Abstract
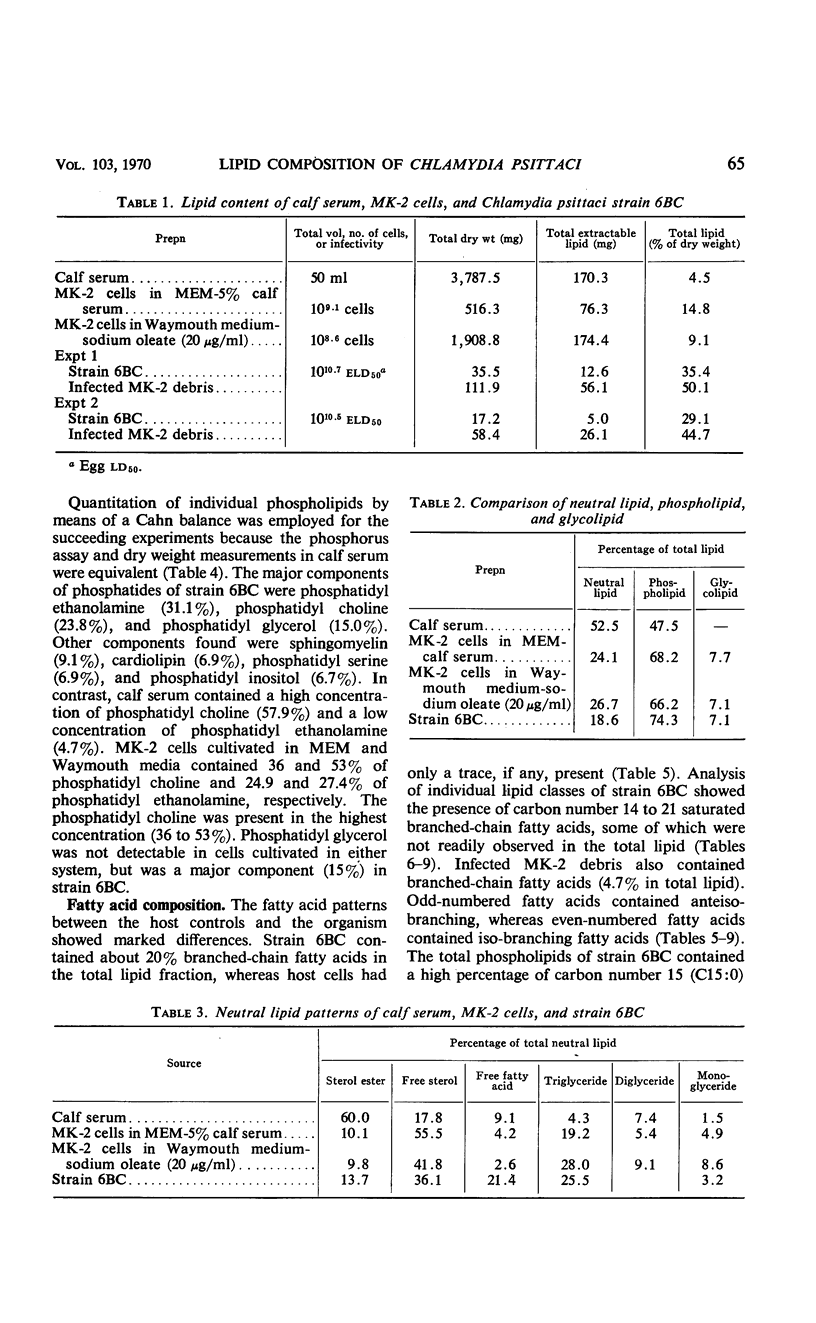
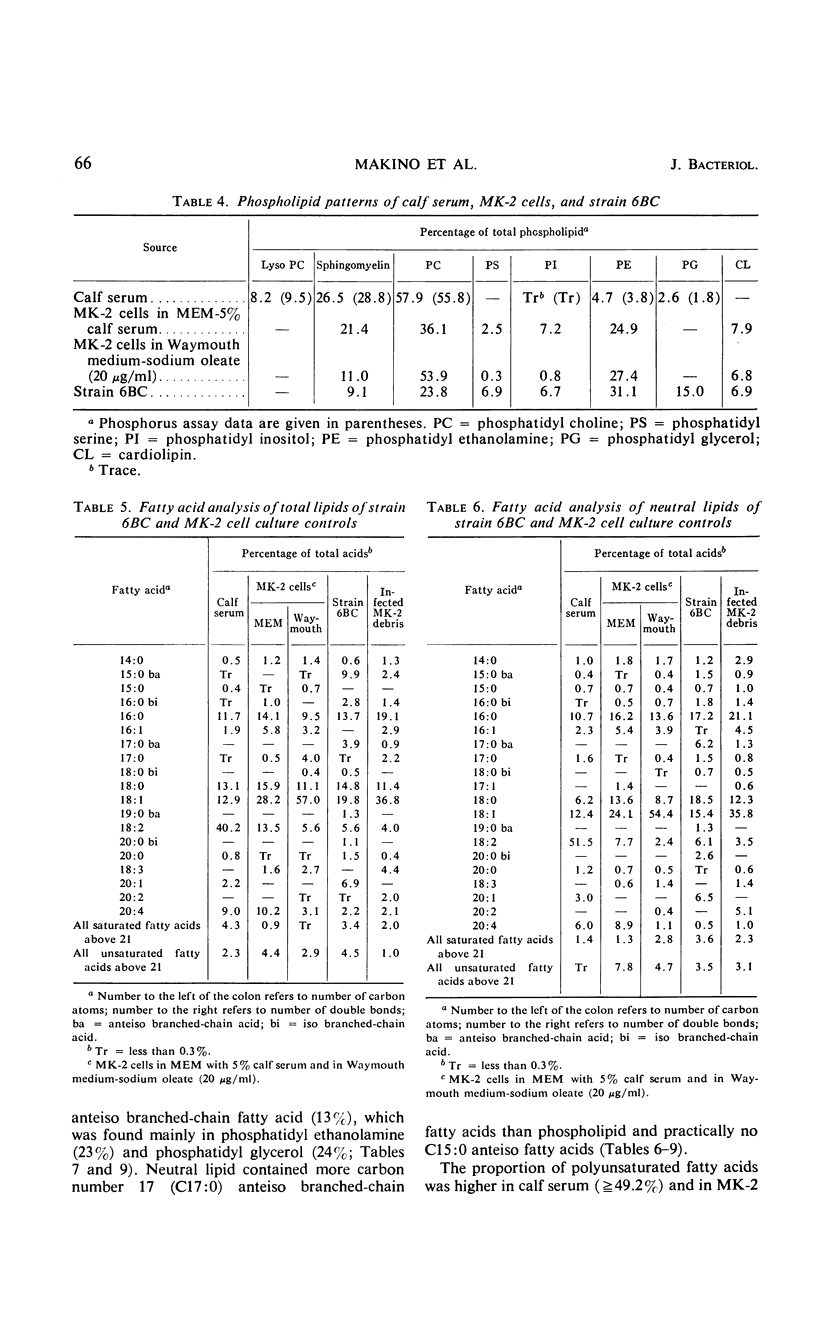
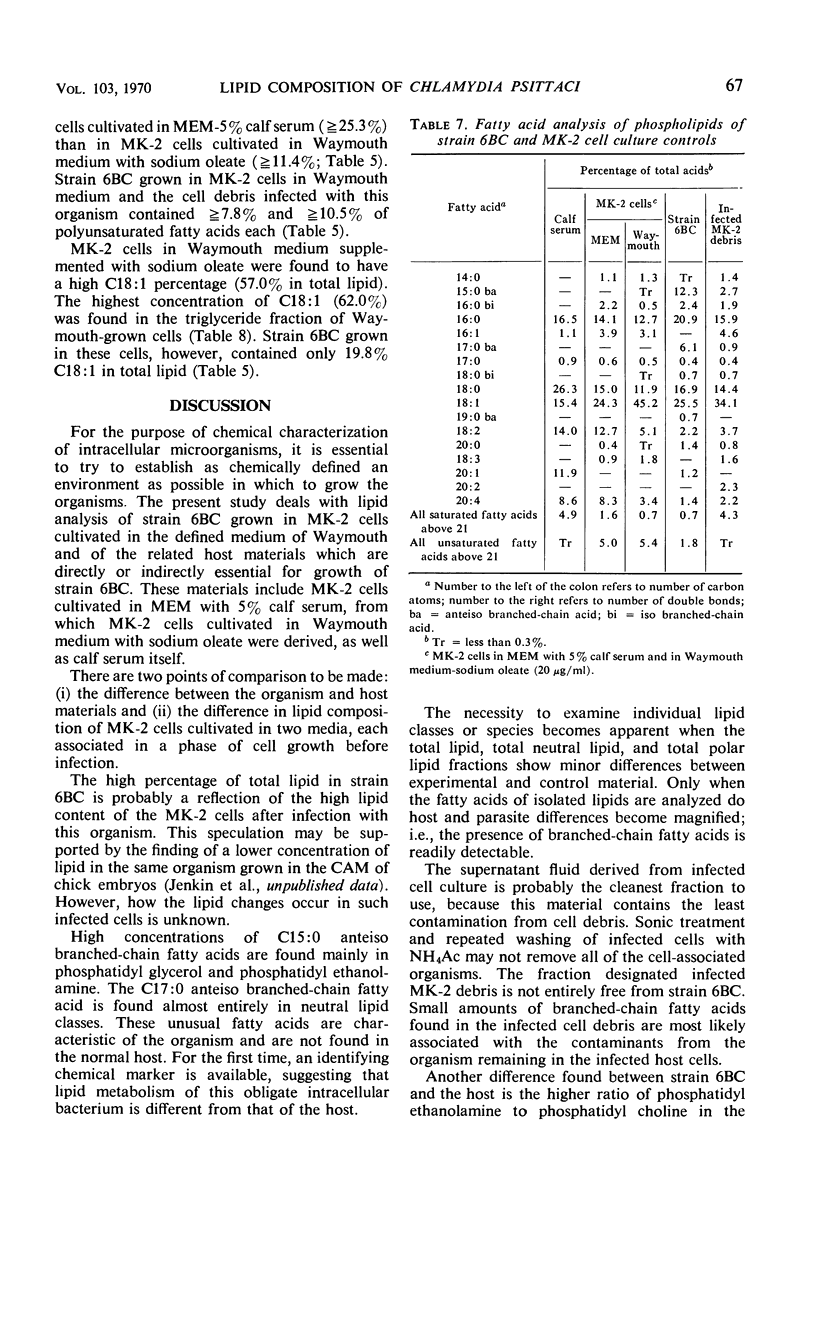
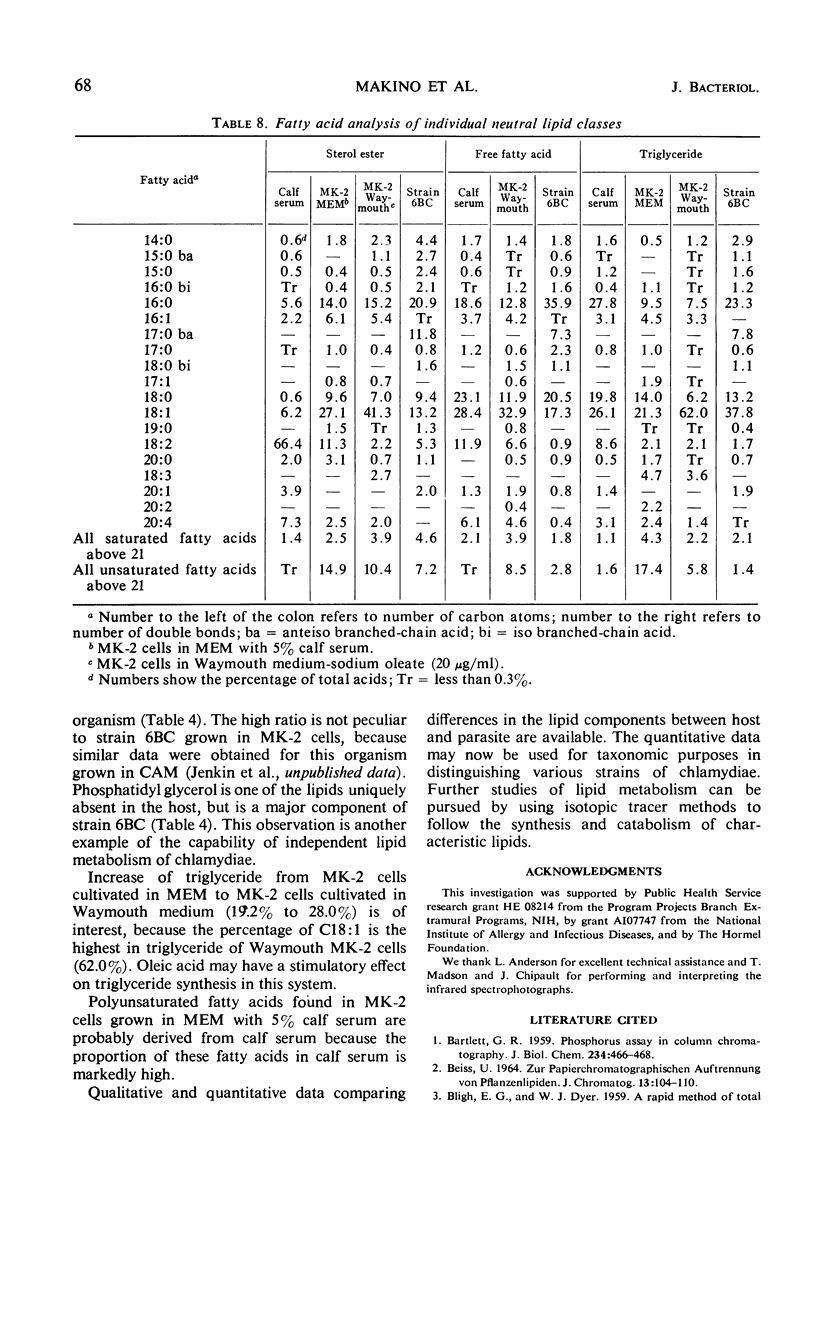
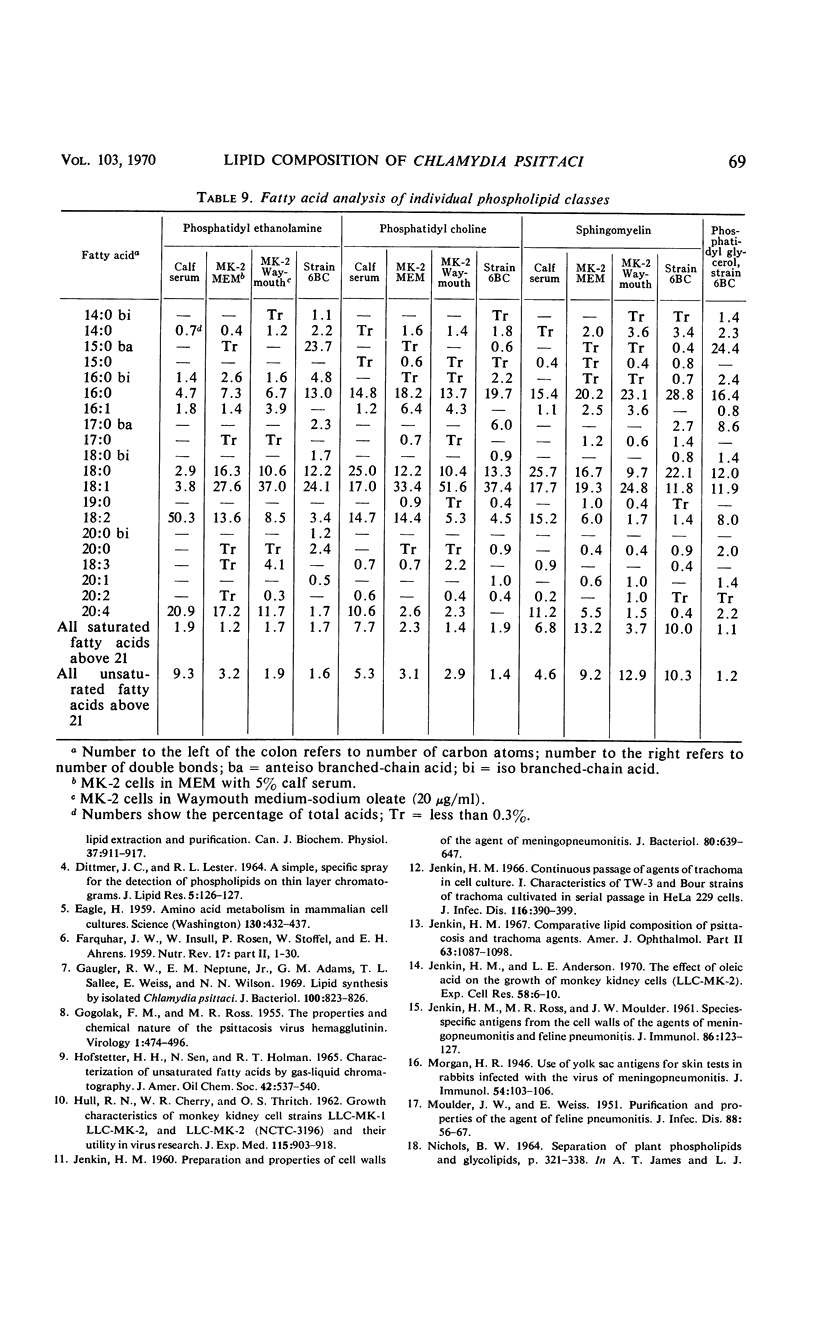
The lipid compositions of (i) monkey kidney (MK-2) cells cultivated in Eagle's minimal essential medium (MEM) with 5% calf serum, (ii) MK-2 cells cultivated in Waymouth medium supplemented with 20 μg of sodium oleate and 2 mg of bovine albumin per ml, (iii) Chlamydia psittaci strain 6BC grown in the latter host system, and (iv) calf serum were compared. Strain 6BC contains 31% phosphatidyl ethanolamine (PE) and 15% phosphatidyl glycerol (PG), whereas the host cell contains almost the same amount of PE (27%) and no PG. A high concentration of total lipid was observed in strain 6BC (29 to 34%), whereas MK-2 cells contain only 9 to 15% and calf serum contains 4.5% total lipid. The fatty acids of the total lipid from strain 