Abstract
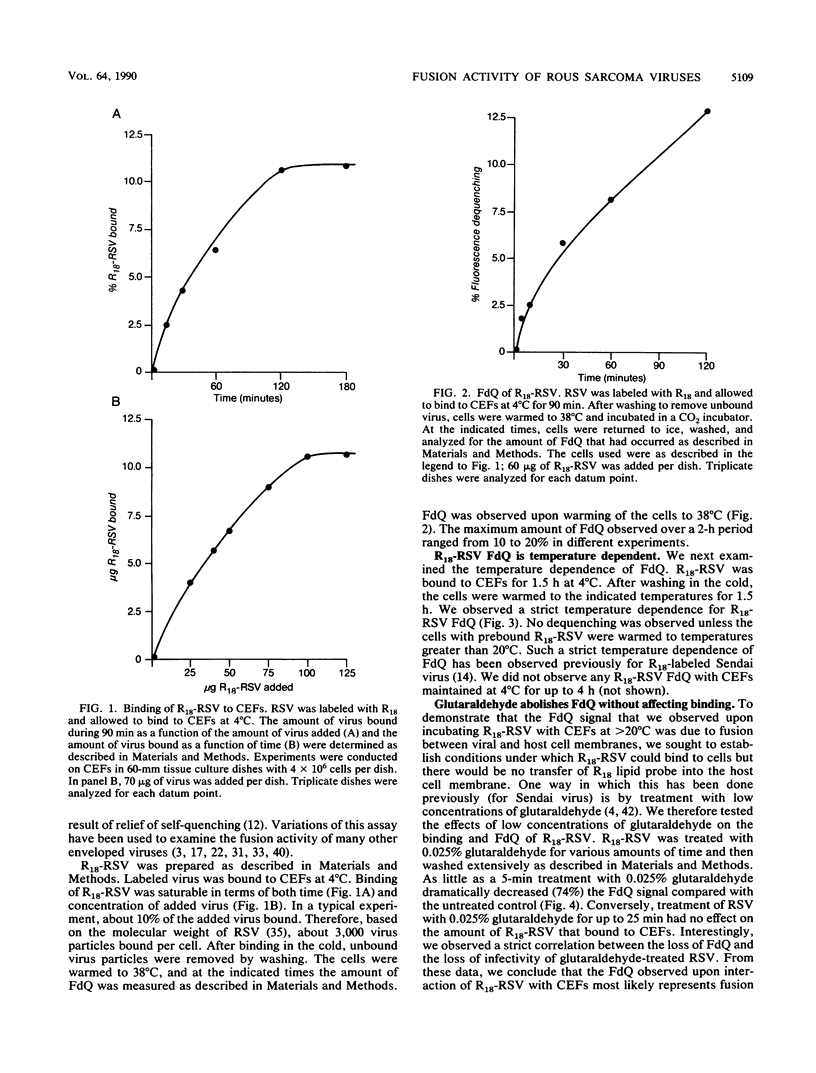
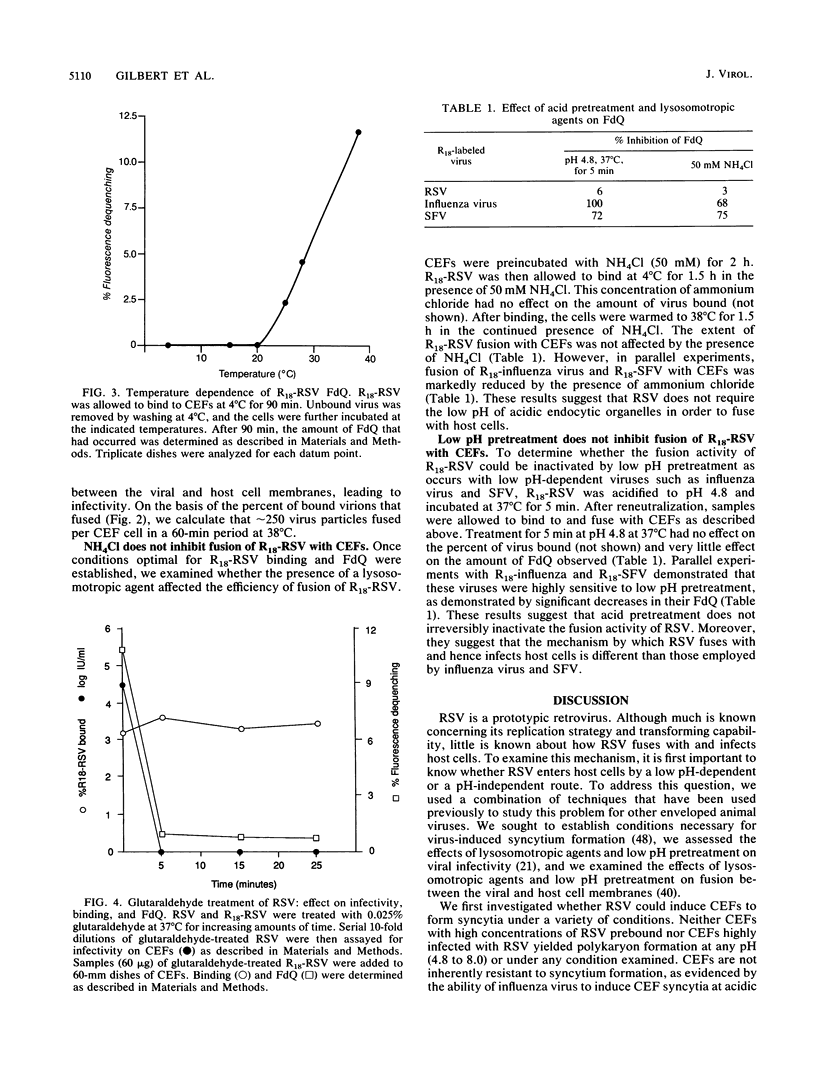
We investigated whether Rous sarcoma virus (RSV) infects cells through a pH-independent or a low-pH-dependent pathway. To do this, the effects of lysosomotropic agents and acid pretreatment on RSV infectivity of, and fusion with, chicken embryo fibroblasts (CEFs) were studied. High concentrations of lysosomotropic agents (ammonium chloride and monensin) did not inhibit virus infectivity: equal titers of RSV were produced in the presence and absence of these agents. Similarly, low-pH pretreatment did not inhibit RSV infectivity. In parallel experiments, lysosomotropic agents and acid pretreatment completely abolished the ability of influenza virus to infect CEFs. To monitor the fusion activity of RSV directly, the viral membrane was labeled with the fluorescent lipid probe octadecyl rhodamine at a self-quenching concentration. Upon fusion with a host cell, the probe is diluted in the cell membrane, resulting in fluorescence dequenching (D. Hoekstra, T. de Boer, K. Klappe, and J. Wilschut, Biochemistry 23:5675-5681, 1984). In this assay, fusion of RSV with CEFs was found to occur in both a time-dependent and a strictly temperature-dependent fashion. No fusion occurred unless cells with prebound virus were warmed to temperatures greater than 20 degrees C. Fusion, but not binding, was abolished if virus was pretreated with low concentrations of glutaraldehyde. High concentrations of ammonium chloride had no effect on fusion of RSV with CEFs but greatly diminished the ability of influenza virus and Semliki Forest virus to fuse with CEFs. Similarly, acid pretreatment of RSV had no effect on fusion with CEFs while markedly inhibiting fusion of both influenza and Semliki Forest viruses. Collectively, our results show that RSV fusion with and hence infection of CEFs does not require exposure of the virus to low pH. In this respect, RSV resembles another retrovirus, human immunodeficiency virus.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen K. B., Nexø B. A. Entry of murine retrovirus into mouse fibroblasts. Virology. 1983 Feb;125(1):85–98. doi: 10.1016/0042-6822(83)90065-x. [DOI] [PubMed] [Google Scholar]
- Arvan P., Rudnick G., Castle J. D. Osmotic properties and internal pH of isolated rat parotid secretory granules. J Biol Chem. 1984 Nov 10;259(21):13567–13572. [PubMed] [Google Scholar]
- Blumenthal R., Bali-Puri A., Walter A., Covell D., Eidelman O. pH-dependent fusion of vesicular stomatitis virus with Vero cells. Measurement by dequenching of octadecyl rhodamine fluorescence. J Biol Chem. 1987 Oct 5;262(28):13614–13619. [PubMed] [Google Scholar]
