Abstract
The L type calcium channel agonist (±)Bay K 8644 has been reported to cause characteristic motor abnormalities in adult mice. The current study shows that administration of this drug can also cause the unusual phenomenon of self-injurious biting, particularly when given to young mice. Self-biting is provoked by injecting small quantities of (±)Bay K 8644 directly into the lateral ventricle of the brain, suggesting a central effect of the drug. Similar behaviors can be provoked by administration of another L type calcium channel agonist, FPL 64176. The self-biting provoked by (±)Bay K 8644 can be inhibited by pretreating the mice with dihydropyridine L type calcium channel antagonists such as nifedipine, nimodipine, or nitrendipine. However, self-biting is not inhibited by nondihydropyridine antagonists including diltiazem, flunarizine, or verapamil. The known actions of (±)Bay K 8644 as an L type calcium channel agonist, the reproduction of similar behavior with another L type calcium channel agonist, and the protection afforded by certain L type calcium channel antagonists implicate calcium channels in the mediation of the self-biting behavior. This phenomenon provides a model for studying the neurobiology of this unusual behavior.
Self-biting (SB) and other self-injurious behavior (SIB) may occur in a number of different human disorders (1, 2). They are most commonly seen in developmentally disabled individuals with severe mental retardation or autism. These behaviors also occur in specific neurogenetic diseases such as Lesch–Nyhan syndrome, Cornelia de Lange syndrome, Rett's syndrome, Tourette's syndrome, and neuroacanthocytosis. The neurobiology of these peculiar behaviors is incompletely understood, and several animal models have been developed to study pathophysiology and potential treatments (3). For example, SB and SIB may be provoked in rodents after high doses or chronic administration of drugs that promote dopamine release such as amphetamine, GBR-12909, and pemoline (4–8). SB and SIB also occur in rodents after stimulation of dopamine receptors if brain dopamine neurons are destroyed during early development (9). Clonidine (10, 11) and the methylxanthines caffeine and theophylline can also provoke these behaviors at high doses, though the mechanism by which they do so remains unclear (6, 12, 13).
To date, calcium channels have received little attention as mediators or potential therapeutic targets for SB or SIB. Calcium channels are expressed throughout the nervous system and in other organs, where they play an important role in stimulus–response coupling. Several calcium channel subtypes are currently recognized by their different pharmacological and electrophysiological properties (14). The L type calcium channel is voltage-gated and allows a transient influx of calcium in response to cell membrane depolarization. Within the brain, these channels are expressed at particularly high levels in the striatum, cortex, and hippocampus (15–18).
(±)Bay K 8644 functions as an L type calcium channel activator that increases calcium fluxes in response to depolarizing stimuli (19–21). In rodents, this drug has been reported to produce characteristic motor abnormalities including impaired ambulation, twisting and stretching movements, transient limb extension, back arching, spasticity, ataxia, or catatonia (22–28). Some studies have anecdotally noted the occurrence of SIB with this drug (23–25, 27), though this phenomenon has received little attention. The current study shows that (±)Bay K 8644 will reliably provoke SB and SIB under certain conditions in mice, providing a tool to study the neurobiology of this unusual behavior.
Methods
Animals.
C57BL/6J mice were obtained from The Jackson Laboratory and maintained in the Johns Hopkins Medical Institutions animal care facilities for at least 5 days before testing. They were kept on a 12-h light/dark cycle with free access to food and water. All animal procedures were conducted in strict accordance with published guidelines from the National Institutes of Health.
Materials.
(±)Bay K 8644, the agonist enantiomer (−)Bay K 8644, the antagonist enantiomer (+)Bay K 8644, and FPL 64176 (Research Biochemicals, Natick, MA) are poorly soluble in aqueous solutions. For subcutaneous administration, the drugs were dissolved in ethanol at a concentration of 20 mg/ml and then mixed with an equal volume of Tween 80 (Sigma). This preparation was diluted at least 10-fold with distilled water and injected subcutaneously at a final volume of 10 ml/kg. For intracerebral injections, the drugs were dissolved in dimethyl sulfoxide.
