Abstract
Brain-derived neurotrophic factor (BDNF) has trophic effects on serotonergic (5-HT) neurons in the central nervous system. However, the role of endogenous BDNF in the development and function of these neurons has not been established in vivo because of the early postnatal lethality of BDNF null mice. In the present study, we use heterozygous BDNF+/− mice that have a normal life span and show that these animals develop enhanced intermale aggressiveness and hyperphagia accompanied by significant weight gain in early adulthood; these behavioral abnormalities are known to correlate with 5-HT dysfunction. Forebrain 5-HT levels and fiber density in BDNF+/− mice are normal at an early age but undergo premature age-associated decrements. However, young adult BDNF+/− mice show a blunted c-fos induction by the specific serotonin releaser-uptake inhibitor dexfenfluramine and alterations in the expression of several 5-HT receptors in the cortex, hippocampus, and hypothalamus. The heightened aggressiveness can be ameliorated by the selective serotonin reuptake inhibitor fluoxetine. Our results indicate that endogenous BDNF is critical for the normal development and function of central 5-HT neurons and for the elaboration of behaviors that depend on these nerve cells. Therefore, BDNF+/− mice may provide a useful model to study human psychiatric disorders attributed to dysfunction of serotonergic neurons.
The neurotrophin brain-derived neurotrophic factor (BDNF) influences the phenotype, structural plasticity, and perhaps survival of central serotonergic (5-HT) neurons (1–3). Disturbances in brain 5-HT systems have been implicated in psychiatric syndromes characterized by behavioral dyscontrol, such as obsessive-compulsive disorder, bulimia, chronic impulsivity/aggression, and violent suicide (4–7). Many of these psychiatric syndromes are being treated with compounds that augment 5-HT neurotransmission in the brain, including selective serotonin reuptake inhibitors and 5-HT receptor agonists (8, 9). Pharmacological studies indicate that exogenously administered BDNF has trophic effects on 5-HT neurons. For example, BDNF administration increases 5-HT metabolism in the brain (3) and stimulates the local sprouting of 5-HT fibers in the cerebral cortex and spinal cord (2, 10, 11). The enhancement of 5-HT neurotransmission by exogenous BDNF potentiates several behaviors regulated by serotonin (12, 13) and has antidepressant-like effects in animal models of depression (14). However, the role of endogenous BDNF in the normal development and function of 5-HT neurons has not been determined.
A major obstacle in elucidating the role of endogenous BDNF has been the early postnatal lethality of BDNF−/− mice. However, recent studies confirmed the presence of several nonlethal, functional defects within the peripheral and central nervous systems of heterozygous animals with one functional BDNF allele, suggesting that BDNF is haploinsufficient. For example, targeted mutation of the BDNF locus causes deficits in hippocampal synaptic function and mechanosensitivity and in the development of the peripheral nervous system in heterozygous as well as homozygous BDNF null mice (15–17). In the present study, we use heterozygous BDNF+/− mice to examine whether a deficiency in BDNF during the development and maturation of brain serotonergic neurons leads to abnormalities in the structure and function of these nerve cells and in behaviors that depend on central 5-HT neurotransmission.
Materials and Methods
Mice.
BDNF+/− mice generated as described (18) were back-crossed for 10–12 generations to a C57BL/6 genetic background, a strategy resulting in great reduction of the genetic heterogeneity present in the original 129 Sv-C57BL/6 mixed background. Forebrain BDNF mRNA and protein levels in these mice were ≈50% of the wild-type (WT) (data not shown; refs. 15, 16, and 19). Unless otherwise stated, mice were group-housed under standard conditions, three to five per cage with food and water available ad libitum and were maintained on a 12-h light/dark cycle. Mice were fed a standard chow diet containing 9% crude fat (PMI Nutrition, Brentwood, MO) and were treated in compliance with National Institutes of Health guidelines for animal care and use. Except for the studies on intermale aggression, all experiments included matched numbers of male and female mice, and no significant sex differences were found in any measures.
Evaluation of Forebrain 5-HT Innervation.
Brain tissue was processed for 5-HT immunocytochemistry as described (2) by using an antibody directed against serotonin (1:15,000; Incstar, Stillwater, MN). For neurochemical measures, fresh-frozen brain areas were assayed for levels of 5-HT and its metabolite 5-hydroxyindoleacetic acid (5-HIAA) by using HPLC with electrochemical detection as described (20).
c-Fos Induction.
WT or BDNF+/− mice at 3–6 months of age were administered dexfenfluramine (dFen) (3 or 10 mg/kg, i.p.) or vehicle; 2 h later, mice were killed, and brain tissue was processed for c-Fos immunocytochemistry as described (2), using an antibody directed against the c-fos N-peptide (AB-5; Oncogene Science; diluted 1:20,000).
Reverse Transcription–PCR.
