Summary
The anxiolytic effects of neuropeptide Y (NPY) are mediated in part by the central nucleus of the amygdala (CeA), a brain region involved in the regulation of alcohol-drinking behaviors. Centrally administered NPY suppresses alcohol drinking in subpopulations of rats vulnerable to the development of high alcohol-drinking behavior. The purpose of the current study was to determine the role of NPY in the CeA on elevated alcohol drinking produced by alcohol dependence. Adult male Wistar rats were trained to respond for 10% w/v alcohol in an operant situation with the use of a supersaccharin fading procedure. Following stabilization of responding, rats were divided into two groups matched for intake and given daily access to either alcohol-containing (9.2% v/v) liquid diet or an isocaloric control diet. Following extended access to the diet and reliable separation of operant responding between dependent and non-dependent rats during 6-hr withdrawal tests, all rats were implanted bilaterally with cannulae aimed at the CeA. Rats were then infused with 4 NPY doses (0.0, 0.25, 0.5, 1.0 μg/0.5 μl aCSF) in a within-subjects Latin-square design during acute withdrawal and tested for operant alcohol responding 30 minutes later. Alcohol-dependent rats exhibited higher operant alcohol responding than non-dependent rats when infused with vehicle, but responding was similar in the two groups following infusion of all doses of NPY. These results indicate that NPY abolishes dependence-induced elevations in alcohol drinking and implicate the recruitment of limbic NPY systems in the motivational drive to consume alcohol following the transition to dependence.
Keywords: Neuropeptide Y, Amygdala, Ethanol, Alcohol Dependence, Operant Self-administration, Alcohol Withdrawal
Alcohol dependence produces pathological changes in brain, thought to include significant perturbations in brain NPY-anxiety circuits, and these changes likely contribute to the negative affective state that defines alcohol abstinence and drives the negative reinforcing effects of alcohol during relapse drinking (Valdez & Koob, 2004). Neuropeptide Y (NPY) decreases anxiety-like behavior in rats in a multitude of behavioral assays (Heilig et al., 1989, 1992; Broqua et al., 1995; Britton et al., 1997; Sajdyk et al., 1999), and these anxiety-reducing effects of NPY are mediated by the amygdala (Heilig et al., 1993; Sajdyk et al., 1999). NPY administered into the amygdala of alcohol-preferring (P) rats, selectively bred for high alcohol preference, suppresses alcohol drinking only in rats that have endured periods of imposed alcohol abstinence (Gilpin et al.,2008). At a slightly finer level of anatomical resolution, the central nucleus of the amygdala (CeA) is involved in the regulation of anxiety (Davis, 1992), and it is thought that the anxiolytic effects of NPY are at least partially mediated by the CeA (Heilig et al., 1993). Since the CeA is also involved in the regulation of alcohol-drinking behaviors (McBride et al., 2002), the present investigation sought to determine the effects of exogenous NPY administered into the CeA on alcohol drinking by alcohol-dependent rats.
Intracerebroventricular (ICV) administration of NPY does not affect limited access alcohol intake by Wistar rats (Badia-Elder et al., 2001, 2003; Katner et al., 2002a; Slawecki et al., 2000). However, ICV-administered NPY does effectively reduce limited-access alcohol intake in Wistar rats if they have a history of alcohol dependence produced by chronic intermittent exposure to alcohol vapor (Thorsell et al., 2005a, 2005b). ICV NPY also suppresses alcohol intake in rats selectively bred for high alcohol preference, but does not alter alcohol intake in their low-preferring counterparts (Badia-Elder et al. 2001, 2003). The suppressive effects of ICV-administered NPY on ethanol drinking in P rats is enhanced and prolonged following periods of imposed alcohol abstinence (Gilpin et al., 2003, 2005).