Behavior Testing.
Initial dose-response profiles were obtained with an escalating-dose paradigm, where behavioral assessments were conducted after subcutaneous injections of saline, 2 mg/kg, 4 mg/kg, 8 mg/kg, and 12 mg/kg (±)Bay K 8644 at daily intervals. Independent groups of mice were examined at three different ages: weanling (3–4 weeks), young adult (4–5 weeks), and full adult (6–8 weeks). To assess the potential contributions of the repeated dosing paradigm, another group of 10 mice was given the same drug dose on five separate occasions.
Behavior was also examined after microinjection of (±)Bay K 8644 or dimethyl sulfoxide directly into the brain. A total of five animals were examined at each of the following doses: 10, 25, 50, and 100 μg of (±)Bay K 8644. Animals were anesthetized with methoxyflurane, and the surface of the skull was partially exposed with a 1-cm midline longitudinal incision. Vehicle or drug was administered by inserting a 28-gauge needle though the skull into the lateral ventricle by using coordinates estimated from an atlas of the mouse brain (29). The needle was blunted and shortened to provide a consistent injection depth of 3 mm from the surface of the skull. Injections were 2.5 mm anterior to bregma and 1 mm lateral to the saggital suture. Animals began to awaken 5 min after discontinuation of methoxyflurane anesthesia and were usually ambulatory within 10 min.
For behavioral assessments, mice were placed singly in 20 × 30-cm clear plastic boxes similar to the home cage. The mice were observed for 1 min each at 10-min intervals for 1 h by using a behavioral sampling method (30). SB and SIB were recorded independently. To prevent unnecessary pain or suffering, any animal that caused tissue injury or bleeding was immediately anesthetized with methoxyflurane and given 20 mg/kg nifedipine subcutaneously. This treatment reliably terminated all SIB.
Aggression was assessed by three methods. The first involved the resident–intruder paradigm, where the frequency of attack behavior by a drug-treated aggressor was recorded (31). In the second paradigm, 10 mice were placed individually in a test cage and a wooden stick was presented at the snout five separate times for 2–3 s each. The number of mice biting the stick and the frequency of bites were recorded. In the third paradigm, 10 mice were each lowered individually by their tails onto a foam platform on five separate trials. The number of mice biting the platform and the frequency of bites were recorded.
Data Analysis.
SB and SIB scores were analyzed by calculating the percentage of scoring intervals in which the target behavior occurred (30). Aggression scores were analyzed by calculating the percentage of biting behavior in each paradigm. Because all target behaviors were reduced or eliminated by administration of nifedipine to prevent self-injury, any animal given nifedipine received the last scores recorded for the remainder of the test period. Average scores were then compared by ANOVA, with subsequent Tukey's tests where appropriate.
Results
SB and SIB After (±)Bay K 8644.
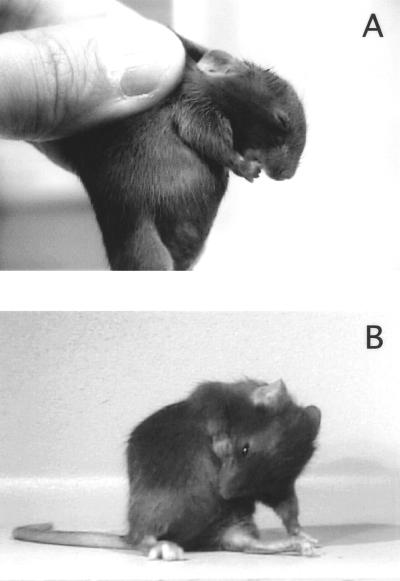
When placed in the test cage, untreated or vehicle-treated mice engaged in their typical behaviors including ambulation, rearing, sniffing, manipulating bedding, and occasional grooming. Normal grooming of the limbs or abdomen was readily distinguished from SB and SIB, which were never observed in untreated or vehicle-treated mice. At a dose of 2 mg/kg (±)Bay K 8644, exaggerated grooming behaviors such as licking or stroking the fur were not observed, and SB occurred rarely. As the dose increased, SB became more frequent, again without any other signs of excessive grooming. SB typically began within 10 min of drug administration, peaked at 20–30 min, and eventually waned and disappeared by 50–120 min. The mice typically bit their forepaws, shoulders, or abdomen (Fig. 1). The animals would sometimes vocalize and dart after biting, suggesting that they felt pain; and at higher doses, SB persisted and would lead to severe skin and bone injury if allowed to proceed.