First strand cDNA was synthesized from total cellular RNA by using the SuperScript Preamplification System (GIBCO/BRL). AmpliWax (Perkin–Elmer) gem-facilitated hot-start PCR was performed with the following 5-HT receptor-specific 32P-end-labeled primers: 5-HT1A 5′-ACCATCTACTCCACTTTCGGCG-3′ [sense (s)], 5′-TTCACTGTCTTCCTCTCACGGG-3′ [antisense (as)]; 5-HT2A, 5′-CCAACCTCTCCTGCGAAGGG-3′ (s), 5′-GCGGCTATGGTGAATGGGG-3′ (as); 5-HT1B, 5′-AGGAGCAGGGTATTCAGTGCG-3′ (s), 5′-TGTCCAGCGTCCAGTGACCG-3′ (as); 5-HT2C, 5′-CCTACGCCGTCAAACCCTG-3′ (s) and 5′-GCCTTCCCACAAAGCACCGACAG-3′ (as). As a control for RNA input, levels of glyceraldehyde-3-phosphate dehydrogenase were assayed by using the primer sequences 5′-ACCACAGTCCATGCCATCAC-3′ (s) and 5′-TCCACCACCCTGTTGCTGTA-3′ (as). Amplification was conducted in the linear range, and the amplified products were resolved on 1.6% agarose gels. Gels were apposed to a Storage Phosphor Screen (Molecular Dynamics), and PCR products were quantified with imagequant software (Molecular Dynamics). PCR products were identified by size and restriction digest analysis. For each 5-HT receptor subtype, PCR amplifications from BDNF+/− and WT littermates (6–9 months of age) were processed together, and the amplified products were resolved on the same gel. The data were calculated as the ratio of 5-HT-receptor to glyceraldehyde-3-phosphate dehydrogenase mRNA levels and were expressed as a percentage of the mean value found in WT controls.
Resident-Intruder Aggression Assay.
For isolation-induced aggression, male resident test mice were isolated for at least 4 weeks. Cages were changed once per week, but not during the week preceding testing. Aggressive behaviors in 2.5- to 4.5-month-old test mice were monitored during 5-min exposures to WT C57BL/6 male intruder mice that had been group-housed (five per cage) and carefully matched with resident mice for body weight. Five test sessions were conducted (one trial per day). The latency to first biting attack and the total number of biting attacks were recorded from videotapes of each test session. For mice that failed to attack, the latency was scored as 5 min. In a separate experiment, WT and BDNF+/− mice were chronically administered either fluoxetine (Flx) (5 mg/kg/day s.c.) or vehicle, initiated at the start of the 4-week isolation and continued through the testing period. After three conditioning sessions, attack latencies and numbers were monitored during two additional testing trials; because measures did not differ between the two testing trials, data were collapsed for analysis.
Measurement of Food Intake.
Several groups of mice, beginning at 1–5 months of age, were monitored for food intake over a 2- to 6-month period by using powder feeders containing ground standard chow. Mice were housed one per cage and were given free access to food and water.
Open Field Testing.
Locomotor activity was quantified by using an open-field arena with an automated optical animal activity system (Digiscan RXYZCM, Omnitech Electronics, Columbus, OH). Measures of horizontal activity (number of horizontal beam interruptions), total distance (centimeters), vertical activity (number of vertical beam interruptions), and center time (seconds) were obtained over a 10-min period.
Results
Brain Serotonin Abnormalities in BDNF+/− Mice.
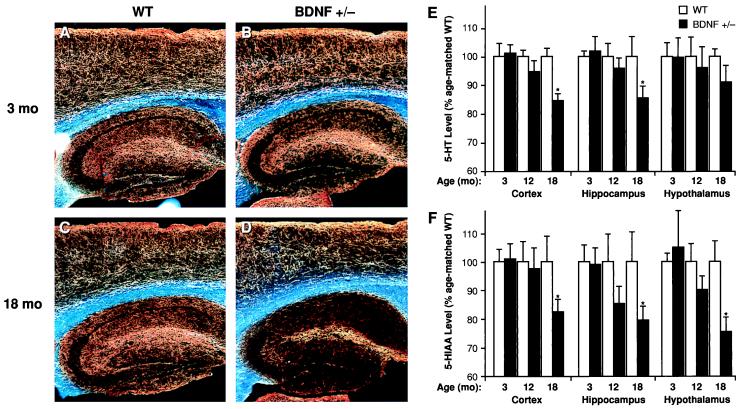
BDNF+/− mice at 3–6 months of age did not show any obvious structural damage to 5-HT axons, as assessed by 5-HT immunocytochemistry and HPLC analysis of total tissue content of 5-HT and its metabolite 5-hydroxyindoleacetic acid (5-HIAA). Forebrain 5-HT axon densities appeared normal, and there were no significant differences in the levels of 5-HT or 5-HIAA or in the 5-HIAA/5-HT ratios in neocortex, hippocampus, or hypothalamus between 3-month-old BDNF+/− mice and WT littermates (Fig. 1). Likewise, 10-day-old homozygous BDNF−/− null mice did not exhibit any apparent differences in forebrain 5-HT-immunoreactive fibers when compared with WT controls (not shown). However, a progressive loss of 5-HT axons was observed in 12- to 18-month-old BDNF+/− mice compared with age-matched WT littermates (Fig. 1 C and D); in support of the morphological observations, forebrain levels of 5-HT and 5-HIAA were significantly lower in older BDNF+/− mice relative to WT mice of the same age (Fig. 1 E and F). Moreover, the 5-HIAA/5-HT ratio was lower in the hypothalamus of 18-month-old BDNF+/− mice (0.49 ± 0.02) relative to age-matched WT mice (0.61 ± 0.05; P < 0.05), suggesting a decreased turnover of 5-HT in this brain area. These patterns suggest that endogenous BDNF is not required for the survival or structural integrity of 5-HT neurons during development and in early adult life, but may be required for the structural maintenance of these neurons in advanced age.
Figure 1.