Infusion of NPY into the CeA does not affect limited-access alcohol drinking by Wistar rats that do not have a history of alcohol dependence (Katner et al., 2002b). Intra-CeA infusion of a viral vector encoding prepro-NPY reduces continuous access-alcohol drinking by Long-Evans rats deemed to be “anxious” according to behavior on an elevated plus maze (Primeaux et al. 2006). Furthermore, in Wistar rats with a history of dependence and multiple abstinence periods, viral vector-induced amygdala NPY overexpression reduces anxiety-like behavior and produces long-term suppressions of alcohol drinking (Thorsell et al., 2007). In P rats with a long history of alcohol consumption, infusions of NPY aimed at the CeA suppress alcohol drinking only by P rats that have endured periods of imposed alcohol abstinence (Gilpin et al., 2008). Finally, P rats with a brief self-administration history consume less alcohol following NPY infusion into the CeA, and also following increases in NPY activity in the CeA produced via alterations in cAMP-responsive element-binding protein (CREB) function (Pandey et al. 2005).
The purpose of the present study was to examine the effects of exogenous NPY administered into the CeA on alcohol drinking during acute withdrawal by alcohol-dependent rats. It was hypothesized that alcohol dependence would produce increases in alcohol drinking relative to non-dependent controls, and that NPY would abolish those increases.
Methods
Animals
31 adult male Wistar rats obtained from Charles River (Kingston, NY) were used in this experiment. The average body weight of rats at the start of operant training was 275.8±7.7 grams. Animals were group-housed at the start of operant training, and subsequently single-housed (to monitor daily liquid diet intake by individual rats and also to reduce the likelihood of cannula loss following surgery; see below) in standard plastic cages with wood chip bedding under a 12 hr light/12 hr dark cycle (lights off at 10 AM). Animals were given ad libitum access to food and water throughout except during experimental drinking sessions. All procedures were conducted in the dark cycle and met the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
The subjects in the present study received extended access to liquid diet (details below) as part of an experiment that examined parametric aspects of blood-alcohol levels and behavior as they relate to alcohol dependence produced by the liquid diet. Because the aims of the parametric study are beyond the scope of the present investigation, the details of those data are to be presented elsewhere (manuscript in preparation) in combination with other data from our lab that address similar parametrics in the vapor dependence model.
Drugs
Neuropeptide Y (Sigma-Aldrich, St. Louis, MO) was dissolved in artificial cerebrospinal fluid (aCSF) at various concentrations such that a volume of 0.5μl aCSF contained 0.0, 0.25, 0.5, or 1.0μg NPY. These microgram doses are equivalent to picomolar doses of NPY (equivalent to 59, 118, 235 pmol, respectively).
Stereotaxic Surgeries
Surgical implantation of cannulae was conducted using aseptic procedures. Rats were anesthetized via inhalation of isoflourane (IsoFlo, Abbott Laboratories, North Chicago, IL) before and during surgery. The incision area of the scalp was shaved, the rat was placed in a Kopf stereotaxic instrument, and a sagittal incision (approximately 2 cm long) was made in the midline exposing the surface of the skull. Two holes were drilled through the skull targeted above the left and right central amygdaloid nuclei according to the appropriate stereotaxic coordinates and a guide cannula was implanted. The stereotaxic coordinates were determined according to Paxinos and Watson (1998). The coordinates relative to bregma were (AP-2.6, ML±4.2, DV-5.2) from skull surface. Microinjection cannulae components (Plastics One Inc., Roanoke, VA) included guide cannulae (26 gauge), internal injection cannulae (33 gauge), and dummy cannulae (33 gauge). The injection cannula extended 1.0 mm beyond the tip of the guide cannula when inserted. At all times, except when infusions were conducted, the dummy cannulae, cut to the same length as the guide cannula, were maintained in the guide cannulae. Four stainless steel screws were inserted into the skull at positions around the cannula implant site. Cranioplastic cement was applied over the open surface of the skull covering both the screws and the guide cannula. The incision was closed around the implants and the dummy cannula was inserted. Immediately after surgery, antibiotic ointment was applied to the surgical wound area. Surgeries occurred following extended access to liquid diet (43 days; see below). The rats were monitored during seven days of recovery to determine that the animal had resumed normal activity such as mobility, and consumption of liquid diet and water.
