Abstract
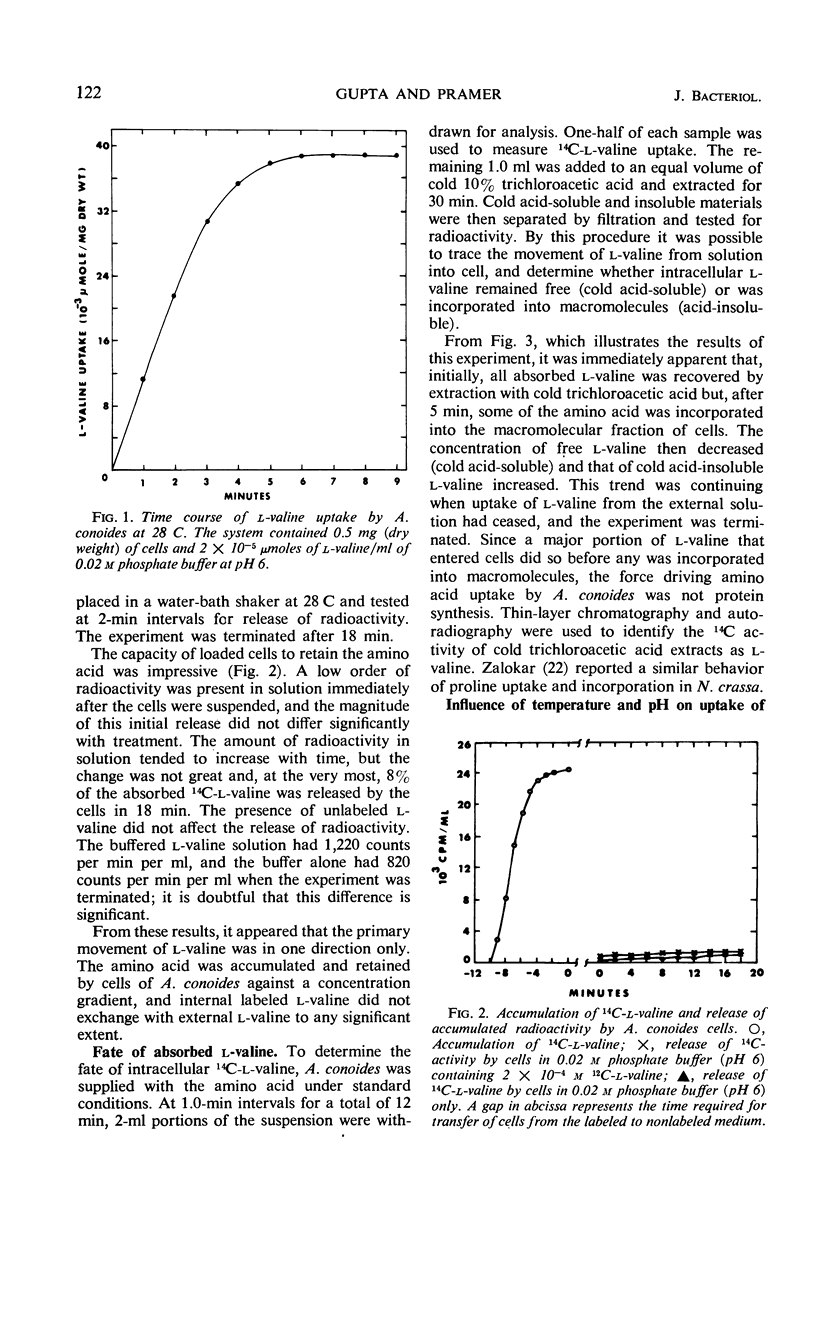
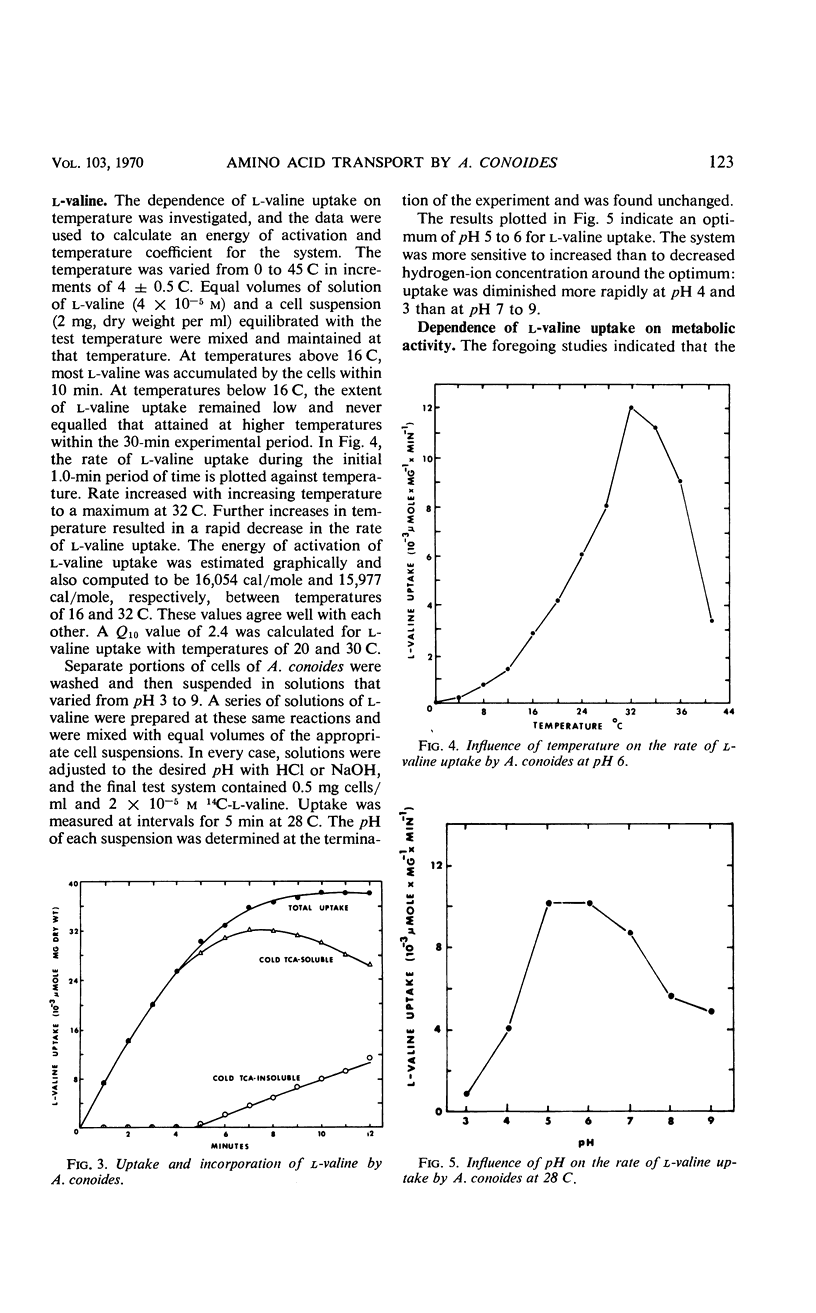
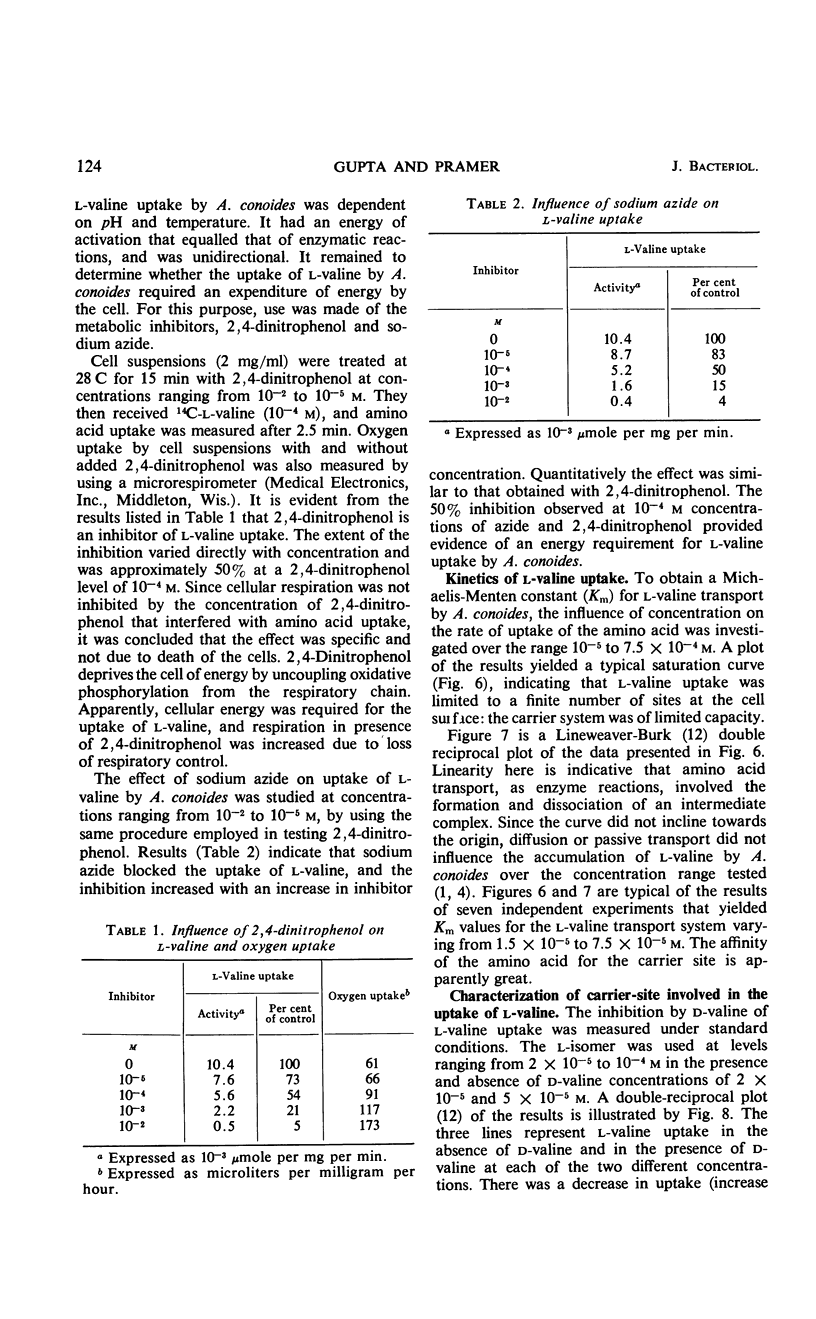
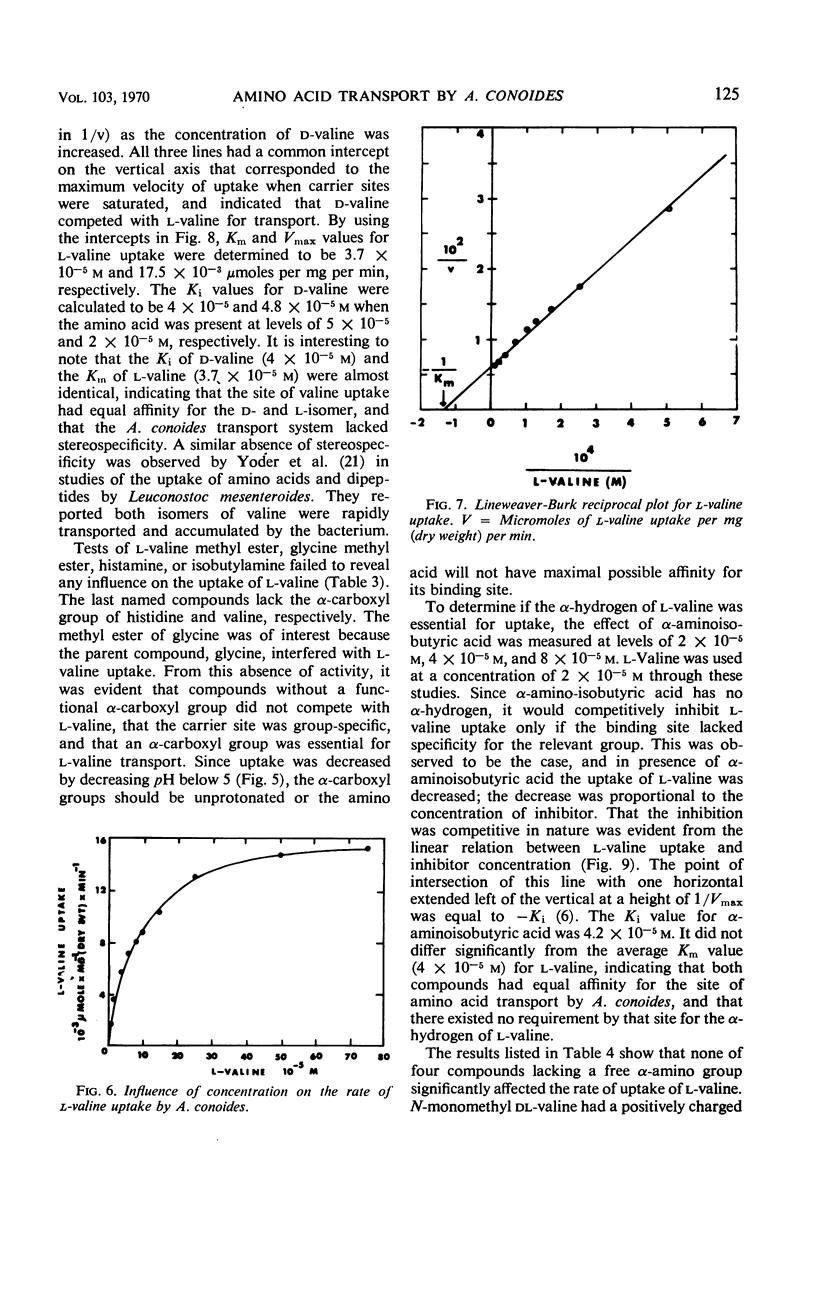
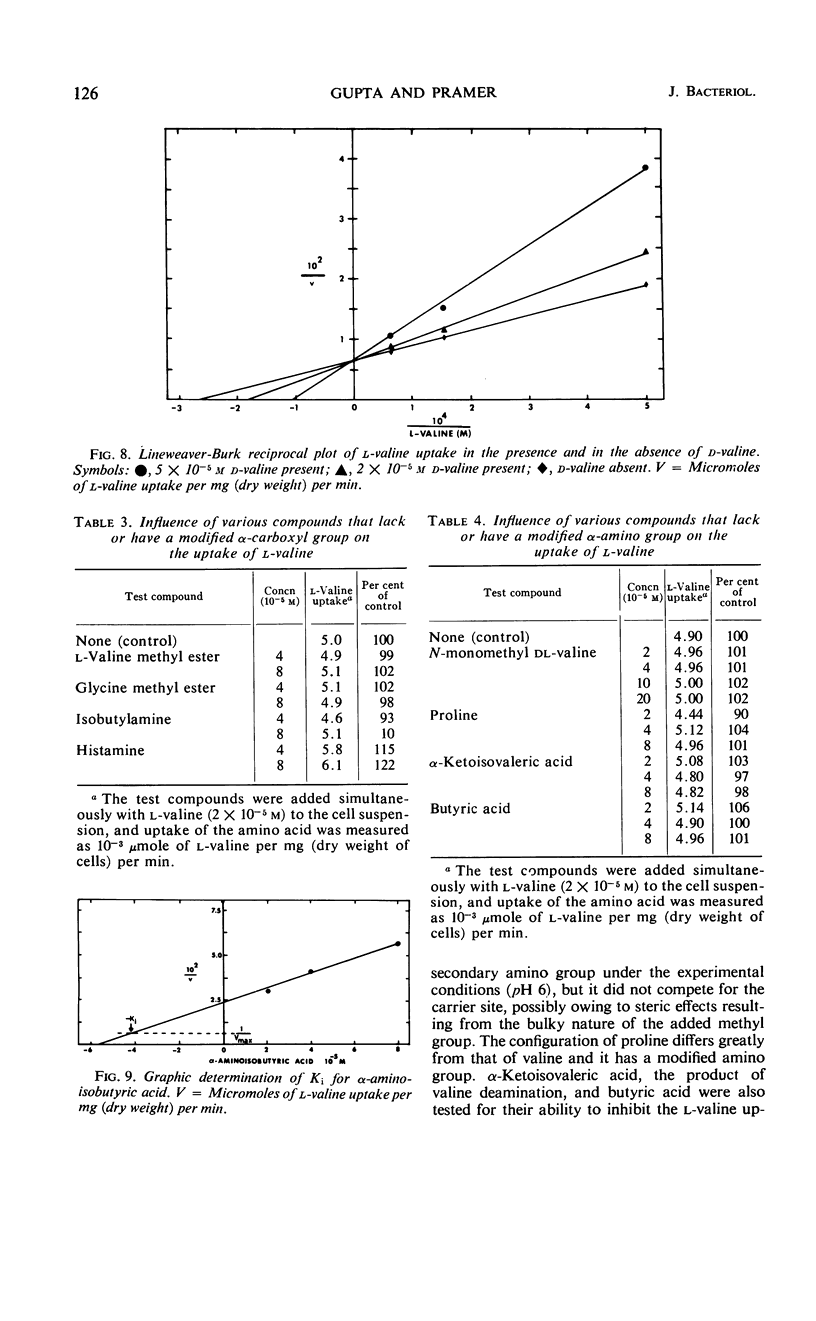
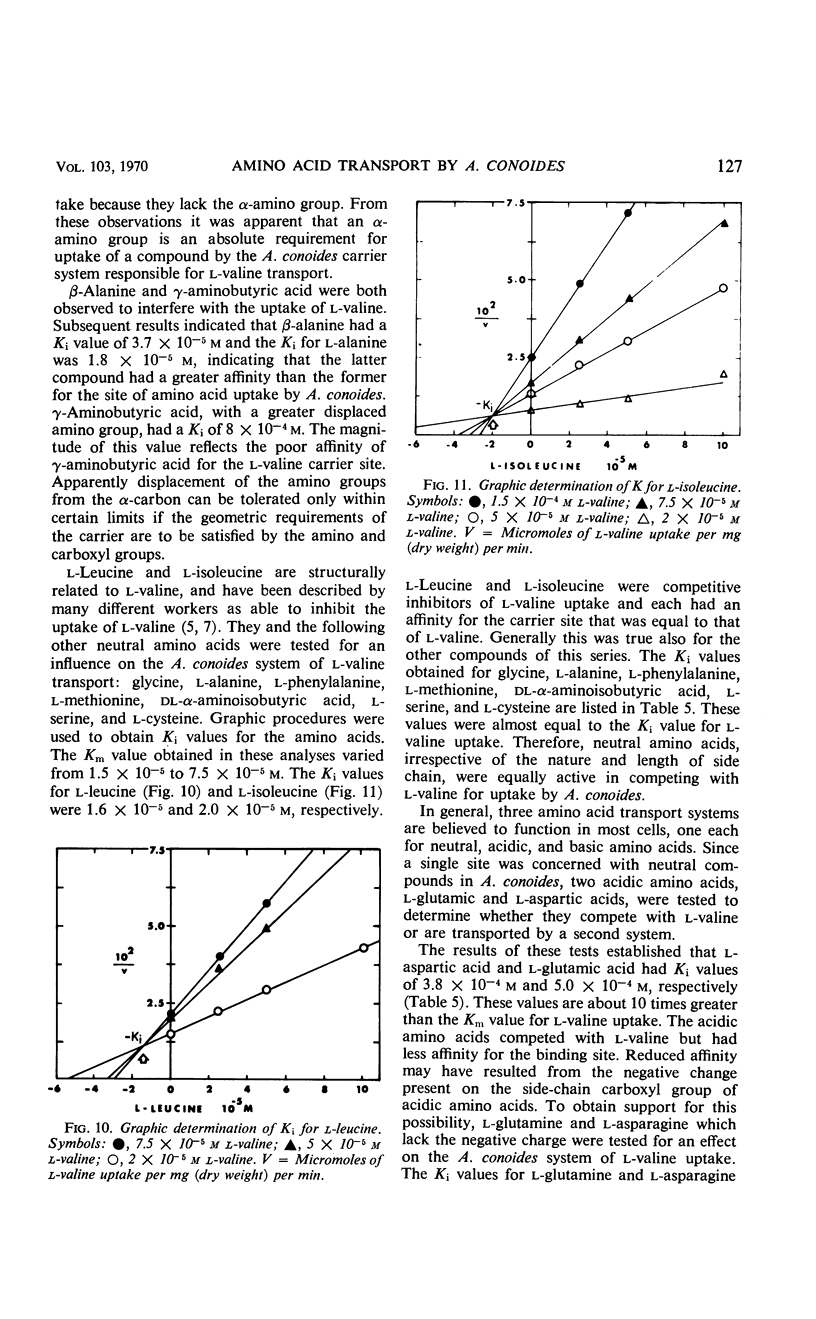
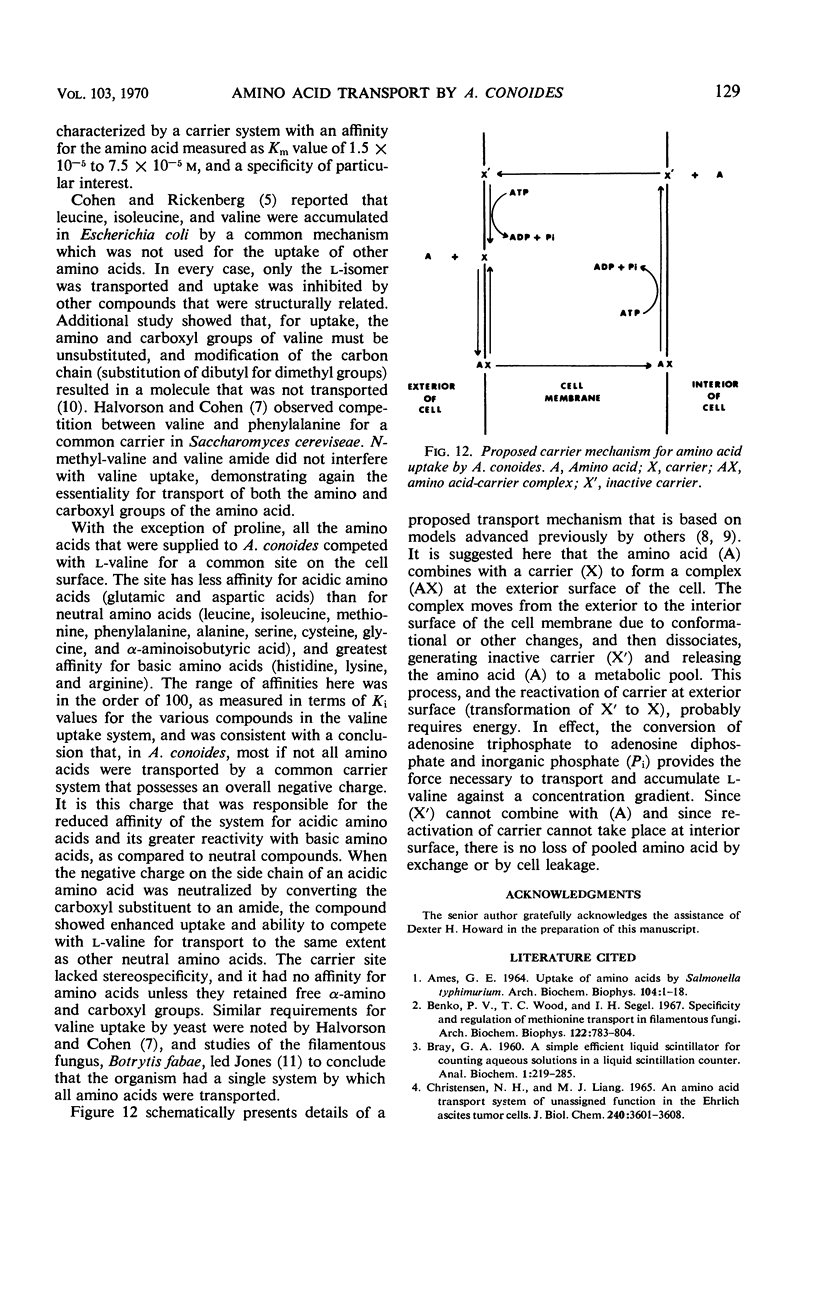
Uptake of l-valine by germinated spores of Arthrobotrys conoides has all the characteristics of a system of transport that requires an expenditure of energy by the cells. It is dependent on temperature and has an energy of activation of 16,000 cal/mole. Uptake is optimal at pH 5 to 6. l-Valine accumulated against a concentration gradient and is not lost from the cells by leakage or exchange. The process requires energy