Abstract
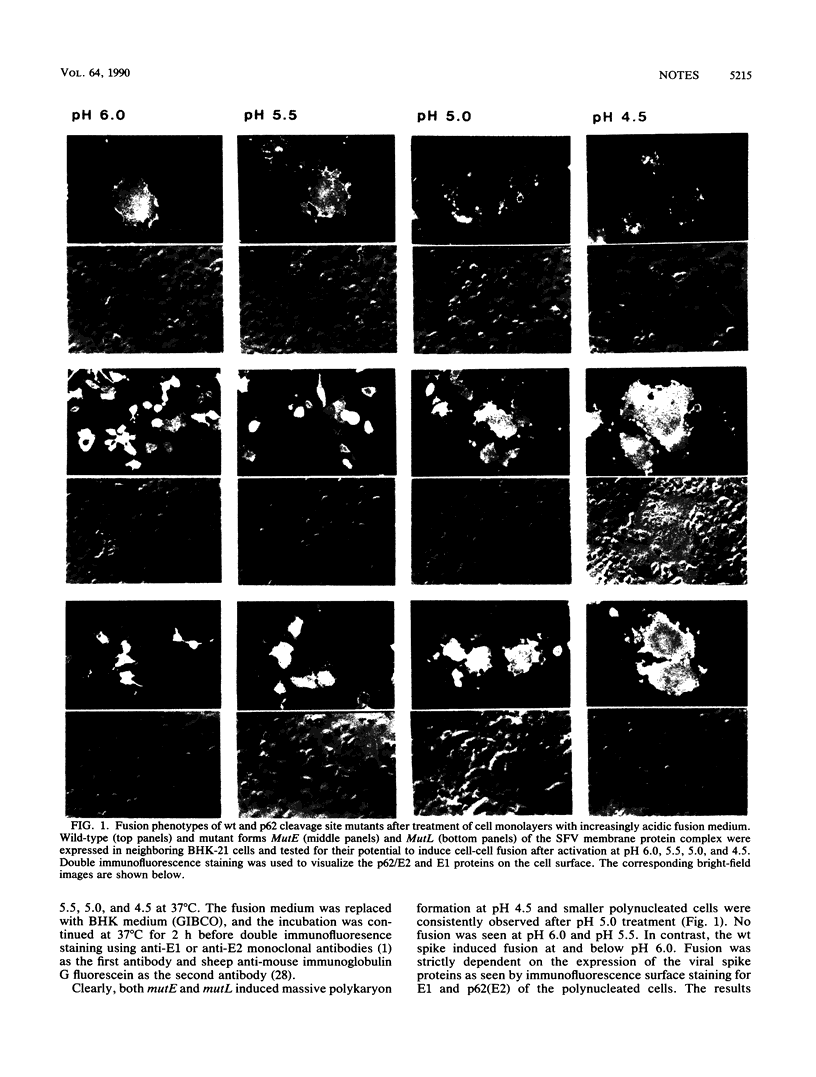
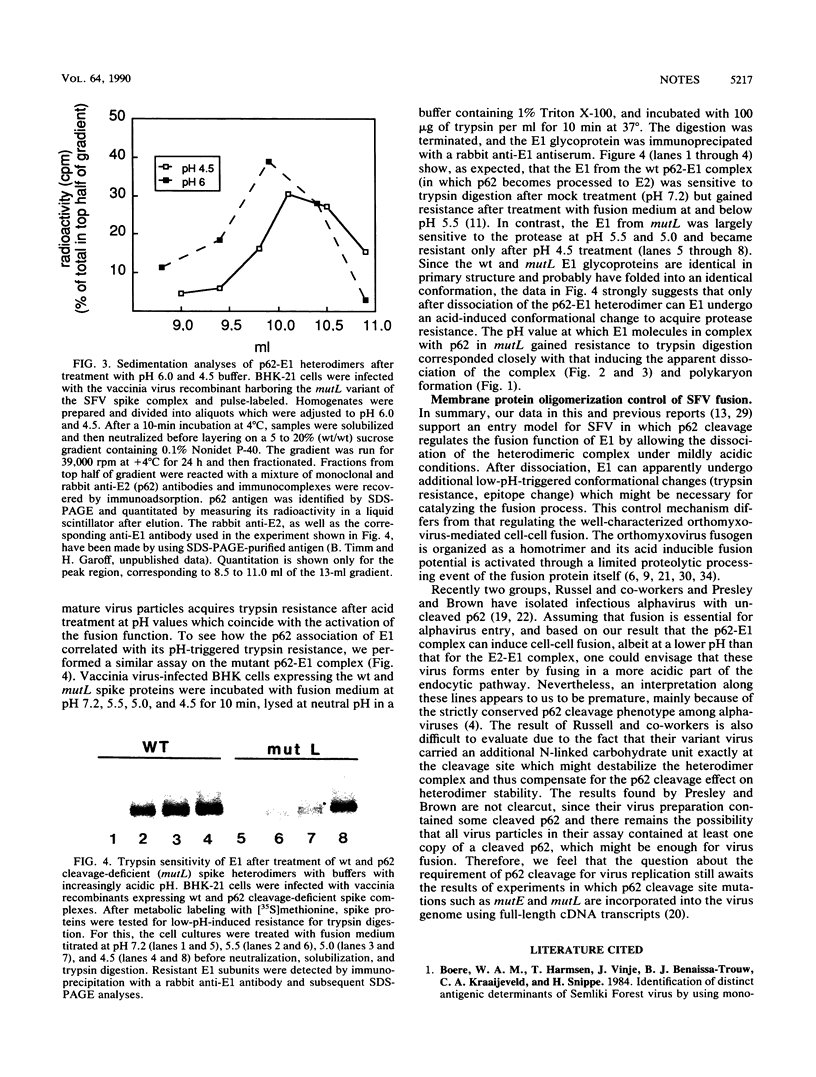
We have recently shown, using cleavage-deficient mutants of the p62-E1 membrane protein complex of Semliki Forest virus that p62 cleavage to E2 is necessary for the activation of the fusion function of the complex at pH 5.8 (a pH optimal for virus fusion) (M. Lobigs and H. Garoff, J. Virol. 64:1233-1240, 1990). In this study, we show that the mutant precursor complexes can be induced to activate membrane fusion when treated with more acidic buffers (pH 5.0 and 4.5), which also appear to dissociate most of the p62-E1 complexes and change the conformation of the E1 subunit (the supposed fusion protein of Semliki Forest virus into a form which is resistant to trypsin digestion. These data suggest that p62 cleavage is not essential for membrane fusion per se but that the crucial event activating this process seems to be the apparent dissociation of the heterodimer, which in turn is facilitated by the spike precursor cleavage.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boggs W. M., Hahn C. S., Strauss E. G., Strauss J. H., Griffin D. E. Low pH-dependent Sindbis virus-induced fusion of BHK cells: differences between strains correlate with amino acid changes in the E1 glycoprotein. Virology. 1989 Apr;169(2):485–488. doi: 10.1016/0042-6822(89)90178-5. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Dalgarno L., Rice C. M., Strauss J. H. Ross River virus 26 s RNA: complete nucleotide sequence and deduced sequence of the encoded structural proteins. Virology. 1983 Aug;129(1):170–187. doi: 10.1016/0042-6822(83)90404-x. [DOI] [PubMed] [Google Scholar]
- Doms R. W., Helenius A. Quaternary structure of influenza virus hemagglutinin after acid treatment. J Virol. 1986 Dec;60(3):833–839. doi: 10.1128/jvi.60.3.833-839.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed E. O., Risser R. The role of envelope glycoprotein processing in murine leukemia virus infection. J Virol. 1987 Sep;61(9):2852–2856. doi: 10.1128/jvi.61.9.2852-2856.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoff H., Kondor-Koch C., Riedel H. Structure and assembly of alphaviruses. Curr Top Microbiol Immunol. 1982;99:1–50. doi: 10.1007/978-3-642-68528-6_1. [DOI] [PubMed] [Google Scholar]