Serotonergic innervation and neurochemical levels of 5-HT and 5-HIAA in the forebrain of BDNF+/− or WT mice. Darkfield photomicrographs (A–D) of 5-HT-immunoreactive axons (sagittal sections) in WT (A and C) and BDNF+/− mice (B and D) at 3 months (A and B) or 18 months (C and D) of age. There is a normal 5-HT axon density in neocortex and hippocampus of younger BDNF+/− mice but a loss of axons in 18-month-old BDNF+/− mice relative to WT mice of the same age; we also observed a loss of 5-HT axon density in some of the 12-month-old BDNF+/− mice relative to age-matched WT controls (not shown). The total tissue contents of 5-HT (E) and its metabolite 5-HIAA (F) in the neocortex, hippocampus, and hypothalamus of BDNF+/− and WT littermates at 3, 12, or 18 months of age were measured by HPLC (n = 6–9/group). Data is expressed as a percentage of the mean value found in age-matched WT controls. Repeated measures ANOVA revealed significant reductions in the levels of 5-HT (F1,16 = 7.42, P < 0.05) and 5-HIAA (F1,16 = 8.04, P < 0.05) in 18-month-old BDNF+/− mice relative to WT littermates but not in the younger mutant mice (P > 0.05) in these brain areas. *, P < 0.05.
Although total tissue contents of 5-HT and 5-HIAA were normal in the younger mutant mice, these measures might not reflect functional perturbations in 5-HT neurotransmission. Moreover, recent pharmacological studies suggest that BDNF administration modulates the physiology and plasticity of 5-HT neurons (2, 3, 10, 11) while failing to promote the survival of injured 5-HT axons in young adult rats (21). Thus, it was necessary to assess the functional state of 5-HT neurons in BDNF+/− mice during earlier adulthood (in 3- to 9-month-old mice) before the appearance of structural 5-HT deficits in the aged animals.
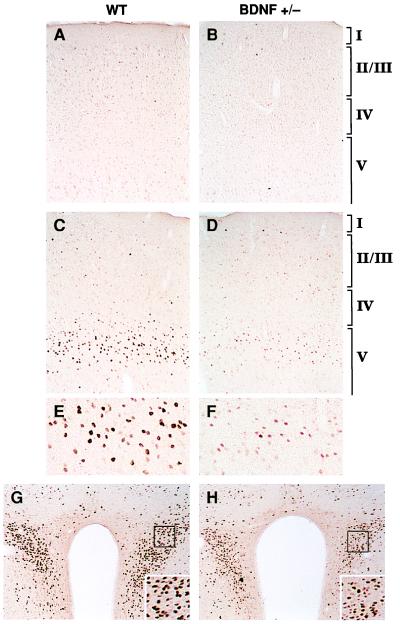
As a measure of 5-HT release by terminals, we studied the transcriptional activation of the immediate early gene c-fos in response to dFen administration in 3-to 6-month-old BDNF+/− mice. The induction of c-Fos has been used in vivo to measure neuronal activation in response to a variety of stimuli (22), including drugs such as dFen that activate specific neurotransmitter systems. dFen stimulates a Ca2+-dependent, exocytotic release of serotonin from 5-HT nerve terminals while also inhibiting 5-HT reuptake (23). This compound induces c-Fos expression exclusively through 5-HT release at several brain sites, including the frontal cortex, cingulate cortex, and the paraventricular nucleus of the hypothalamus (24), thus providing a measure of 5-HT neurotransmission at these sites. Baseline expression of c-Fos assessed by immunocytochemistry in WT, vehicle-treated mice was absent or low in the above areas; BDNF+/− mice showed similar patterns (Fig. 2 A and B). dFen (3 or 10 mg/kg, i.p.) administered to WT mice caused a dose-dependent increase in c-Fos expression in neurons with a regional pattern of activation that is characteristic for rodents (25, 26). Although dFen elicited a robust induction of c-Fos in the lateral frontal (LF) cortex and paraventricular nucleus of WT mice, this response was blunted in BDNF+/− mice (Fig. 2 C–H). BDNF+/− mice also showed an attenuated c-Fos response in the bed nucleus of the stria terminalis, the central nucleus of the amygdala, parts of the caudate-putamen, the shell region of the nucleus accumbens, and the midline thalamic nuclei. In some areas, such as the cingulate cortex and in caudal areas of neocortex, BDNF+/− mice showed c-Fos inductions comparable to that of WT mice. Consistent with previous observations (25, 26), dFen did not induce c-Fos expression in the hippocampus of WT mice, despite evidence that this compound stimulates hippocampal 5-HT release (27); in contrast to the lack of response in the WT, BDNF+/− mice displayed a moderate c-Fos activation in pyramidal neurons of the hippocampus after dFen administration (not shown). Thus, our results suggest that BDNF+/− mice show anomalous patterns of neuronal activation upon dFen-induced 5-HT release: i.e., a decreased activation of many target areas, including the frontal cortex and paraventricular nucleus, and an excessive activation of the hippocampus.
Figure 2.