Figure 1.
Typical biting of the paw (A) or abdomen (B) associated with 8 mg/kg (±)Bay K 8644.
Though the majority of biting was directed toward the animal's own body, some mice occasionally bit the bedding material or cage walls during testing. Other mice engaged in vacuous chewing movements with nothing in the mouth. When disturbed, however, violent biting was often directed toward the inciting stimulus.
Influence of Dose and Mouse Age.
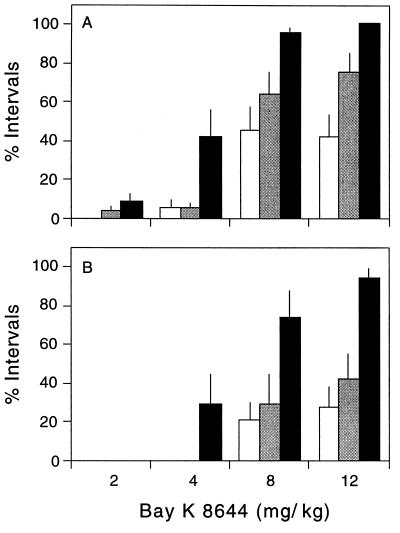
The influence of escalating doses of (±)Bay K 8644 was quantified in mice of different ages. The drug produced a dose-dependent increase in SB and SIB at all ages tested, but weanling mice engaged in SB and SIB far more frequently than adult mice (Fig. 2). A qualitative difference among animals of different ages was also observed. Weanling mice would often engage quietly and continuously in SB, eventually producing tissue injury. In adult mice, SB was often followed by vocalization and darting behavior, with the result that tissue injury was less common. These results show that the appearance of SB and SIB depends markedly on drug dose and mouse age.
Figure 2.
Influence of age and dose. Three independent groups of 10 mice each were tested at different ages: 3 weeks (black bars), 4–5 weeks (gray bars), and 6–8 weeks (white bars). Bars depict the average (± SEM) percentage of 10-min intervals in which SB (A) or SIB (B) were observed. Data were analyzed by a two-way ANOVA with age and dose as the main factors. For SB, there was a significant effect for both age (F = 27.2; P < 0.001) and dose (F = 65.6; P < 0.001). For SIB, there was also a significant effect for both age (F = 20.4; P < 0.001) and dose (F = 24.9; P < 0.001).
Desensitization with Repeated Dosing.
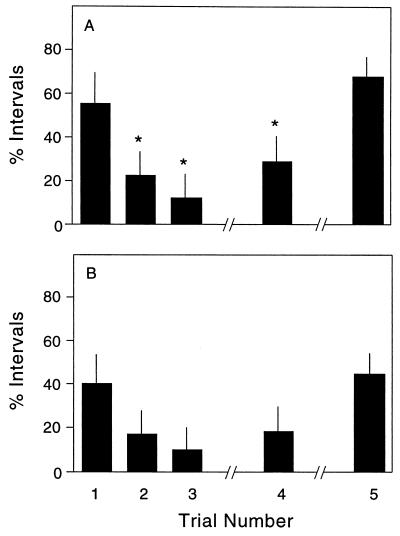
Several drugs capable of provoking SB and SIB in animals are associated with augmented behavioral responses with repeated testing, a phenomenon known as priming or sensitization (7, 32). To determine whether the SB and SIB observed at high doses of (±)Bay K 8644 were due to a priming effect rather than an increase in dose, a single group of drug-naïve adult mice was tested with the same dose of 8 mg/kg on five separate occasions. After the first trial, SB was observed during 55% and SIB during 40% of the scoring intervals (Fig. 3). The second trial, given 1 day later, was associated with approximately half the SB and SIB observed after the first trial. A third trial given on the third day was associated with a further reduction in both behaviors. However, these behaviors were at least partially restored in a fourth trial performed after 1 week of recovery and were fully restored in a fifth trial after 1 month of recovery. These results indicate desensitization of SB and SIB with repeated treatment with (±)Bay K 8644.