- Garten W., Bosch F. X., Linder D., Rott R., Klenk H. D. Proteolytic activation of the influenza virus hemagglutinin: The structure of the cleavage site and the enzymes involved in cleavage. Virology. 1981 Dec;115(2):361–374. doi: 10.1016/0042-6822(81)90117-3. [DOI] [PubMed] [Google Scholar]
- Harter C., James P., Bächi T., Semenza G., Brunner J. Hydrophobic binding of the ectodomain of influenza hemagglutinin to membranes occurs through the "fusion peptide". J Biol Chem. 1989 Apr 15;264(11):6459–6464. [PubMed] [Google Scholar]
- Kielian M., Helenius A. pH-induced alterations in the fusogenic spike protein of Semliki Forest virus. J Cell Biol. 1985 Dec;101(6):2284–2291. doi: 10.1083/jcb.101.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondor-Koch C., Burke B., Garoff H. Expression of Semliki Forest virus proteins from cloned complementary DNA. I. The fusion activity of the spike glycoprotein. J Cell Biol. 1983 Sep;97(3):644–651. doi: 10.1083/jcb.97.3.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobigs M., Garoff H. Fusion function of the Semliki Forest virus spike is activated by proteolytic cleavage of the envelope glycoprotein precursor p62. J Virol. 1990 Mar;64(3):1233–1240. doi: 10.1128/jvi.64.3.1233-1240.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M., Helenius A. Virus entry into animal cells. Adv Virus Res. 1989;36:107–151. doi: 10.1016/S0065-3527(08)60583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune J. M., Rabin L. B., Feinberg M. B., Lieberman M., Kosek J. C., Reyes G. R., Weissman I. L. Endoproteolytic cleavage of gp160 is required for the activation of human immunodeficiency virus. Cell. 1988 Apr 8;53(1):55–67. doi: 10.1016/0092-8674(88)90487-4. [DOI] [PubMed] [Google Scholar]
- Mellman I., Fuchs R., Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- Morrison T. G. Structure, function, and intracellular processing of paramyxovirus membrane proteins. Virus Res. 1988 May;10(2-3):113–135. doi: 10.1016/0168-1702(88)90010-x. [DOI] [PubMed] [Google Scholar]
- Omar A., Koblet H. Semliki Forest virus particles containing only the E1 envelope glycoprotein are infectious and can induce cell-cell fusion. Virology. 1988 Sep;166(1):17–23. doi: 10.1016/0042-6822(88)90141-9. [DOI] [PubMed] [Google Scholar]