Induction of c-Fos immunoreactivity 2 h after dFen administration (3 or 10 mg/kg, i.p.) in 3- to 6-month-old WT and BDNF+/− mice (n = 4–5/group). In LF cortex (A–F), c-Fos immunoreactivity was low or absent after a vehicle injection in WT (A) or BDNF+/− (B) mice. dFen (10 mg/kg) elicited a robust induction of c-Fos immunoreactivity in the upper part of layer V in LF cortex of WT mice (C; higher magnification shown in E), which is in precise register with a dense band of 5-HT2A/2C receptors and a dense plexus of 5-HT axon terminals found in this zone (43). This induction was blunted in BDNF+/− mice, as indicated by the lower number and staining intensity of c-Fos immunoreactive neurons (D; higher magnification in F). The lower dose of dFen (3 mg/kg) elicited a weak-to-moderate c-Fos induction in LF cortex of WT mice whereas no response was observed in BDNF+/− mice at this dose (not shown). dFen-induced activation of c-Fos was also attenuated in the paraventricular nucleus of the hypothalamus in BDNF+/− mice (H) relative to WT controls (G), a brain area suggested to mediate the anorectic effects of dFen (39).
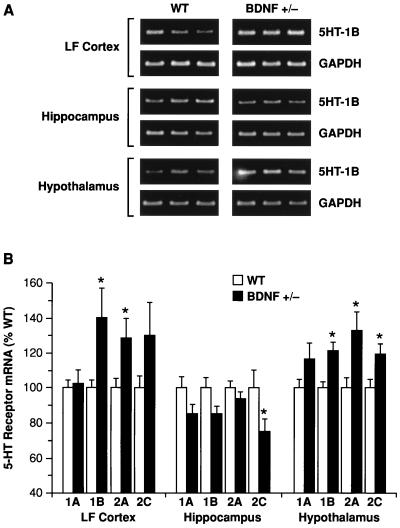
Abnormalities in the patterns of neuronal activation by 5-HT in BDNF+/− mice may reflect perturbations in 5-HT signaling—e.g., a deficient 5-HT release or transduction via 5-HT receptors. At least 15 different subtypes of 5-HT receptors mediate the diverse physiologic effects of serotonin in the brain (28). To determine whether 5-HT receptor expression was perturbed in the brains of BDNF+/− mice, we used a semiquantitative reverse transcription–PCR methodology and focused on the mRNA levels of four postsynaptic 5-HT receptors (5-HT1A, 5-HT1B, 5-HT2A, and 5-HT2C) in LF cortex, hippocampus, and hypothalamus of 6- to 9-month-old BDNF+/− and WT mice. These receptors play a prominent role in the regulation of mood, aggression/impulsivity, and feeding behavior (4–8, 26, 29–32). Repeated-measures ANOVA revealed significant changes in mRNA levels for these receptors in the brains of BDNF+/− mice that varied by brain region (Fig. 3): when compared with WT littermates, BDNF+/− mice showed significantly higher 5-HT receptor mRNA levels in LF cortex (F1,12 = 5.72, P < 0.05) and hypothalamus (F1,12 = 32.8, P < 0.001) whereas lower levels were found in the hippocampus (F1,12 = 6.41, P < 0.05). Post hoc comparisons pointed to particular alterations in 5-HT1B, 5-HT2A, and 5-HT2C mRNA levels, as depicted in Fig. 3. These results indicate that postsynaptic 5-HT receptor expression is altered in the brains of BDNF+/− mice. These receptor perturbations may represent a primary defect in the 5-HT system of BDNF-deficient mice or may reflect compensatory adaptations in response to anomalies in presynaptic 5-HT function.
Figure 3.
Reverse transcription–PCR analysis of 5-HT1A, 5-HT1B, 5-HT2A, and 5-HT2C receptor mRNA levels in lateral frontal cortex (LF Cortex), hippocampus, and hypothalamus of WT and BDNF+/− mice. (A) Example of ethidium bromide-stained agarose gels demonstrating 5-HT1B and corresponding glyceraldehyde-3-phosphate dehydrogenase (GAPDH) amplication products (≈330 and 450 bp, respectively) in the indicated brain areas of three WT and three BDNF+/− mice. (B) 5-HT receptor mRNA levels were measured in WT and BDNF+/− mice (6–9 months of age) by reverse transcription–PCR (see Materials and Methods; n = 6–7/group). There were significant (ANOVA, P < 0.05) up-regulations of 5HT1B and 5HT2A receptors in LF cortex and hypothalamus, an up-regulation of the 5HT2C receptor in hypothalamus, and a down-regulation of the 5HT2C receptor in the hippocampus of BDNF+/− mice relative to WT controls; a marginal (P = 0.05–0.1) up-regulation of the 5HT2C receptor was found in LF cortex, and marginal down-regulations of the 5HT1A and 5HT1B receptors were found in the hippocampus of BDNF+/− mice. *, P < 0.05.
Behavioral Abnormalities in BDNF+/− Mice.
An extensive body of research indicates a role for central 5-HT neurotransmission in the control of aggressive behavior and appetite/food intake (5–9, 26, 29–35); moreover, both behaviors and their link to 5-HT functioning have been well characterized in rodent models. Thus, we investigated whether the 5-HT abnormalities in the brains of BDNF+/− mice are associated with altered aggressiveness and/or eating behaviors.