Procedure
Operant Alcohol Self-administration Training
The operant chambers (Coulbourn Instruments, Allentown, PA) utilized in the present study had two retractable levers located 4 cm above a grid floor and 4.5 cm to either side of a two-well acrylic drinking cup. Operant responses and resultant fluid deliveries were recorded by custom software running on a PC computer. A single lever-press activated a 15 rpm Razel syringe pump (Stanford, CT) that delivered 0.1 ml of fluid to the appropriate well over a period of 0.5 s. Lever presses that occurred during the 0.5 s of pump activation were not recorded and did not result in fluid delivery. Operant chambers were individually housed in sound-attenuated ventilated cubicles to minimize environmental disturbances.
Wistar rats were trained to respond for a “supersaccharin” solution (3% glucose and 0.125% saccharin; Valenstein et al., 1967) versus water in a concurrent, two-lever, free-choice contingency during daily 30-min sessions. This procedure eliminates the need for any food or water restriction during operant training. Lever-presses were reinforced on a continuous fixed ratio-1 (FR1) schedule such that each response resulted in delivery of 0.1 ml of fluid. Following the second session of operant training with supersaccharin, 10% (w/v) ethanol was added and then sweeteners gradually removed from the experimental solution across days. Upon completion of this fading procedure, Wistar rats were allowed 26 sessions of operant responding for 10% (w/v) ethanol versus water. Operant responding was stable and reliable for these rats by the 26th day of operant responding. Wistar rats were divided into two groups based on mean intakes across the final 6 days of this baseline period: rats to receive alcohol-containing liquid diet (dependent, n=16; mean ± SEM ethanol intake prior to vapor = 0.48 ± 0.13 g/kg), and rats to receive control liquid diet with no alcohol (non-dependent; n=15; mean ± SEM ethanol intake prior to vapor = 0.52 ± 0.07 g/kg).
Alcohol-Liquid Diet Exposure
Immediately prior to the start of alcohol-liquid diet exposure, lab chow was removed from cages. From that point forward, the sole source of nutrition available to rats in the home cage was the alcohol- or control-liquid diet, although water was still available to all rats ad libitum. One liter of the palatable alcohol-liquid diet contained 3 g vitamins (MP Biomedicals, LLC, Solon, OH), 5 g salt (ICN Biomedicals, Inc., Aurora, OH), 92 ml 95% v/v ethanol, 711 ml Boost (High-protein chocolate-flavored nutritional energy drink, Columbus, IN), and 197 ml water; one liter of control-liquid diet was similar except that it contained 126 g sucrose (isocalorcially matched to alcohol-liquid diet; Sigma-Aldrich, St. Louis, MO) instead of 95% v/v ethanol. One liter of liquid diet contained 1225 calories (505 calories derived from ethanol/sucrose, and 720 calories derived from Boost), and the Boost in one liter of diet contained 18 g total fat, 99 g total carbohydrates, and 45 g protein. Control diet availability for non-dependent rats was yoked to 24-hr intakes of alcohol-liquid diet by dependent rats on the previous day, and standardized for body weights. At these concentrations, rats derived 41% of their caloric intake from ethanol/sucrose. Because these rats were part of a parametric liquid-diet dependence study (data presented elsewhere; see Subjects section), rats had access to liquid diet for an extended period of time (43 days) prior to surgical implantation of cannulae. This alcohol liquid-diet procedure is sufficient to produce somatic (Majchrowicz, 1975) and motivational (Overstreet et al., 2004) signs of dependence in rats after much shorter periods of access. To determine blood-alcohol levels (BALs) achieved during alcohol-liquid diet consumption, blood samples were collected from all rats at various time points during the circadian cycle on days that rats were not tested.