- Chejanovsky N., Zakai N., Amselem S., Barenholz Y., Loyter A. Membrane vesicles containing the Sendai virus binding glycoprotein, but not the viral fusion protein, fuse with phosphatidylserine liposomes at low pH. Biochemistry. 1986 Aug 26;25(17):4810–4817. doi: 10.1021/bi00365a014. [DOI] [PubMed] [Google Scholar]
- Doms R. W., Helenius A., White J. Membrane fusion activity of the influenza virus hemagglutinin. The low pH-induced conformational change. J Biol Chem. 1985 Mar 10;260(5):2973–2981. [PubMed] [Google Scholar]
- Dorner A. J., Coffin J. M. Determinants for receptor interaction and cell killing on the avian retrovirus glycoprotein gp85. Cell. 1986 May 9;45(3):365–374. doi: 10.1016/0092-8674(86)90322-3. [DOI] [PubMed] [Google Scholar]
- Einfeld D., Hunter E. Oligomeric structure of a prototype retrovirus glycoprotein. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8688–8692. doi: 10.1073/pnas.85.22.8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Gething M. J., Doms R. W., York D., White J. Studies on the mechanism of membrane fusion: site-specific mutagenesis of the hemagglutinin of influenza virus. J Cell Biol. 1986 Jan;102(1):11–23. doi: 10.1083/jcb.102.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollins S. W., Porterfield J. S. The uncoating and infectivity of the flavivirus West Nile on interaction with cells: effects of pH and ammonium chloride. J Gen Virol. 1986 Sep;67(Pt 9):1941–1950. doi: 10.1099/0022-1317-67-9-1941. [DOI] [PubMed] [Google Scholar]
- Hirst G. K. THE AGGLUTINATION OF RED CELLS BY ALLANTOIC FLUID OF CHICK EMBRYOS INFECTED WITH INFLUENZA VIRUS. Science. 1941 Jul 4;94(2427):22–23. doi: 10.1126/science.94.2427.22. [DOI] [PubMed] [Google Scholar]
- Hoekstra D., Klappe K., Hoff H., Nir S. Mechanism of fusion of Sendai virus: role of hydrophobic interactions and mobility constraints of viral membrane proteins. Effects of polyethylene glycol. J Biol Chem. 1989 Apr 25;264(12):6786–6792. [PubMed] [Google Scholar]
- Hoekstra D., Klappe K., de Boer T., Wilschut J. Characterization of the fusogenic properties of Sendai virus: kinetics of fusion with erythrocyte membranes. Biochemistry. 1985 Aug 27;24(18):4739–4745. doi: 10.1021/bi00339a005. [DOI] [PubMed] [Google Scholar]
- Hoekstra D., de Boer T., Klappe K., Wilschut J. Fluorescence method for measuring the kinetics of fusion between biological membranes. Biochemistry. 1984 Nov 20;23(24):5675–5681. doi: 10.1021/bi00319a002. [DOI] [PubMed] [Google Scholar]
