is required for packaging. J Virol. 1986 Jul;59(1):163–167. doi: 10.1128/jvi.59.1.163-167.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandjian E. W., Méric C. A procedure for Northern blot analysis of native RNA. Anal Biochem. 1986 Nov 15;159(1):227–232. doi: 10.1016/0003-2697(86)90332-5. [DOI] [PubMed] [Google Scholar]
- Kohl N. E., Emini E. A., Schleif W. A., Davis L. J., Heimbach J. C., Dixon R. A., Scolnick E. M., Sigal I. S. Active human immunodeficiency virus protease is required for viral infectivity. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4686–4690. doi: 10.1073/pnas.85.13.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korb J., Trávnícek M., Ríman J. The oncornavirus maturation process: quantitative correlation between morphological changes and conversion of genomic virion RNA. Intervirology. 1976;7(4-5):211–224. doi: 10.1159/000149954. [DOI] [PubMed] [Google Scholar]
- Kotler M., Danho W., Katz R. A., Leis J., Skalka A. M. Avian retroviral protease and cellular aspartic proteases are distinguished by activities on peptide substrates. J Biol Chem. 1989 Feb 25;264(6):3428–3435. [PubMed] [Google Scholar]
- Kotler M., Katz R. A., Skalka A. M. Activity of avian retroviral protease expressed in Escherichia coli. J Virol. 1988 Aug;62(8):2696–2700. doi: 10.1128/jvi.62.8.2696-2700.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapatto R., Blundell T., Hemmings A., Overington J., Wilderspin A., Wood S., Merson J. R., Whittle P. J., Danley D. E., Geoghegan K. F. X-ray analysis of HIV-1 proteinase at 2.7 A resolution confirms structural homology among retroviral enzymes. Nature. 1989 Nov 16;342(6247):299–302. doi: 10.1038/342299a0. [DOI] [PubMed] [Google Scholar]
- Le Grice S. F., Ette R., Mills J., Mous J. Comparison of the human immunodeficiency virus type 1 and 2 proteases by hybrid gene construction and trans-complementation. J Biol Chem. 1989 Sep 5;264(25):14902–14908. [PubMed] [Google Scholar]
- Le Grice S. F., Mills J., Mous J. Active site mutagenesis of the AIDS virus protease and its alleviation by trans complementation. EMBO J. 1988 Aug;7(8):2547–2553. doi: 10.1002/j.1460-2075.1988.tb03103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuthardt A., Le Grice S. F. Biosynthesis and analysis of a genetically engineered HIV-1 reverse transcriptase/endonuclease polyprotein in Escherichia coli. Gene. 1988 Aug 15;68(1):35–42. doi: 10.1016/0378-1119(88)90596-3. [DOI] [PubMed] [Google Scholar]
- Lu A. H., Soong M. M., Wong P. K. Maturation of Moloney murine leukemia virus. Virology. 1979 Feb;93(1):269–274. doi: 10.1016/0042-6822(79)90297-6. [DOI] [PubMed] [Google Scholar]
- Luftig R. B., Yoshinaka Y. Rauscher leukemia virus populations enriched for "immature" virions contain increased amounts of P70, the gag gene product. J Virol. 1978 Jan;25(1):416–421. doi: 10.1128/jvi.25.1.416-421.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M., Jaskólski M., Rao J. K., Leis J., Wlodawer A. Crystal structure of a retroviral protease proves relationship to aspartic protease family. Nature. 1989 Feb 9;337(6207):576–579. doi: 10.1038/337576a0. [DOI] [PubMed] [Google Scholar]
- Moelling K., Scott A., Dittmar K. E., Owada M. Effect of p15-associated protease from an avian RNA tumor virus on avian virus-specific polyprotein precursors. J Virol. 1980 Feb;33(2):680–688. doi: 10.1128/jvi.33.2.680-688.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mous J., Heimer E. P., Le Grice S. F. Processing protease and reverse transcriptase from human immunodeficiency virus type I polyprotein in Escherichia coli. J Virol. 1988 Apr;62(4):1433–1436. doi: 10.1128/jvi.62.4.1433-1436.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méric C., Darlix J. L., Spahr P. F. It is Rous sarcoma virus protein P12 and not P19 that binds tightly to Rous sarcoma virus RNA. J Mol Biol. 1984 Mar 15;173(4):531–538. doi: 10.1016/0022-2836(84)90396-6. [DOI] [PubMed] [Google Scholar]
- Méric C., Goff S. P. Characterization of Moloney murine leukemia virus mutants with single-amino-acid substitutions in the Cys-His box of the nucleocapsid protein. J Virol. 1989 Apr;63(4):1558–1568. doi: 10.1128/jvi.63.4.1558-1568.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méric C., Gouilloud E., Spahr P. F. Mutations in Rous sarcoma virus nucleocapsid protein p12 (NC): deletions of Cys-His boxes. J Virol. 1988 Sep;62(9):3328–3333. doi: 10.1128/jvi.62.9.3328-3333.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méric C., Spahr P. F. Rous sarcoma virus nucleic acid-binding protein p12 is necessary for viral 70S RNA dimer formation and packaging. J Virol. 1986 Nov;60(2):450–459. doi: 10.1128/jvi.60.2.450-459.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panet A., Baltimore D. Characterization of endonuclease activities in Moloney murine leukemia virus and its replication-defective mutants. J Virol. 1987 May;61(5):1756–1760. doi: 10.1128/jvi.61.5.1756-1760.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panet A., Baltimore D., Hanafusa T. Quantitation of avian RNA tumor virus reverse transcriptase by radioimmunoassay. J Virol. 1975 Jul;16(1):146–152. doi: 10.1128/jvi.16.1.146-152.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C., Ho B. K., Chang T. W., Chang N. T. Role of human immunodeficiency virus type 1-specific protease in core protein maturation and viral infectivity. J Virol. 1989 Jun;63(6):2550–2556. doi: 10.1128/jvi.63.6.2550-2556.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepinsky R. B., Cappiello D., Wilkowski C., Vogt V. M. Chemical crosslinking of proteins in avian sarcoma and leukemia viruses. Virology. 1980 Apr 15;102(1):205–210. doi: 10.1016/0042-6822(80)90081-1. [DOI] [PubMed] [Google Scholar]