supplied by the metabolic reactions that are inhibited by catalytic amounts of 2,4-dinitrophenol and azide. The kinetics of the system are consistent with a mechanism of transport that depends on a limited number of sites on the cell surface, and the Michaelis constant for the system is 1.5 × 10−5 to 7.5 × 10−5m. Modification of the amino or carboxyl group abolishes l-valine uptake. The process is competitively inhibited by d-valine, glycine, and other neutral amino acids (Ki = 1.5 × 10−5 to 4.0 × 10−5m), indicating a lack of stereospecificity, and also indicating that aliphatic side chain is not required for binding with the carrier. The transport system has less affinity for acidic amino acids (glutamic and aspartic acids) than neutral amino acids, and a greater affinity for basic amino acids (histidine, lysine, and arginine). The range of affinity is in the order of 100, as measured in terms of Ki values for various compounds. The data presented provide suggestive evidence that the uptake by A. conoides of all amino acids except proline is mediated by a single carrier system that possesses an overall negative charge.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES G. F. UPTAKE OF AMINO ACIDS BY SALMONELLA TYPHIMURIUM. Arch Biochem Biophys. 1964 Jan;104:1–18. doi: 10.1016/s0003-9861(64)80028-x. [DOI] [PubMed] [Google Scholar]
- COHEN G. N., RICKENBERG H. V. Concentration spécifique réversible des amino acides chez Escherichia coli. Ann Inst Pasteur (Paris) 1956 Nov;91(5):693–720. [PubMed] [Google Scholar]
- Christensen H. N., Liang M. An amino acid transport system of unassigned function in the Ehrlich ascites tumor cell. J Biol Chem. 1965 Sep;240(9):3601–3608. [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALVORSON H. O., COHEN G. N. Incorporation des amino-acides endogènes et exogènes dans les protéines de la levure. Ann Inst Pasteur (Paris) 1958 Jul;95(1):73–87. [PubMed] [Google Scholar]
- HEINZ E., WALSH P. M. Exchange diffusion, transport, and intracellular level of amino acids in Ehrlich carcinoma cells. J Biol Chem. 1958 Dec;233(6):1488–1493. [PubMed] [Google Scholar]