6BC contain branched-chain acids. These fatty acids were found mostly in PE (33.0%) and PG (37.0%). No branched-chain fatty acid was found in the MK-2 cells. There was an increase in triglyceride content when MK-2 cells cultivated in MEM (19.2%) were compared with cells cultivated in Waymouth medium (28.0%). A high concentration (62.0%) of octadecenoic acid (C18:1) was found in the triglyceride of MK-2 cells cultivated in Waymouth medium. The level of polyunsaturated fatty acids observed in MK-2 cells cultivated in Waymouth medium (10.8%) and in the chlamydiae grown in these cells (13.3%) was low compared with the level in MK-2 cells (28.8%) cultivated in MEM with 5% calf serum and the level in calf serum itself (50.8%). A higher ratio of sterol ester to free sterol was found in calf serum than in MK-2 cells or in chlamydiae. Host contribution to lipid composition of strain 6BC is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BEISS U. ZUR PAPIERCHROMATOGRAPHISCHEN AUFTRENNUNG VON PFLANZENLIPIDEN. J Chromatogr. 1964 Jan;13:104–110. doi: 10.1016/s0021-9673(01)95079-4. [DOI] [PubMed] [Google Scholar]
- DITTMER J. C., LESTER R. L. A SIMPLE, SPECIFIC SPRAY FOR THE DETECTION OF PHOSPHOLIPIDS ON THIN-LAYER CHROMATOGRAMS. J Lipid Res. 1964 Jan;5:126–127. [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- FARQUHAR J. W., INSULL W., Jr, ROSEN P., STOFFEL W., AHRENS E. H., Jr The analysis of fatty acid mixtures by gas-liquid chromatography; construction and operation of an ionization chamber instrument. Nutr Rev. 1959 Aug;17(8 Suppl):1–30. [PubMed] [Google Scholar]
- GOGOLAK F. M., ROSS M. R. The properties and chemical nature of the psittacosis virus hemagglutinin. Virology. 1955 Dec;1(5):474–496. doi: 10.1016/0042-6822(55)90038-6. [DOI] [PubMed] [Google Scholar]