Figure 3.
Influence of repeated doses. A single group of 10 drug-naïve adult mice was tested with 8 mg/kg (±)Bay K 8644 on five separate trials. The first three trials were administered at 1-day intervals. The fourth trial was given after a 1-week delay, and the fifth trial after a 1-month delay. Data show the average percentage of 10-min intervals in which SB (A) or SIB (B) were observed (± SEM). ANOVA revealed significant differences among trials for SB (F = 62.6; P < 0.001). Asterisks denote significant differences from responses on the first trial on post hoc Tukey's tests. Though SIB showed trends identical to those for SB, the differences were not statistically significant because of the lower frequency and higher variability of this behavior overall (F = 1.4; P > 0.1).
Intracerebral Microinjection Studies.
SB and SIB are sometimes observed in rodents after injury to peripheral nerves, presumably as a consequence of irritating local paresthesias (33). To determine whether the behaviors associated with (±)Bay K 8644 were due to actions in the brain or at peripheral sites, intracerebral microinjections were performed. Injection of 10–100 μg of (±)Bay K 8644 directly into the lateral ventricle of the mouse brain dose-dependently reproduced all of the motor abnormalities reported for subcutaneous administration of the drug (22–28), with SB and SIB emerging at the highest dose (Table 1). These results show that the behaviors provoked by (±)Bay K 8644 are mediated by its effects in the brain rather than peripheral sites.
Table 1.
Intracerebral (±)Bay K 8644
| Mouse group | No. animals with SB | Percentage of time engaged in SB | No. animals with SIB | Percentage of time engaged in SIB |
|---|---|---|---|---|
| Control | 0/5 | 0 | 0/5 | 0 |
| Bay K 8644 | 5/5 | 76.7 ± 18.3* | 2/5 | 30.0 ± 20.7 |
Asterisk denotes P < 0.01, where the percentage of time animals were engaged in SB or SIB in control and treated animals was compared by ANOVA.
Inhibition with Calcium Antagonists.
Two experiments were performed to verify that the behaviors observed with (±)Bay K 8644 were due to activation of L type calcium channels and not due to some nonspecific side effect. First, it was shown that SB and SIB could be elicited with 2–12 mg/kg of the active agonist enantiomer (−)Bay K 8644 (not shown). The (+)Bay K 8644 enantiomer, which functions as a weak antagonist, had no obvious effect on mouse behavior in doses of 2–12 mg/kg. The overall effects of the racemic preparation are likely to reflect calcium channel agonism, because the agonist isomer is approximately 16-fold more potent than the antagonist isomer (34).
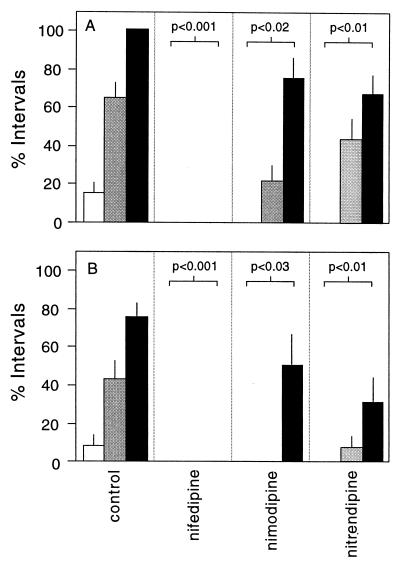
Second, mice were pretreated with one of several different L type calcium channel antagonists 5 min before (±)Bay K 8644. Nifedipine, nimodipine, and nitrendipine are L type calcium channel antagonists that have a dihydropyridine structure. Nifedipine (20 mg/kg) completely prevented the SB and SIB associated with (±)Bay K 8644 (Fig. 4 A and B). Nimodipine (20 mg/kg) and nitrendipine (20 mg/kg) also attenuated both behaviors, though less effectively than nifedipine (Fig. 4 A and B). In contrast to the protective effect of the dihydropyridine antagonists, three nondihydropyridine antagonists, diltiazem, (20 mg/kg), flunarizine (20 mg/kg), and verapamil (20 mg/kg) failed to influence either behavior significantly (not shown). These results show that SB and SIB after (±)Bay K 8644 can be attenuated with dihydropyridine L type calcium channel antagonists but not with nondihydropyridine antagonists.
