Abstract
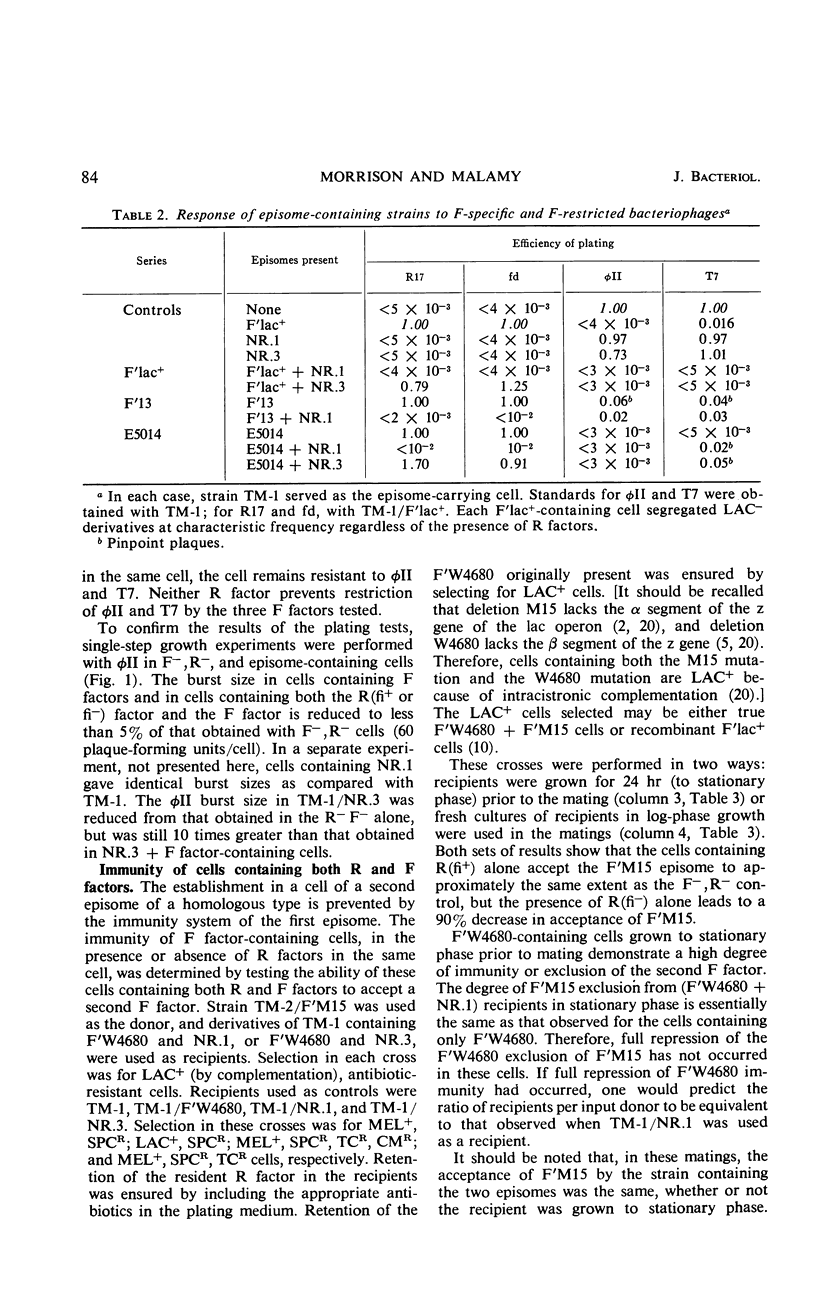
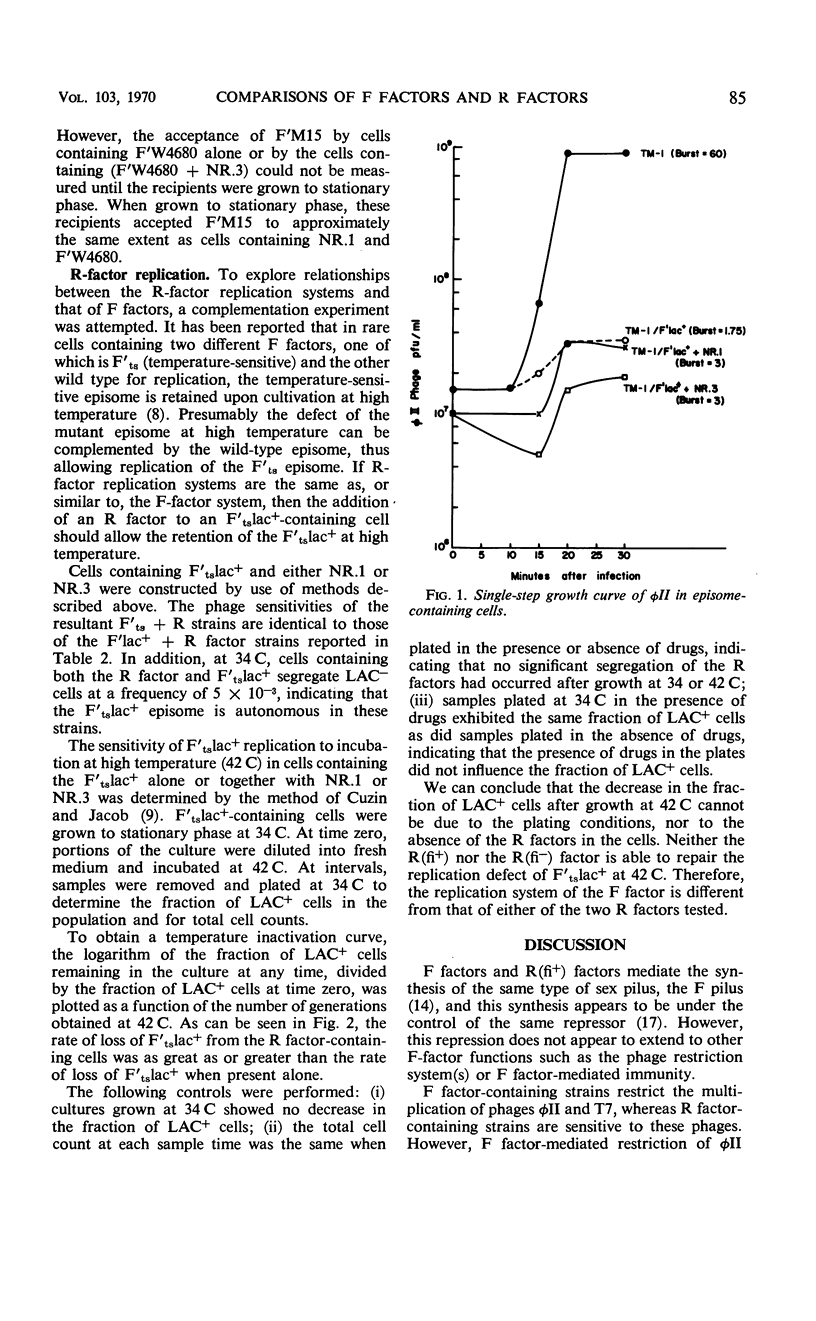
The similarity of sex pili mediated by F factors and R(fi+) factors and the ability of R(fi+) factors to control by repression the functioning of pilus genes encoded by the F factor suggested that F factors and R(fi+) factors are closely related. Further comparisons of the episomal properties of F factors and R(fi+) factors, however, indicated many differences. F factors contain information for a restriction system for phages φII and T7. Cells containing R factors are sensitive to these phages. Furthermore, R(fi+) factors do not repress the F factor φII restriction system in cells containing both an R(fi+) factor and an F factor. R factors and F factors are heteroimmune episomes. In addition, an R(fi+) factor in cells containing both an R factor and an F factor does not fully repress the expression of F-factor immunity to an incoming second F factor. R-factor and F-factor replication systems are not identical. Wild-type F-factor replication genes will complement the mutant Fts114lac+ replication genes in cells containing two F factors. The Fts114lac+ episome is retained when these cells are grown at 42 C; however, cells containing an R(fi+) factor and Fts114lac+ lose the Fts114lac+ when grown at 42 C, at the same rate as cells containing only the Fts114lac+. The replication system of the R(fi+) factor will not complement the mutant Fts114lac+ replication system.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECKWITH J. R. A DELETION ANALYSIS OF THE LAC OPERATOR REGION IN ESCHERICHIA COLI. J Mol Biol. 1964 Mar;8:427–430. doi: 10.1016/s0022-2836(64)80206-0. [DOI] [PubMed] [Google Scholar]