- Presely J. F., Brown D. T. The proteolytic cleavage of PE2 to envelope glycoprotein E2 is not strictly required for the maturation of Sindbis virus. J Virol. 1989 May;63(5):1975–1980. doi: 10.1128/jvi.63.5.1975-1980.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice C. M., Levis R., Strauss J. H., Huang H. V. Production of infectious RNA transcripts from Sindbis virus cDNA clones: mapping of lethal mutations, rescue of a temperature-sensitive marker, and in vitro mutagenesis to generate defined mutants. J Virol. 1987 Dec;61(12):3809–3819. doi: 10.1128/jvi.61.12.3809-3819.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruigrok R. W., Aitken A., Calder L. J., Martin S. R., Skehel J. J., Wharton S. A., Weis W., Wiley D. C. Studies on the structure of the influenza virus haemagglutinin at the pH of membrane fusion. J Gen Virol. 1988 Nov;69(Pt 11):2785–2795. doi: 10.1099/0022-1317-69-11-2785. [DOI] [PubMed] [Google Scholar]
- Russell D. L., Dalrymple J. M., Johnston R. E. Sindbis virus mutations which coordinately affect glycoprotein processing, penetration, and virulence in mice. J Virol. 1989 Apr;63(4):1619–1629. doi: 10.1128/jvi.63.4.1619-1629.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger S., Schlesinger M. J. Formation of Sindbis virus proteins: identification of a precursor for one of the envelope proteins. J Virol. 1972 Nov;10(5):925–932. doi: 10.1128/jvi.10.5.925-932.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid S., Fuchs R., Kielian M., Helenius A., Mellman I. Acidification of endosome subpopulations in wild-type Chinese hamster ovary cells and temperature-sensitive acidification-defective mutants. J Cell Biol. 1989 Apr;108(4):1291–1300. doi: 10.1083/jcb.108.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K., Warren G. Semliki Forest virus: a probe for membrane traffic in the animal cell. Adv Protein Chem. 1984;36:79–132. doi: 10.1016/S0065-3233(08)60296-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel J. J., Bayley P. M., Brown E. B., Martin S. R., Waterfield M. D., White J. M., Wilson I. A., Wiley D. C. Changes in the conformation of influenza virus hemagglutinin at the pH optimum of virus-mediated membrane fusion. Proc Natl Acad Sci U S A. 1982 Feb;79(4):968–972. doi: 10.1073/pnas.79.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman L. S., Holmes K. V. Proteolytic cleavage of peplomeric glycoprotein E2 of MHV yields two 90K subunits and activates cell fusion. Adv Exp Med Biol. 1984;173:25–35. doi: 10.1007/978-1-4615-9373-7_3. [DOI] [PubMed] [Google Scholar]