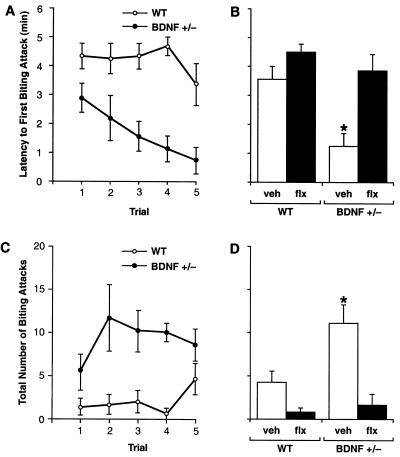
To investigate aggressive behavior, we measured offensive intermale fighting in BDNF+/− and WT mice by using a resident-intruder paradigm (29). Offensive aggression was measured by determining the latency to first biting attack and total number of attacks instigated by isolated resident mice during the first 5 min of exposure to a WT intruder mouse. BDNF+/− residents attacked the intruder more rapidly than did WT residents, with significantly shorter attack latencies apparent as early as the first trial (Fig. 4A). The biting attack latencies of BDNF+/− mice became progressively shorter over successive trials whereas WT mice displayed fairly constant attack latencies over the course of testing. The number of biting attacks was also significantly higher in the BDNF+/− group (Fig. 4C). Although not quantified, the type of aggressive behavior was strikingly different between BDNF+/− and WT mice. WT residents initially appeared cautious of the intruder while exploring their environment and engaging in social interest behaviors directed at the intruder; later, they instigated offensive behaviors (chasing, tail rattling) and biting attacks. In contrast, BDNF+/− residents appeared agitated and intolerant of the intruder and engaged in offensive behaviors at the onset of testing. In addition, the severity and duration of biting attacks were greater in BDNF+/− mice. These results suggest that BDNF+/− mice are more aggressive and possibly more irritable and impulsive than WT mice. Such behaviors have been linked to deficient 5-HT neurotransmission in the brain (5, 6, 8, 9, 29) and are found in mice with a targeted disruption of the 5-HT1B receptor (35).
Figure 4.
Offensive intermale aggression in BDNF+/− and WT littermates as assessed in a resident-intruder assay (n = 8–12/group; 2.5–4.5 months of age). (A) Latency to first biting attack. (C) Number of biting attacks measured during five consecutive trials. Repeated measures ANOVA revealed significant differences between the BDNF+/− and WT groups for both biting attack latency (F1,14 = 13.67, P < 0.005) and number of attacks (F1,14 = 9.98, P < 0.01), evident as early as the first trial. (B and D) Chronic Flx treatment in BDNF+/− mice (BDNF+/− flx) normalized both the attack latency (B) and number of attacks (D) to levels found in WT mice administered either vehicle (WT veh) or Flx (WT flx). *, P < 0.05 (ANOVA; different from all other treatment groups whereas no differences were found between the other three groups).
To assess whether the aggressive phenotype of BDNF+/− mice could be alleviated by strategies that augment 5-HT neurotransmission in the brain, we treated mutant and WT mice with the selective serotonin reuptake inhibitor Flx. Chronic Flx treatment (5 mg/kg/day, s.c., initiated 4 weeks before testing) normalized the attack latencies and number of biting attacks in BDNF+/− mice to levels seen in WT controls (Fig. 4 B and D). Locomotor activity in an open-field test (36) was not reduced by Flx treatment (horizontal activity, Flx-treated BDNF+/− mice, 2,033 ± 171 units; vehicle-treated WT mice, 2,133 ± 155, P > 0.05; total distance traveled, flx- BDNF+/−, 1,098 ± 255 cm; veh-WT, 1,304 ± 124, P > 0.05), a pattern indicating that the effects of Flx on aggression were not caused by sedation. These findings suggest that the overaggressiveness in BDNF+/− mice may be linked to acute perturbations in central 5-HT neurotransmission, rather than irrevocable developmental compensations.
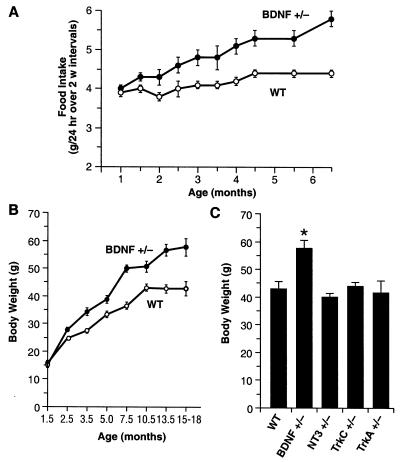
Brain 5-HT systems have been strongly implicated in the neural regulation of appetite. Impairment of 5-HT neurotransmission can lead to bulimia and obesity whereas 5-HT direct and indirect agonists are potent appetite suppressants (31, 33, 34). To assess eating behavior in BDNF+/− mice, we measured daily food intake of 1-to 7-month-old BDNF+/− and WT mice (Fig. 5A). BDNF+/− mice showed an age-related increase in food intake beginning at 6 weeks of age. By 4–7 months of age, BDNF+/− mice were consuming ≈25% more food than their WT littermates. Chronic Flx treatment (5 mg/kg/day) partially suppressed the daily food intake of BDNF+/− mice relative to vehicle-treated mutants (not shown).
Figure 5.
BDNF+/− mice develop hyperphagia and elevated body mass. (A) Daily food intake (g/day) of BDNF+/− and WT mice (n = 12–13/group). Values indicate the mean ± SEM of daily food intake averaged over a 2-week interval. Food intake of BDNF+/− mice was significantly higher than in age-matched WT controls (ANOVA, P < 0.05). (B) Growth curves for BDNF+/− and WT mice (n = 110–115/group) indicate that BDNF+/− mice have a significantly higher body weight than WT littermates, beginning at 10 weeks of age (ANOVA, P < 0.001). (C) Body weight of 15-month-old WT, BDNF+/−, and mice heterozygous for other members of the neurotrophin (NT-3+/−) or neurotrophin receptor family (trkA+/− and trkC+/−). *, P < 0.05 compared with WT.