- HOKIN L. E., HOKIN M. R. Phosphatidic acid metabolism and active transport of sodium. Fed Proc. 1963 Jan-Feb;22:8–18. [PubMed] [Google Scholar]
- LITWACK G., PRAMER D. Absorption of antibiotics by plant cells. III. Kinetics of streptomycin uptake. Arch Biochem Biophys. 1957 Jun;68(2):396–402. doi: 10.1016/0003-9861(57)90371-5. [DOI] [PubMed] [Google Scholar]
- Mayshak J., Yoder O. C., Beamer K. C., Shelton D. C. Inhibition and transport kinetic studies involving L-leucine, L-valine, and their dipeptides in Leuconostoc mesenteroides. Arch Biochem Biophys. 1966 Jan;113(1):189–194. doi: 10.1016/0003-9861(66)90173-1. [DOI] [PubMed] [Google Scholar]
- PRAMER D., KUYAMA S. SYMPOSIUM ON BIOCHEMICAL BASES OF MORPHOGENESIS IN FUNGI. II. NEMIN AND THE NEMATODE-TRAPPING FUNGI. Bacteriol Rev. 1963 Sep;27:282–292. doi: 10.1128/br.27.3.282-292.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRAMER D. NEMATODE- TRAPPING FUNGI. AN INTRIGUING GROUP OF CARNIVOROUS PLANTS INHABIT THE MICROBIAL WORLD. Science. 1964 Apr 24;144(3617):382–388. doi: 10.1126/science.144.3617.382. [DOI] [PubMed] [Google Scholar]
- PRAMER D., STOLL N. R. Nemin: a morphogenic substance causing trap formation by predaceous fungi. Science. 1959 Apr 10;129(3354):966–967. doi: 10.1126/science.129.3354.966. [DOI] [PubMed] [Google Scholar]
- SOLOMON A. K. The permeability of the human erythrocyte to sodium and potassium. J Gen Physiol. 1952 May;36(1):57–110. doi: 10.1085/jgp.36.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley W. R., Matchett W. H. Tryptophan transport in Neurospora crassa. I. Specificity and kinetics. J Bacteriol. 1966 Dec;92(6):1698–1705. doi: 10.1128/jb.92.6.1698-1705.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder O. C., Beamer K. C., Shelton D. C. Membrane specificity of Leuconostoc mesenteroides for the stereoisomeric forms of glycine and valine dipeptides. Can J Biochem. 1967 Feb;45(2):213–220. doi: 10.1139/o67-024. [DOI] [PubMed] [Google Scholar]
- ZALOKAR M. Kinetics of amino acid uptake and protein synthesis in Neurospora. Biochim Biophys Acta. 1961 Jan 29;46:423–432. doi: 10.1016/0006-3002(61)90573-x. [DOI] [PubMed] [Google Scholar]