- BRINTON C. C., Jr, GEMSKI P., Jr, CARNAHAN J. A NEW TYPE OF BACTERIAL PILUS GENETICALLY CONTROLLED BY THE FERTILITY FACTOR OF E. COLI K 12 AND ITS ROLE IN CHROMOSOME TRANSFER. Proc Natl Acad Sci U S A. 1964 Sep;52:776–783. doi: 10.1073/pnas.52.3.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN G. N., RICKENBERG H. V. Concentration spécifique réversible des amino acides chez Escherichia coli. Ann Inst Pasteur (Paris) 1956 Nov;91(5):693–720. [PubMed] [Google Scholar]
- COOK A., LEDERBERG J. Recombination studies of lactose nonfermenting mutants of Escherichia coli K-12. Genetics. 1962 Oct;47:1335–1353. doi: 10.1093/genetics/47.10.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss R., 3rd Bacterial conjugation. Annu Rev Microbiol. 1969;23:69–136. doi: 10.1146/annurev.mi.23.100169.000441. [DOI] [PubMed] [Google Scholar]
- Cuzin F., Jacob F. Association stable de deux épisomes F différents dans un clone d'Escherichia coli. Ann Inst Pasteur (Paris) 1967 Aug;113(2):145–155. [PubMed] [Google Scholar]
- Cuzin F., Jacob F. Mutations de l'épisome F d'Escherichia coli K 12. II. Mutants à réplication thermosensible. Ann Inst Pasteur (Paris) 1967 Apr;112(4):397–418. [PubMed] [Google Scholar]
- Cuzin F. Un bactériophage spécifique du type sexuel F- d'Escherichia coli K 12. C R Acad Sci Hebd Seances Acad Sci D. 1965 Jun 14;260(24):6482–6485. [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- Lawn A. M., Meynell G. G., Meynell E., Datta N. Sex pili and the classification of sex factors in the enterobacteriaceae. Nature. 1967 Oct 28;216(5113):343–346. doi: 10.1038/216343a0. [DOI] [PubMed] [Google Scholar]
- Meynell E., Datta N. Mutant drug resistant factors of high transmissibility. Nature. 1967 May 27;214(5091):885–887. doi: 10.1038/214885a0. [DOI] [PubMed] [Google Scholar]