In conjunction with the chronic hyperphagia, BDNF+/− mice developed an age-dependent obesity, occurring gradually over the lifespan of the animals (Fig. 5B). Significant differences in body weight between BDNF+/− and WT mice were first evident by 2–4 months of age. By 12–18 months of age, the body weight of BDNF+/− mice was on average 34% higher than that of their WT littermates. Body length of 3- or 12-month-old BDNF+/− mice was not significantly different from age-matched WT controls (3-month-old BDNF+/−, 9.8 ± 0.2 cm, versus WT, 9.9 ± 0.1, P > 0.05; 12-month-old BDNF+/−, 10.8 ± 0.1, versus WT, 10.8 ± 0.1, P > 0.05), implicating an increased body fat content rather than a linear increase in growth or size as the primary cause for the increased body mass. Mice heterozygous for other neurotrophins or neurotrophin receptors (NT-3+/−, TrkC+/−, or TrkA+/−) did not show significant deviations in body weight by 15 months of age (Fig. 5C), demonstrating the specificity of the BDNF gene for influencing this phenotype. To determine whether reduced locomotor activity contributed to the obesity of BDNF+/− mice, we measured the activity of the mice in the open-field test. No significant differences were detected between mutant and WT mice in horizontal activity (BDNF+/−, 2,218 ± 115 units, versus WT, 2238 ± 189; P > 0.05) and total distance traveled (BDNF+/−, 1,271 ± 77 cm, versus WT, 1,412 ± 147; P > 0.05), a pattern indicating normal levels in locomotor activity. However, BDNF+/− mice engaged in significantly less vertical activity (rearing) and spent more time in the center of the testing chamber than did WT mice (not shown). Finally, the serum insulin level was about doubled in 12-month-old BDNF+/− mice (15.1 ± 1.9 ng/ml) relative to that of WT littermates (8.1 ± 1.6; P < 0.05). Interestingly, this constellation of behavioral/metabolic phenotypes in BDNF+/− mice is remarkably similar to that characterized in 5-HT2C receptor null mice that develop chronic hyperphagia and obesity associated with insulin resistance and type 2 diabetes (30, 32).
Discussion
Our results suggest that a partial impairment in BDNF expression causes physiological disturbances in central 5-HT neurons in early adulthood and leads to a structural deterioration of these neurons in advanced age. The early functional deficits in the 5-HT system of heterozygous BDNF null mice are revealed by the blunted neuronal activation in response to dFen-induced 5-HT release and by the altered expression of several postsynaptic 5-HT receptors in forebrain areas such as the frontal cortex, hippocampus, and hypothalamus. These functional deficits suggest impaired 5-HT signaling mechanisms in the younger BDNF+/− mice (evident before the age-related loss of 5-HT axons) and are associated with exaggerated aggressiveness and bulimia (excessive appetite/food intake). In human and animals studies, both aggressive behavior and bulimia are closely linked to deficient 5-HT neurotransmission within complex brain circuits involving the hypothalamus and limbic cortex (5, 6, 29, 31, 33, 34). The heightened aggressiveness in BDNF+/− mice can be ameliorated by chronic treatment with the selective serotonin reuptake inhibitor Flx, a strategy that augments 5-HT neurotransmission in the brain. Our data indicate that endogenous BDNF is not required for the survival or structural integrity of 5-HT neurons during development and in early adult life. However, normal BDNF levels are required for the proper functioning of the 5-HT system. These results represent in vivo evidence that the 5-HT system is highly sensitive to endogenous levels of BDNF. Thus, even partial disruptions in BDNF expression lead to perturbations in 5-HT neuronal functioning and in behavioral abnormalities associated with this neurotransmitter system.
Serotonin-releasing drugs such as fenfluramine and dFen have been widely used in clinical studies as a probe for brain 5-HT functioning. For example, severe depression and aggressiveness in humans correlate with a blunted prolactin response to fenfluramine, a pattern suggesting reduced 5-HT activity within brain circuits (37). Consistent with these observations in human subjects, BDNF+/− mice show a blunted c-Fos response to dFen in widespread cortical/limbic areas, including the frontal cortex and hypothalamus. Hippocampal neurons display an opposite pattern; i.e., they are excessively activated by dFen in the mutants. These anomalous patterns of neuronal activation by 5-HT release could reflect disturbed pre- or postsynaptic 5-HT mechanisms. Future studies that directly assess presynaptic 5-HT functioning (e.g., by in vivo microdialysis) in BDNF+/− mice may clarify this issue.
Postsynaptic 5-HT receptor expression is also disturbed in the brains of BDNF+/− mice and involve several receptor subtypes in at least three forebrain regions. The dysregulation of multiple 5-HT receptors at multiple brain sites may impair the functioning of the 5-HT system in BDNF+/− mice and may lead to abnormalities in impulse regulation and eating behaviors. Nearly identical behavioral abnormalities are found in mice with targeted disruptions of the 5-HT1B (35) and 5-HT2C (30, 32) receptors, two receptors found perturbed in BDNF+/− mice. Serotonin receptor alterations in BDNF+/− mice may also represent compensatory adaptations in response to presynaptic 5-HT dysfunction, i.e., 5-HT receptor up-regulation in response to a decreased availability of presynaptic 5-HT in frontal cortex and hypothalamus. In this regard, it is interesting to note the inverse relationship of a blunted c-Fos response combined with 5-HT receptor up-regulation in frontal cortex and hypothalamus versus the opposite pattern in the hippocampus of BDNF+/− mice. Interestingly, alterations in the levels of 5-HT receptors in the brains of violent suicide victims—i.e., increased 5HT2 binding in prefrontal cortex and decreased 5HT1 binding in hippocampus (7, 38)—are strikingly similar to the abnormal patterns of 5-HT receptor expression found in BDNF-deficient mice. Chronic antidepressant treatment can reverse these receptor alterations by down-regulating 5-HT2 binding in prefrontal cortex and sensitizing 5-HT1A receptors in the hippocampus (39, 40) and, in doing so, ameliorate behavioral abnormalities in humans and experimental animals. The results of these studies are in congruence with the effects of Flx on behavioral abnormalities of BDNF-deficient mice.