- Pepinsky R. B. Localization of lipid-protein and protein-protein interactions within the murine retrovirus gag precursor by a novel peptide-mapping technique. J Biol Chem. 1983 Sep 25;258(18):11229–11235. [PubMed] [Google Scholar]
- Pepinsky R. B., Mattaliano R. J., Vogt V. M. Structure and processing of the p2 region of avian sarcoma and leukemia virus gag precursor polyproteins. J Virol. 1986 Apr;58(1):50–58. doi: 10.1128/jvi.58.1.50-58.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepinsky R. B., Vogt V. M. Purification and properties of a fifth major viral gag protein from avian sarcoma and leukemia viruses. J Virol. 1983 Feb;45(2):648–658. doi: 10.1128/jvi.45.2.648-658.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prats A. C., Roy C., Wang P. A., Erard M., Housset V., Gabus C., Paoletti C., Darlix J. L. cis elements and trans-acting factors involved in dimer formation of murine leukemia virus RNA. J Virol. 1990 Feb;64(2):774–783. doi: 10.1128/jvi.64.2.774-783.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prats A. C., Sarih L., Gabus C., Litvak S., Keith G., Darlix J. L. Small finger protein of avian and murine retroviruses has nucleic acid annealing activity and positions the replication primer tRNA onto genomic RNA. EMBO J. 1988 Jun;7(6):1777–1783. doi: 10.1002/j.1460-2075.1988.tb03008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrschneider J. M., Diggelmann H., Ogura H., Friis R. R., Bauer H. Defective cleavage of a precursor polypeptide in a temperature-sensitive mutant of avian sarcoma virus. Virology. 1976 Nov;75(1):177–187. doi: 10.1016/0042-6822(76)90016-7. [DOI] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Skalka A. M. Retroviral proteases: first glimpses at the anatomy of a processing machine. Cell. 1989 Mar 24;56(6):911–913. doi: 10.1016/0092-8674(89)90621-1. [DOI] [PubMed] [Google Scholar]
- Stoltzfus C. M., Snyder P. N. Structure of B77 sarcoma virus RNA: stabilization of RNA after packaging. J Virol. 1975 Nov;16(5):1161–1170. doi: 10.1128/jvi.16.5.1161-1170.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J., Wong R. N. Evolution in the structure and function of aspartic proteases. J Cell Biochem. 1987 Jan;33(1):53–63. doi: 10.1002/jcb.240330106. [DOI] [PubMed] [Google Scholar]
- Torruella M., Gordon K., Hohn T. Cauliflower mosaic virus produces an aspartic proteinase to cleave its polyproteins. EMBO J. 1989 Oct;8(10):2819–2825. doi: 10.1002/j.1460-2075.1989.tb08428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M., Pepinsky R. B., Southard L. E. Primary structure of p19 species of avian sarcoma and leukemia viruses. J Virol. 1985 Oct;56(1):31–39. doi: 10.1128/jvi.56.1.31-39.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M., Wight A., Eisenman R. In vitro cleavage of avian retrovirus gag proteins by viral protease p15. Virology. 1979 Oct 15;98(1):154–167. doi: 10.1016/0042-6822(79)90534-8. [DOI] [PubMed] [Google Scholar]
- Voynow S. L., Coffin J. M. Truncated gag-related proteins are produced by large deletion mutants of Rous sarcoma virus and form virus particles. J Virol. 1985 Jul;55(1):79–85. doi: 10.1128/jvi.55.1.79-85.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills J. W., Craven R. C., Achacoso J. A. Creation and expression of myristylated forms of Rous sarcoma virus gag protein in mammalian cells. J Virol. 1989 Oct;63(10):4331–4343. doi: 10.1128/jvi.63.10.4331-4343.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N., Baltimore D. Relationship of retrovirus polyprotein cleavages to virion maturation studied with temperature-sensitive murine leukemia virus mutants. J Virol. 1978 Jun;26(3):750–761. doi: 10.1128/jvi.26.3.750-761.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaka Y., Luftig R. B. In vitro phosphorylation of murine leukemia virus proteins: specific phosphorylation of Pr65gag, the precursor of the internal core antigens. Virology. 1982 Jan 15;116(1):181–195. doi: 10.1016/0042-6822(82)90412-3. [DOI] [PubMed] [Google Scholar]
- Yoshinaka Y., Luftig R. B. Murine leukemia virus morphogenesis: cleavage of P70 in vitro can be accompanied by a shift from a concentrically coiled internal strand ("immature") to a collapsed ("mature") form of the virus core. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3446–3450. doi: 10.1073/pnas.74.8.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka K., Honma H., Zushi M., Kondo S., Togashi S., Miyake T., Shiba T. Virus-like particle formation of Drosophila copia through autocatalytic processing. EMBO J. 1990 Feb;9(2):535–541. doi: 10.1002/j.1460-2075.1990.tb08140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Helm K. Cleavage of Rous sarcoma viral polypeptide precursor into internal structural proteins in vitro involves viral protein p15. Proc Natl Acad Sci U S A. 1977 Mar;74(3):911–915. doi: 10.1073/pnas.74.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]