- Timm B., Kondor-Koch C., Lehrach H., Riedel H., Edström J. E., Garoff H. Expression of viral membrane proteins from cloned cDNA by microinjection into eukaryotic cell nuclei. Methods Enzymol. 1983;96:496–511. doi: 10.1016/s0076-6879(83)96043-3. [DOI] [PubMed] [Google Scholar]
- Wahlberg J. M., Boere W. A., Garoff H. The heterodimeric association between the membrane proteins of Semliki Forest virus changes its sensitivity to low pH during virus maturation. J Virol. 1989 Dec;63(12):4991–4997. doi: 10.1128/jvi.63.12.4991-4997.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton S. A., Ruigrok R. W., Martin S. R., Skehel J. J., Bayley P. M., Weis W., Wiley D. C. Conformational aspects of the acid-induced fusion mechanism of influenza virus hemagglutinin. Circular dichroism and fluorescence studies. J Biol Chem. 1988 Mar 25;263(9):4474–4480. [PubMed] [Google Scholar]
- White J., Helenius A. pH-dependent fusion between the Semliki Forest virus membrane and liposomes. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3273–3277. doi: 10.1073/pnas.77.6.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Kartenbeck J., Helenius A. Fusion of Semliki forest virus with the plasma membrane can be induced by low pH. J Cell Biol. 1980 Oct;87(1):264–272. doi: 10.1083/jcb.87.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Matlin K., Helenius A. Cell fusion by Semliki Forest, influenza, and vesicular stomatitis viruses. J Cell Biol. 1981 Jun;89(3):674–679. doi: 10.1083/jcb.89.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley D. C., Skehel J. J. The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annu Rev Biochem. 1987;56:365–394. doi: 10.1146/annurev.bi.56.070187.002053. [DOI] [PubMed] [Google Scholar]
- Ziemiecki A., Garofff H. Subunit composition of the membrane glycoprotein complex of Semliki Forest virus. J Mol Biol. 1978 Jul 5;122(3):259–269. doi: 10.1016/0022-2836(78)90189-4. [DOI] [PubMed] [Google Scholar]
- de Curtis I., Simons K. Dissection of Semliki Forest virus glycoprotein delivery from the trans-Golgi network to the cell surface in permeabilized BHK cells. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8052–8056. doi: 10.1073/pnas.85.21.8052. [DOI] [PMC free article] [PubMed] [Google Scholar]