In concert, a less-than-total deletion of endogenous BDNF causes marked 5-HT abnormalities in the brain and is associated with 5-HT-sensitive behavioral disturbances in the regulation of aggression and appetite. These observations suggest a profound dependency of 5-HT neurons on this neurotrophin. BDNF expression is rapidly and dynamically regulated by various physiological stimuli, including alterations in neuronal activity (41) and psychosocial stressors (42). Thus, alterations in BDNF expression induced by physiological or complex environmental/psychological conditions may lead to dysfunction of central 5-HT systems and may cause behavioral changes, especially in the realm of aggression and impulsivity. BDNF+/− mice may provide a useful model to study these complex interactions and shed light onto psychiatric disorders associated with brain 5-HT dysfunction.
Acknowledgments
We thank E. Sterneck for advice and critical comment on behavioral studies. 5-HT receptor cDNA probes were generously provided by L. Tecott, M. Toth, and R. Hen. Research was sponsored by the National Cancer Institute, Department of Health and Human Services, under contract with Advanced BioScience Laboratories (W.E.L, V.C., S.W.R., and L.T.); by National Institutes of Mental Health Grant R29MH85433 (L.A.M.); and by a Javits Neuroscience Investigator Award, NIH NS 10580 (V.E.K.).
Abbreviations
- BDNF
brain-derived neurotrophic factor
- 5-HT
serotonin
- dFen
dexfenfluramine
- Flx
fluoxetine
- WT
wild type
- s
sense
- as
antisense
- 5-HIAA
5-hydroxyindoleacetic acid
- LF cortex
lateral frontal cortex
References
- 1.Eaton M J, Staley J K, Globus M Y, Whittemore S R. Dev Biol. 1995;170:169–182. doi: 10.1006/dbio.1995.1205. [DOI] [PubMed] [Google Scholar]
- 2.Mamounas L A, Blue M E, Siuciak J A, Altar C A. J Neurosci. 1995;15:7929–7939. doi: 10.1523/JNEUROSCI.15-12-07929.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siuciak J A, Boylan C, Fritsche M, Altar C A, Lindsay R M. Brain Res. 1996;710:11–20. doi: 10.1016/0006-8993(95)01289-3. [DOI] [PubMed] [Google Scholar]
- 4.Baumgarten H G, Grozdanovic Z. Pharmacopsychiatry. 1995;28, Suppl. 2:73–79. doi: 10.1055/s-2007-979623. [DOI] [PubMed] [Google Scholar]
- 5.Hen R. Neuron. 1996;16:17–21. doi: 10.1016/s0896-6273(00)80019-7. [DOI] [PubMed] [Google Scholar]
- 6.Berman M E, Tracy J I, Coccaro E F. Clin Psychol Rev. 1997;17:651–665. doi: 10.1016/s0272-7358(97)00039-1. [DOI] [PubMed] [Google Scholar]
- 7.Mann J J. Nat Med. 1998;4:25–30. doi: 10.1038/nm0198-025. [DOI] [PubMed] [Google Scholar]
- 8.Stanislav S W, Fabre T, Crismon M L, Childs A. J Clin Psychopharmacol. 1994;14:126–130. [PubMed] [Google Scholar]
- 9.Fuller R W. Neuropsychopharmacology. 1996;14:77–81. doi: 10.1016/0893-133X(95)00110-Y. [DOI] [PubMed] [Google Scholar]
- 10.Xu X M, Guenard V, Kleitman N, Aebischer P, Bunge M B. Exp Neurol. 1995;134:261–272. doi: 10.1006/exnr.1995.1056. [DOI] [PubMed] [Google Scholar]
- 11.Bregman B S, McAtee M, Dai H N, Kuhn P L. Exp Neurol. 1997;148:475–494. doi: 10.1006/exnr.1997.6705. [DOI] [PubMed] [Google Scholar]
- 12.Siuciak J A, Altar C A, Wiegand S J, Lindsay R M. Brain Res. 1994;633:326–330. doi: 10.1016/0006-8993(94)91556-3. [DOI] [PubMed] [Google Scholar]
- 13.Pelleymounter M A, Cullen M J, Wellman C L. Exp Neurol. 1995;131:229–238. doi: 10.1016/0014-4886(95)90045-4. [DOI] [PubMed] [Google Scholar]
- 14.Siuciak J A, Lewis D R, Wiegand S J, Lindsay R M. Pharmacol Biochem Behav. 1997;56:131–137. doi: 10.1016/S0091-3057(96)00169-4. [DOI] [PubMed] [Google Scholar]
- 15.Ernfors P, Lee K F, Jaenisch R. Nature (London) 1994;368:147–150. doi: 10.1038/368147a0. [DOI] [PubMed] [Google Scholar]
- 16.Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Proc Natl Acad Sci USA. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carroll P, Lewin G R, Koltzenburg M, Toyka K V, Thoenen H. Nat Neurosci. 1998;1:42–46. doi: 10.1038/242. [DOI] [PubMed] [Google Scholar]