- Gaugler R. W., Neptune E. M., Adams G. M., Sallee T. L., Weiss E., Wilson N. N. Lipid synthesis by isolated Chlamydia psittaci. J Bacteriol. 1969 Nov;100(2):823–826. doi: 10.1128/jb.100.2.823-826.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLMAN R. T., HOFSTETTER H. H. THE FATTY ACID COMPOSITION OF THE LIPIDS FROM BOVINE AND PORCINE REPRODUCTIVE TISSUES. J Am Oil Chem Soc. 1965 Jun;42:540–544. doi: 10.1007/BF02540098. [DOI] [PubMed] [Google Scholar]
- HULL R. N., CHERRY W. R., TRITCH O. J. Growth characteristics of monkey kidney cell strains LLC-MK1, LLC-MK2, and LLC-MK2(NCTC-3196) and their utility in virus research. J Exp Med. 1962 May 1;115:903–918. doi: 10.1084/jem.115.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKIN H. M. Preparation and properties of cell walls of the agent of meningopneumonitis. J Bacteriol. 1960 Nov;80:639–647. doi: 10.1128/jb.80.5.639-647.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKIN H. M., ROSS M. R., MOULDER J. W. Species-specific antigens from the cell walls of the agents of meningopneumonitis and feline pneumonitis. J Immunol. 1961 Feb;86:123–127. [PubMed] [Google Scholar]