Figure 4.
Influence of pretreatment with calcium channel antagonists. Independent groups of drug-naïve mice were pretreated with saline or 20 mg/kg of diltiazem, flunarizine, nifedipine, nimodipine, nitrendipine, or verapamil. They then received 2 mg/kg (white bars), 4 mg/kg (gray bars), or 8 mg/kg (black bars) of (±)Bay K 8644. Bars show the average percentage of 10-min intervals in which SB (A) and SIB (B) were observed among groups of eight mice (± SEM). Data were analyzed by multivariate ANOVA with (±)Bay K 8644 dose, pretreatment condition, and target behavior as the main variables. The overall model indicated significant interactive effects for (±)Bay K 8644 dose and pretreatment condition for both SB (F = 4.4; P < 0.001) and SIB (F = 1.9; P < 0.05). Pretreatment with nifedipine, nimodipine, or nitrendipine significantly reduced SB and SIB in comparison with the control group (individual P values shown in the figure), whereas pretreatment with diltiazem, flunarizine, or verapamil had no significant effect (not shown).
Behavioral Effects of FPL 64176.
If the behavioral effects observed with (±)Bay K 8644 are due to calcium channel activation, then other calcium channel activators would be expected to produce similar effects. The L type calcium channel activator FPL 64176 (20, 35) produced motor abnormalities essentially identical to those observed with (±)Bay K 8644 with two exceptions. First, the motor disability seemed more severe with FPL 64176 than with (±)Bay K 8644 in the same dosage ranges. Second, toxicity was significant, because more than half of the animals did not survive treatment with the highest dose. Despite the more severe motor disability and toxicity, 8 of 10 animals treated with 12 mg/kg FPL 64176 displayed SB (not shown).
Aggressive Behavior.
In addition to SB and SIB, mice treated with (±)Bay K 8644 seemed unusually aggressive, because they would often direct violent biting toward the examiner or any disturbing stimulus. The frequently used resident–intruder paradigm (31) provided no evidence for increased aggression, perhaps because the concomitant motor dysfunction inhibited expression in this paradigm (results not shown). However, aggressive biting could be measured via other paradigms. When presented with a wooden stick at the snout, normal mice pretreated with vehicle typically sniffed the stick or fled to another part of the cage. Only 1 of 10 mice bit the stick briefly before fleeing. In contrast, 9 of 10 mice treated with 8 mg/kg (±)Bay K 8644 bit the stick (Table 2). In addition, these mice often failed to release their jaws after biting; and they could be physically lifted from the cage floor by their teeth.
Table 2.
Aggressive biting behavior
| Mouse group | No. animals biting stick | Percentage of total stick bites | No. animals biting foam | Percentage of total foam bites |
|---|---|---|---|---|
| Control | 1/10 | 2.0 ± 2.1 | 4/10 | 10.0 ± 4.7 |
| Bay K 8644 | 9/10 | 42.0 ± 10.2* | 10/10 | 70.0 ± 9.0* |
Asterisks denote P < 0.01, where the percentage of stick or foam bites in control and treated animals was compared by ANOVA.