- Hughes S., Kosik E. Mutagenesis of the region between env and src of the SR-A strain of Rous sarcoma virus for the purpose of constructing helper-independent vectors. Virology. 1984 Jul 15;136(1):89–99. doi: 10.1016/0042-6822(84)90250-2. [DOI] [PubMed] [Google Scholar]
- Hunter E., Hill E., Hardwick M., Bhown A., Schwartz D. E., Tizard R. Complete sequence of the Rous sarcoma virus env gene: identification of structural and functional regions of its product. J Virol. 1983 Jun;46(3):920–936. doi: 10.1128/jvi.46.3.920-936.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klappe K., Wilschut J., Nir S., Hoekstra D. Parameters affecting fusion between Sendai virus and liposomes. Role of viral proteins, liposome composition, and pH. Biochemistry. 1986 Dec 16;25(25):8252–8260. doi: 10.1021/bi00373a019. [DOI] [PubMed] [Google Scholar]
- Kowalski M., Potz J., Basiripour L., Dorfman T., Goh W. C., Terwilliger E., Dayton A., Rosen C., Haseltine W., Sodroski J. Functional regions of the envelope glycoprotein of human immunodeficiency virus type 1. Science. 1987 Sep 11;237(4820):1351–1355. doi: 10.1126/science.3629244. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marsh M., Helenius A. Virus entry into animal cells. Adv Virus Res. 1989;36:107–151. doi: 10.1016/S0065-3527(08)60583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure M. O., Marsh M., Weiss R. A. Human immunodeficiency virus infection of CD4-bearing cells occurs by a pH-independent mechanism. EMBO J. 1988 Feb;7(2):513–518. doi: 10.1002/j.1460-2075.1988.tb02839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller N., Hutt-Fletcher L. M. A monoclonal antibody to glycoprotein gp85 inhibits fusion but not attachment of Epstein-Barr virus. J Virol. 1988 Jul;62(7):2366–2372. doi: 10.1128/jvi.62.7.2366-2372.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrow S., Hahn B. H., Shaw G. M., Gallo R. C., Wong-Staal F., Wolf H. Computer-assisted analysis of envelope protein sequences of seven human immunodeficiency virus isolates: prediction of antigenic epitopes in conserved and variable regions. J Virol. 1987 Feb;61(2):570–578. doi: 10.1128/jvi.61.2.570-578.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison T. G. Structure, function, and intracellular processing of paramyxovirus membrane proteins. Virus Res. 1988 May;10(2-3):113–135. doi: 10.1016/0168-1702(88)90010-x. [DOI] [PubMed] [Google Scholar]
- Notter M. F., Leary J. F., Balduzzi P. C. Adsorption of Rous sarcoma virus to genetically susceptible and resistant chicken cells studied by laser flow cytometry. J Virol. 1982 Mar;41(3):958–964. doi: 10.1128/jvi.41.3.958-964.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PURCHASE H. G., OKAZAKI W. MORPHOLOGY OF FOCI PRODUCED BY STANDARD PREPARATION OF ROUS SARCOMA VIRUS. J Natl Cancer Inst. 1964 Mar;32:579–586. [PubMed] [Google Scholar]