- Jenkin H. M., Anderson L. E. The effect of oleic acid on the growth of monkey kidney cells (LLC-MK2). Exp Cell Res. 1970 Jan;59(1):6–10. doi: 10.1016/0014-4827(70)90616-6. [DOI] [PubMed] [Google Scholar]
- Jenkin H. M. Comparative lipid composition of psittacosis and trachoma agents. Am J Ophthalmol. 1967 May;63(5 Suppl):1087–1098. doi: 10.1016/0002-9394(67)94087-1. [DOI] [PubMed] [Google Scholar]
- Jenkin H. M. The continuous passage of agents of trachoma in cell culture. I. Characteristics of TW-3 and Bour strains of trachoma cultivated in serial passage in HeLa 229 cells. J Infect Dis. 1966 Jun;116(3):390–399. doi: 10.1093/infdis/116.3.390. [DOI] [PubMed] [Google Scholar]
- MOULDER J. W., WEISS E. Purification and properties of the agent of feline pneumonitis. J Infect Dis. 1951 Jan-Feb;88(1):56–67. doi: 10.1093/infdis/88.1.56. [DOI] [PubMed] [Google Scholar]
- Siakotos A. N. Analytical separation of nonlipid water soluble substances and gangliosides from other lipids by dextran gel column chromatography. J Am Oil Chem Soc. 1965 Nov;42(11):913–919. doi: 10.1007/BF02632444. [DOI] [PubMed] [Google Scholar]
- Skipski V. P., Peterson R. F., Barclay M. Quantitative analysis of phospholipids by thin-layer chromatography. Biochem J. 1964 Feb;90(2):374–378. doi: 10.1042/bj0900374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAMURA A., HIGASHI N. PURIFICATION AND CHEMICAL COMPOSITION OF MENINGOPNEUMONITIS VIRUS. Virology. 1963 Aug;20:596–604. doi: 10.1016/0042-6822(63)90284-8. [DOI] [PubMed] [Google Scholar]
- WAYMOUTH C. Rapid proliferation of sublines of NCTC clone 929 (strain L) mouse cells in a simple chemically defined medium (MB 752/1). J Natl Cancer Inst. 1959 May;22(5):1003–1017. doi: 10.1093/jnci/22.5.1003. [DOI] [PubMed] [Google Scholar]