- 18.Liebl D J, Tessarollo L, Palko M E, Parada L F. J Neurosci. 1997;17:9113–9121. doi: 10.1523/JNEUROSCI.17-23-09113.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolbeck R, Bartke I, Eberle W, Barde Y A. J Neurochem. 1999;72:1930–1938. doi: 10.1046/j.1471-4159.1999.0721930.x. [DOI] [PubMed] [Google Scholar]
- 20.Ricaurte G A, Martello A L, Katz J L, Martello M B. J Pharmacol Exp Ther. 1992;261:616–622. [PubMed] [Google Scholar]
- 21.Mamounas, L. A., Altar, C. A., Blue, M. E., Kaplan, D. R., Tessarollo, L. & Lyons, W. E. (2000) J. Neurosci., in press. [DOI] [PMC free article] [PubMed]
- 22.Sagar S M, Sharp F R, Curran T. Science. 1988;240:1328–1331. doi: 10.1126/science.3131879. [DOI] [PubMed] [Google Scholar]
- 23.Gobbi M, Frittoli E, Uslenghi A, Mennini T. Eur J Pharmacol. 1993;238:9–17. doi: 10.1016/0014-2999(93)90499-8. [DOI] [PubMed] [Google Scholar]
- 24.Javed A, Van De Kar L D, Gray T S. Brain Res. 1997;774:94–105. doi: 10.1016/s0006-8993(97)81692-7. [DOI] [PubMed] [Google Scholar]
- 25.Li B H, Rowland N E. Brain Res Bull. 1993;31:43–48. doi: 10.1016/0361-9230(93)90009-z. [DOI] [PubMed] [Google Scholar]
- 26.Lucas J J, Yamamoto A, Scearce-Levie K, Saudou F, Hen R. J Neurosci. 1998;18:5537–5544. doi: 10.1523/JNEUROSCI.18-14-05537.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Assie M B, Koek W. Br J Pharmacol. 1996;119:845–850. doi: 10.1111/j.1476-5381.1996.tb15749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoyer D, Clarke D E, Fozard J R, Hartig P R, Martin G R, Mylecharane E J, Saxena P R, Humphrey P P. Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- 29.Olivier B, Mos J, van Oorschot R, Hen R. Pharmacopsychiatry. 1995;28, Suppl. 2:80–90. doi: 10.1055/s-2007-979624. [DOI] [PubMed] [Google Scholar]
- 30.Tecott L H, Sun L M, Akana S F, Strack A M, Lowenstein D H, Dallman M F, Julius D. Nature (London) 1995;374:542–546. doi: 10.1038/374542a0. [DOI] [PubMed] [Google Scholar]
- 31.Curzon G, Gibson E L, Oluyomi A O. Trends Pharmacol Sci. 1997;18:21–25. doi: 10.1016/s0165-6147(96)01003-6. [DOI] [PubMed] [Google Scholar]
- 32.Nonogaki K, Strack A M, Dallman M F, Tecott L H. Nat Med. 1998;4:1152–1156. doi: 10.1038/2647. [DOI] [PubMed] [Google Scholar]
- 33.Leibowitz S F. Trends Neurosci. 1992;15:491–497. doi: 10.1016/0166-2236(92)90101-d. [DOI] [PubMed] [Google Scholar]
- 34.Simansky K J. Behav Brain Res. 1996;73:37–42. doi: 10.1016/0166-4328(96)00066-6. [DOI] [PubMed] [Google Scholar]
- 35.Saudou F, Amara D A, Dierich A, LeMeur M, Ramboz S, Segu L, Buhot M C, Hen R. Science. 1994;265:1875–1878. doi: 10.1126/science.8091214. [DOI] [PubMed] [Google Scholar]
- 36.Walsh R N, Cummins R A. Psychol Bull. 1976;83:482–504. [PubMed] [Google Scholar]
- 37.O'Keane V, Moloney E, O'Neill H, O'Connor A, Smith C, Dinan T G. Br J Psychiatry. 1992;160:643–646. doi: 10.1192/bjp.160.5.643. [DOI] [PubMed] [Google Scholar]
- 38.Cheetham S C, Crompton M R, Katona C L, Horton R W. Psychopharmacology. 1990;102:544–548. doi: 10.1007/BF02247138. [DOI] [PubMed] [Google Scholar]
- 39.Berendsen H H. Pharmacol Ther. 1995;66:17–37. doi: 10.1016/0163-7258(94)00075-e. [DOI] [PubMed] [Google Scholar]
- 40.Haddjeri N, Blier P, de Montigny C. J Neurosci. 1998;18:10150–10156. doi: 10.1523/JNEUROSCI.18-23-10150.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lindholm D, Castren E, Berzaghi M, Blochl A, Thoenen H. J Neurobiol. 1994;25:1362–1372. doi: 10.1002/neu.480251105. [DOI] [PubMed] [Google Scholar]
- 42.Smith M A, Makino S, Kvetnansky R, Post R M. J Neurosci. 1995;15:1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blue M E, Yagaloff K A, Mamounas L A, Hartig P R, Molliver M E. Brain Res. 1988;453:315–328. doi: 10.1016/0006-8993(88)90172-2. [DOI] [PubMed] [Google Scholar]