- Pinter A., Chen T. E., Lowy A., Cortez N. G., Silagi S. Ecotropic murine leukemia virus-induced fusion of murine cells. J Virol. 1986 Mar;57(3):1048–1054. doi: 10.1128/jvi.57.3.1048-1054.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis J. L., McAtee F. J., Evans L. H. Infectious entry of murine retroviruses into mouse cells: evidence of a postadsorption step inhibited by acidic pH. J Virol. 1985 Sep;55(3):806–812. doi: 10.1128/jvi.55.3.806-812.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond S., Peters G., Dickson C. Mouse mammary tumor virus can mediate cell fusion at reduced pH. Virology. 1984 Mar;133(2):393–402. doi: 10.1016/0042-6822(84)90405-7. [DOI] [PubMed] [Google Scholar]
- Rosenberg N., Baltimore D. The effect of helper virus on Abelson virus-induced transformation of lymphoid cells. J Exp Med. 1978 Apr 1;147(4):1126–1141. doi: 10.1084/jem.147.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheule R. K. Fusion of Sindbis virus with model membranes containing phosphatidylethanolamine: implications for protein-induced membrane fusion. Biochim Biophys Acta. 1987 May 29;899(2):185–195. doi: 10.1016/0005-2736(87)90399-3. [DOI] [PubMed] [Google Scholar]
- Shioda T., Wakao S., Suzu S., Shibuta H. Differences in bovine parainfluenza 3 virus variants studied by sequencing of the genes of viral envelope proteins. Virology. 1988 Feb;162(2):388–396. doi: 10.1016/0042-6822(88)90479-5. [DOI] [PubMed] [Google Scholar]
- Sinangil F., Loyter A., Volsky D. J. Quantitative measurement of fusion between human immunodeficiency virus and cultured cells using membrane fluorescence dequenching. FEBS Lett. 1988 Oct 24;239(1):88–92. doi: 10.1016/0014-5793(88)80551-9. [DOI] [PubMed] [Google Scholar]
- Smith R. E., Bernstein E. H. Production and purification of large amounts of Rous sarcoma virus. Appl Microbiol. 1973 Mar;25(3):346–353. doi: 10.1128/am.25.3.346-353.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. E. Large-scale growth of Rous sarcoma virus. Methods Enzymol. 1979;58:393–403. doi: 10.1016/s0076-6879(79)58154-3. [DOI] [PubMed] [Google Scholar]
- Stegmann T., Booy F. P., Wilschut J. Effects of low pH on influenza virus. Activation and inactivation of the membrane fusion capacity of the hemagglutinin. J Biol Chem. 1987 Dec 25;262(36):17744–17749. [PubMed] [Google Scholar]
- Stegmann T., Doms R. W., Helenius A. Protein-mediated membrane fusion. Annu Rev Biophys Biophys Chem. 1989;18:187–211. doi: 10.1146/annurev.bb.18.060189.001155. [DOI] [PubMed] [Google Scholar]