When slowly lowered by the tail onto a foam platform, normal mice grasped the foam with the forepaws and attempted to escape. Only 4 of 10 normal mice briefly bit the platform on initial contact as well. After treatment with 8 mg/kg (±)Bay K 8644, all 10 mice bit the platform on the majority of attempts. In addition, these mice often tore out large pieces, a behavior never observed in untreated mice (Table 2).
Discussion
The current study shows that (±)Bay K 8644 reliably provokes SB and SIB when given to young mice. The known actions of this drug as an L type calcium channel agonist (20, 21, 35), the reproduction of similar behavior with a structurally unrelated L type calcium channel agonist (FPL 64176), and the protection afforded by dihydropyridine calcium channel antagonists (Fig. 4) implicate L type calcium channels in the mediation of these behaviors. The behaviors are likely to reflect activation of brain calcium channels, because microinjection of smaller doses of (±)Bay K 8644 directly into the brain reproduced the same features observed with subcutaneous administration (Table 1).
Though L type calcium channels are widely expressed in the brain, channels in certain brain regions may be differentially activated by this drug. The susceptibility to activation is likely to depend on several variables, including differences in the quantity of channels expressed, their susceptibility to activation, their efficiency of coupling to subsequent intracellular events, or compensatory mechanisms to an exogenous calcium influx. SB and SIB have most often been associated with dysfunction of the striatum (3). This brain region expresses particularly high levels of L type calcium channels (15–18), suggesting that a preferential action of (±)Bay K 8644 in this brain region may underlie its ability to provoke SB or SIB. A peak in the expression of these channels during the first few weeks of age in brain tissue of normal mice (36) may be responsible for the increased sensitivity of weanling animals in comparison to adults.
Because several prior studies have also implicated abnormalities in dopaminergic (4–9) and serotonergic (37) transmission in the expression of SB and SIB, it is useful to consider a potential effect of (±)Bay K 8644 on these monoamine systems. (±)Bay K 8644 has already been shown to increase the synthesis, release, and turnover of both monoamines in the brain (38–43). Recent studies have also shown a direct association between D1 dopamine receptors and calcium channel activity (44). It seems likely that calcium channel activation with (±)Bay K 8644 might be mediated through activation of brain dopamine or serotonin systems, though more work will be required to determine whether this mediation occurs at the presynaptic or postsynaptic level.
To our knowledge, calcium channels have not received wide attention as potential mediators of SB or SIB in animals or humans. Nor have calcium channel antagonists been considered as potential therapeutic targets. The current study provides a tool for the investigation of the neurobiology of these unusual behaviors. In addition, the wide availability of safe drugs acting at calcium channels may also provide a treatment approach for affected individuals.
Acknowledgments
We would like to thank E. J. Hess and S. G. Reich for reviewing the manuscript. H.A.J. is supported by National Institutes of Health Grant NS01985 and the Lesch–Nyhan Syndrome Children's Research Foundation.