Microinfusions and Operant Tests During Alcohol Liquid Diet
On operant test days, liquid diet bottles were removed and intakes recorded two hours before the start of the dark cycle (8 a.m.). Six hours later (i.e. rats only had 18 hrs access to liquid diet on test days), Wistar rats in the dependent and non-dependent groups were placed in operant chambers and tested for operant alcohol responding during acute withdrawal. Following establishment of stable operant responding across test days in dependent and non-dependent rats, all rats underwent stereotaxic surgery and were implanted bilaterally with cannulae aimed at the CeA. Following surgery, rats were allowed several days to recover, and were then once again tested for baseline operant alcohol responding across multiple days until responding stabilized (3 sessions). All rats were habituated to the infusion procedure with “mock infusion” days (i.e. nothing infused into brain). Once mock infusion responding stabilized and was representative of baseline responding (3 sessions), NPY infusions began.
On NPY test days, rats were infused with one of four NPY doses (0.0, 0.25, 0.5, 1.0μg; equivalent to 59, 118, 235 pmol) six hours following removal of liquid-diet bottles (i.e. six hours into withdrawal) in a within-subjects Latin-square design. A Harvard 33 microinfusion pump was used for all drug infusions at a rate of 0.25μl/minute for a period of two minutes, and the injection cannula was left in the guide cannula for one additional minute to allow for adequate diffusion of the solution. Infusions were delivered to the cannula via polyethylene tubing (PE 20) that was connected to a Hamilton 10 μl syringe.
Immediately following infusions, rats were placed in operant chambers with no levers available. Thirty minutes later, levers were made available and 30-min operant sessions began. Doses were administered to rats in a Latin-square design. Following all experimental procedures, cannulae placements and patency were histologically verified.
Blood-Alcohol Level Determinations
Tail blood was sampled two hours into the dark cycle to determine blood-alcohol levels (BALs) achieved by rats during a period of high liquid diet consumption. Rats were gently restrained while the tip of the tail (2 mm) was cut with a clean razor blade. Tail blood (0.2 ml) was collected and centrifuged. Plasma (5 μl) was used for measurement of BALs using an Analox AM 1 analyzer (Analox Instruments LTD, Lunenberg, MA). The reaction is based on the oxidation of alcohol by alcohol oxidase in the presence of molecular oxygen (alcohol + O2 → acetaldehyde +H2O2). The rate of oxygen consumption is directly proportional to the alcohol concentration. Single point calibrations are done for each set of samples with reagents provided by Analox Instruments (0.025-0.400 g%).
Statistical Analysis
Operant responding, alcohol consumption (g ethanol/kg body weight), and alcohol preference (ethanol consumed/total fluid consumed) during 30-min test sessions were analyzed using two-way repeated-measures analyses of variance (RM ANOVAs) where NPY dose (0.0, 0.25, 0.5, 1.0μg plus 3 mock infusions) was the within-subjects factor and dependence history (dependent vs. non-dependent) was the between-subjects factor. Also, liquid diet intake (ml) by dependent animals only (non-dependent animals were yoked) during the 24 hrs following NPY infusion was analyzed using one-way RM ANOVA where NPY dose (0.0, 0.25, 0.5, 1.0μg plus 3 mock infusions) was the within-subjects factor. Post-hoc comparisons were made using the Student Newman-Keuls test and paired t-test. Statistical significance was set at p<0.05. Ten rats were excluded from post-surgery analyses for reasons that included inaccurate cannulae placements (n=6), lost headcaps during the course of experimentation (n=2), or rats were sacrificed due to poor health (n=2). A single rat was excluded from all data analyses because its operant response datum (following infusion of 1.0 μg NPY) was determined to be an outlier according to the Geigy Extreme Test. Therefore, analysis of operant response data included 10 dependent rats and 10 non-dependent rats.
Results
Alcohol liquid diet intake and dependence-induced drinking
During the 6 days preceding surgical implantation of cannulae, dependent rats consumed a daily average of 86.99 ± 1.29 ml of alcohol liquid diet, which corresponded to 11.07 ± 0.19 g/kg daily ethanol intake. During the 6 days following surgical implantation of cannulae, dependent rats consumed a daily average of 84.61 ± 1.57 ml of alcohol liquid diet, which corresponded to 10.87 ± 0.30 g/kg daily ethanol intake. Non-dependent rats were always given a quantity of control liquid diet that was calorically matched to the intake of dependent rats on the previous day. These calorically matched diets allowed similar body weight gain in dependent (mean body weight = 587.98 ± 16.74 g prior to start of NPY infusions) and non-dependent (mean body weight = 583.02 ± 16.49 g prior to start of NPY infusions) rats.