- Stegmann T., Hoekstra D., Scherphof G., Wilschut J. Fusion activity of influenza virus. A comparison between biological and artificial target membrane vesicles. J Biol Chem. 1986 Aug 25;261(24):10966–10969. [PubMed] [Google Scholar]
- Stegmann T., Morselt H. W., Scholma J., Wilschut J. Fusion of influenza virus in an intracellular acidic compartment measured by fluorescence dequenching. Biochim Biophys Acta. 1987 Nov 2;904(1):165–170. doi: 10.1016/0005-2736(87)90100-3. [DOI] [PubMed] [Google Scholar]
- Stein B. S., Gowda S. D., Lifson J. D., Penhallow R. C., Bensch K. G., Engleman E. G. pH-independent HIV entry into CD4-positive T cells via virus envelope fusion to the plasma membrane. Cell. 1987 Jun 5;49(5):659–668. doi: 10.1016/0092-8674(87)90542-3. [DOI] [PubMed] [Google Scholar]
- Toister Z., Loyter A. The mechanism of cell fusion. II. Formation of chicken erythrocyte polykaryons. J Biol Chem. 1973 Jan 25;248(2):422–432. [PubMed] [Google Scholar]
- Vogt V. M., Eisenman R., Diggelmann H. Generation of avian myeloblastosis virus structural proteins by proteolytic cleavage of a precursor polypeptide. J Mol Biol. 1975 Aug 15;96(3):471–493. doi: 10.1016/0022-2836(75)90174-6. [DOI] [PubMed] [Google Scholar]
- Wallbank A. M., Matter R. E., Klinikowski N. G. 1-Adamantanamine hydrochloride: inhibition of Rous and Esh Sarcoma viruses in cell culture. Science. 1966 Jun 24;152(3730):1760–1761. doi: 10.1126/science.152.3730.1760. [DOI] [PubMed] [Google Scholar]
- Wang L. H., Hanafusa H. Avian sarcoma viruses. Virus Res. 1988 Feb;9(2-3):159–203. doi: 10.1016/0168-1702(88)90030-5. [DOI] [PubMed] [Google Scholar]
- White J. M. Viral and cellular membrane fusion proteins. Annu Rev Physiol. 1990;52:675–697. doi: 10.1146/annurev.ph.52.030190.003331. [DOI] [PubMed] [Google Scholar]
- White J. M., Wilson I. A. Anti-peptide antibodies detect steps in a protein conformational change: low-pH activation of the influenza virus hemagglutinin. J Cell Biol. 1987 Dec;105(6 Pt 2):2887–2896. doi: 10.1083/jcb.105.6.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Kartenbeck J., Helenius A. Membrane fusion activity of influenza virus. EMBO J. 1982;1(2):217–222. doi: 10.1002/j.1460-2075.1982.tb01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Matlin K., Helenius A. Cell fusion by Semliki Forest, influenza, and vesicular stomatitis viruses. J Cell Biol. 1981 Jun;89(3):674–679. doi: 10.1083/jcb.89.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley D. C., Skehel J. J. The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annu Rev Biochem. 1987;56:365–394. doi: 10.1146/annurev.bi.56.070187.002053. [DOI] [PubMed] [Google Scholar]