Abbreviations
- SB
self-biting
- SIB
self-injurious behavior
References
- 1.King B H. Am J Ment Retard. 1993;98:93–112. [PubMed] [Google Scholar]
- 2.Winchel R M, Stanley M. Am J Psychiatr. 1991;148:306–317. doi: 10.1176/ajp.148.3.306. [DOI] [PubMed] [Google Scholar]
- 3.Jinnah H A, Breese G R. In: Biological Aspects of Disease: Contributions from Animal Models. Iannocconne P M, Scarpelli D G, editors. Amsterdam: Harwood; 1997. pp. 93–143. [Google Scholar]
- 4.Sivam S P. Brain Res. 1995;690:259–263. doi: 10.1016/0006-8993(95)00604-o. [DOI] [PubMed] [Google Scholar]
- 5.Lara-Lemus A, Mora M P, Mendez-Franco J, Palomero-Rivero M, Drucker-Colin R. Brain Res. 1997;770:60–64. doi: 10.1016/s0006-8993(97)00746-4. [DOI] [PubMed] [Google Scholar]
- 6.Mueller K, Saboda S, Palmour R, Nyhan W L. Pharmacol Biochem Behav. 1982;17:613–617. doi: 10.1016/0091-3057(82)90332-x. [DOI] [PubMed] [Google Scholar]
- 7.Mueller K, Hollingsworth E, Pettit H. Pharmacol Biochem Behav. 1986;25:933–938. doi: 10.1016/0091-3057(86)90065-1. [DOI] [PubMed] [Google Scholar]
- 8.King B H, Au D, Poland R E. Dev Neurosci. 1995;17:47–52. doi: 10.1159/000111272. [DOI] [PubMed] [Google Scholar]
- 9.Moy S S, Criswell H E, Breese G R. Neurosci Biobehav Rev. 1997;21:425–435. doi: 10.1016/s0149-7634(96)00040-1. [DOI] [PubMed] [Google Scholar]
- 10.Razzak A, Fujiwara M, Ueki S. Eur J Pharmacol. 1975;30:356–359. doi: 10.1016/0014-2999(75)90121-1. [DOI] [PubMed] [Google Scholar]
- 11.Katsuragi T, Ushijima I, Furukawa T. Pharmacol Biochem Behav. 1984;20:943–946. doi: 10.1016/0091-3057(84)90020-0. [DOI] [PubMed] [Google Scholar]
- 12.Ferrer I, Costell M, Grisolia S. FEBS Lett. 1982;141:275–278. doi: 10.1016/0014-5793(82)80065-3. [DOI] [PubMed] [Google Scholar]
- 13.Morgan L L, Schneiderman N, Nyhan W L. Psychon Sci. 1970;19:37–38. [Google Scholar]
- 14.Catterall W A. Annu Rev Biochem. 1995;64:493–531. doi: 10.1146/annurev.bi.64.070195.002425. [DOI] [PubMed] [Google Scholar]
- 15.Marangos P J, Patel J, Miller C, Martino A M. Life Sci. 1982;31:1575–1585. doi: 10.1016/0024-3205(82)90049-2. [DOI] [PubMed] [Google Scholar]
- 16.Quirion R, Lal S, Nair N P, Stratford J G, Ford R M, Olivier A. Prog. Neuropsychopharmacol. Biol. Psychiatr. 1985. 643–649. [DOI] [PubMed] [Google Scholar]
- 17.Hirota K, Lambert D G. Neurosci Lett. 1997;223:169–172. doi: 10.1016/s0304-3940(97)13434-6. [DOI] [PubMed] [Google Scholar]
- 18.Ramkumar V, El-Fakahany E E. Eur J Pharmacol. 1988;146:73–83. doi: 10.1016/0014-2999(88)90488-8. [DOI] [PubMed] [Google Scholar]
- 19.Rampe D, Lacerda A E. J Pharmacol Exp Ther. 1991;259:982–987. [PubMed] [Google Scholar]
- 20.Zheng W, Rampe D, Triggle D J. Mol Pharmacol. 1991;40:734–741. [PubMed] [Google Scholar]
- 21.Triggle D J, Janis R A. Annu Rev Pharmacol Toxicol. 1987;27:347–369. doi: 10.1146/annurev.pa.27.040187.002023. [DOI] [PubMed] [Google Scholar]
- 22.Bolger G T, Weissman B A, Skolnick P. Naunyn-Schmiedeberg's Arch Pharmacol. 1985;328:373–377. doi: 10.1007/BF00692903. [DOI] [PubMed] [Google Scholar]