- Nishimura Y., Ishibashi M., Meynell E., Hirota Y. Specific piliation directed by a fertility factor and a resistance factor of Escherichia coli. J Gen Microbiol. 1967 Oct;49(1):89–98. doi: 10.1099/00221287-49-1-89. [DOI] [PubMed] [Google Scholar]
- Novick R. P. Extrachromosomal inheritance in bacteria. Bacteriol Rev. 1969 Jun;33(2):210–263. doi: 10.1128/br.33.2.210-263.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaife J. Episomes. Annu Rev Microbiol. 1967;21:601–638. doi: 10.1146/annurev.mi.21.100167.003125. [DOI] [PubMed] [Google Scholar]
- Ullmann A., Jacob F., Monod J. Characterization by in vitro complementation of a peptide corresponding to an operator-proximal segment of the beta-galactosidase structural gene of Escherichia coli. J Mol Biol. 1967 Mar 14;24(2):339–343. doi: 10.1016/0022-2836(67)90341-5. [DOI] [PubMed] [Google Scholar]
- WATANABE T., FUKASAWA T. Episome-mediated transfer of drug resistance in Enterobacteriaceae IV. Interactions between resistance transfer factor and F-factor in Escherichia coli K-12. J Bacteriol. 1962 Apr;83:727–735. doi: 10.1128/jb.83.4.727-735.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE T. Infective heredity of multiple drug resistance in bacteria. Bacteriol Rev. 1963 Mar;27:87–115. doi: 10.1128/br.27.1.87-115.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE T., NISHIDA H., OGATA C., ARAI T., SATO S. EPISOME-MEDIATED TRANSFER OF DRUG RESISTANCE IN ENTEROBACTERIACEAE. VII. TWO TYPES OF NATURALLY OCCURRING R FACTORS. J Bacteriol. 1964 Sep;88:716–726. doi: 10.1128/jb.88.3.716-726.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE T., OKADA M. NEW TYPE OF SEX FACTOR-SPECIFIC BACTERIOPHAGE OF ESCHERICHIA COLI. J Bacteriol. 1964 Mar;87:727–736. doi: 10.1128/jb.87.3.727-736.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]