- 23.Peterson R N. Eur J Pharmacol. 1986;130:323–326. doi: 10.1016/0014-2999(86)90286-4. [DOI] [PubMed] [Google Scholar]
- 24.O'Neill S K, Bolger G T. Brain Res Bull. 1988;21:865–872. doi: 10.1016/0361-9230(88)90019-6. [DOI] [PubMed] [Google Scholar]
- 25.Shelton R C, Grebb J A, Freed W J. Brain Res. 1987;402:399–402. doi: 10.1016/0006-8993(87)90054-0. [DOI] [PubMed] [Google Scholar]
- 26.Bourson A, Moser P C, Gower A J, Mir A K. Eur J Pharmacol. 1989;160:339–347. doi: 10.1016/0014-2999(89)90089-7. [DOI] [PubMed] [Google Scholar]
- 27.Bianchi M, Rovati L C, Sacerdote P, Mantegassa P, Panerai A E. Neurosci Res Commun. 1990;6:157–162. [Google Scholar]
- 28.Palmer G C, Stagnitto M L, Ray R K, Knowles M A, Harvey R, Garske G E. Epilepsia. 1993;34:372–380. doi: 10.1111/j.1528-1157.1993.tb02424.x. [DOI] [PubMed] [Google Scholar]
- 29.Franklin K B J, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. New York: Academic; 1997. [Google Scholar]
- 30.Fray P J, Sahakian B J, Robbins T W, Koob G F, Iversen S D. Psychopharmacology. 1980;69:253–259. doi: 10.1007/BF00433091. [DOI] [PubMed] [Google Scholar]
- 31.Guillot P V, Chapouthier G. Behav Brain Res. 1996;77:211–213. doi: 10.1016/0166-4328(95)00163-8. [DOI] [PubMed] [Google Scholar]
- 32.Stodgell C J, Loupe P S, Schroeder S R, Tessel R E. Brain Res. 1998;783:10–18. doi: 10.1016/s0006-8993(97)01128-1. [DOI] [PubMed] [Google Scholar]
- 33.Gorea E, Lombard M C. Neurosci Lett. 1984;48:75–80. doi: 10.1016/0304-3940(84)90291-x. [DOI] [PubMed] [Google Scholar]
- 34.O'Neill S K, McKay D W, Campbell N, Triggle C R, Crowley M, Bolger G T. J Pharmacol Exp Ther. 1990;253:905–910. [PubMed] [Google Scholar]
- 35.Rampe D, Anderson B, Rapien-Pryor V, Li T, Dage R C. J Pharmacol Exp Ther. 1993;265:1125–1130. [PubMed] [Google Scholar]
- 36.Litzinger M J, Grover B B, Saderup S, Abbott J R. Int J Dev Neurosci. 1993;11:17–24. doi: 10.1016/0736-5748(93)90031-8. [DOI] [PubMed] [Google Scholar]
- 37.Pies R W, Popli A P. J Clin Psychiatr. 1995;56:580–588. [PubMed] [Google Scholar]
- 38.Woodward J J, Leslie S W. Brain Res. 1986;370:397–400. doi: 10.1016/0006-8993(86)90502-0. [DOI] [PubMed] [Google Scholar]
- 39.Pileblad E, Carlsson A. Neuropharmacology. 1987;26:101–105. doi: 10.1016/0028-3908(87)90052-9. [DOI] [PubMed] [Google Scholar]
- 40.Bolger G T, Lesieur P, Basile A S, Skolnick P. Brain Res. 1988;438:101–107. doi: 10.1016/0006-8993(88)91328-5. [DOI] [PubMed] [Google Scholar]
- 41.Chaudieu I, Alonso R, Mount H, Quirion R, Boksa P. Eur J Pharmacol. 1992;220:203–209. doi: 10.1016/0014-2999(92)90749-t. [DOI] [PubMed] [Google Scholar]
- 42.Woodward J J, Cook M E, Leslie S W. Proc Natl Acad Sci USA. 1988;85:7389–7393. doi: 10.1073/pnas.85.19.7389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Colado M I, Ormazabal M J, Alfaro M J, Martin M I. J Pharm Pharmacol. 1993;45:220–222. doi: 10.1111/j.2042-7158.1993.tb05537.x. [DOI] [PubMed] [Google Scholar]
- 44.Surmeier D J, Bargas J, Hemmings H C, Nairn A C, Greengard P. Neuron. 1995;14:385–397. doi: 10.1016/0896-6273(95)90294-5. [DOI] [PubMed] [Google Scholar]