Figure 1 shows a scatter plot of liquid diet consumption during the first 2 hours of dark and resultant blood-alcohol levels. Alcohol liquid diet consumption and blood-alcohol levels were significantly correlated in dependent rats, r(18)=0.82, p<0.001. On the day of tail blood collection, dependent rats consumed an average of 15.55 ± 1.59 ml of alcohol liquid diet during the first two hours of liquid diet access; mean resultant BALs were 280.35 ± 28.57 mg%. Tail bloods were also collected from rats drinking control diet, and BALs in control rats were negligible. On a separate day, liquid diet was removed from cages at the start of the dark cycle and, 6 hrs later at the time when rats would normally be tested for operant responding, tail bloods were instead collected from all rats. Blood-alcohol levels were negligible for all rats at that time point, indicating that alcohol was eliminated from blood prior to the start of operant sessions six hours into withdrawal on behavioral test days.
Figure 1.
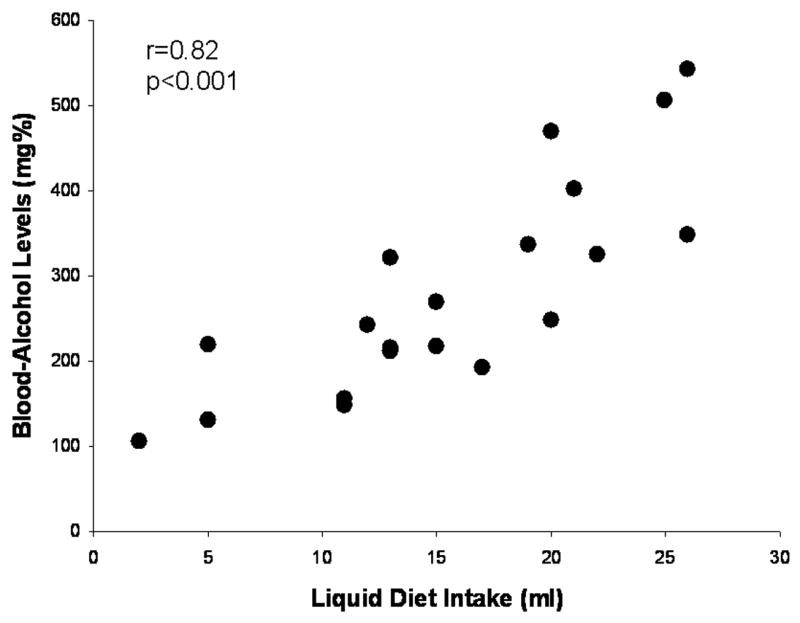
Scatter plot of blood-alcohol levels (BALs) produced by alcohol liquid diet intake (ml) by dependent Wistar rats during the first two hours of the dark cycle on a representative day. There was a significant correlation between liquid diet consumption and BALs in those animals. BALs in animals consuming control diet were negligible. Also, BALs were negligible for all rats six hours into withdrawal on behavioral test days.
Following establishment of baseline operant responding and 6 weeks of access to alcohol (dependent group) or control (non-dependent group) liquid diet, rats were tested for operant alcohol responding on multiple days at the 6-hr withdrawal time point (Figure 2). A two-way (history × test day) RM ANOVA exhibited that dependent rats responded more for alcohol across tests than non-dependent rats, F(1,51)=13.85, p=0.002. There was also a significant interaction effect on operant alcohol responding, F(3,51)=3.35, p=0.026. Post-hoc analyses indicated that dependent rats responded significantly more for alcohol during all three pre-surgery withdrawal tests relative to their own baseline (p<0.05 in all cases) and also relative to non-dependent rats (p<0.05 in all cases). A separate series of two-way RM ANOVAs yielded similar effects on alcohol intake (g/kg).
Figure 2.
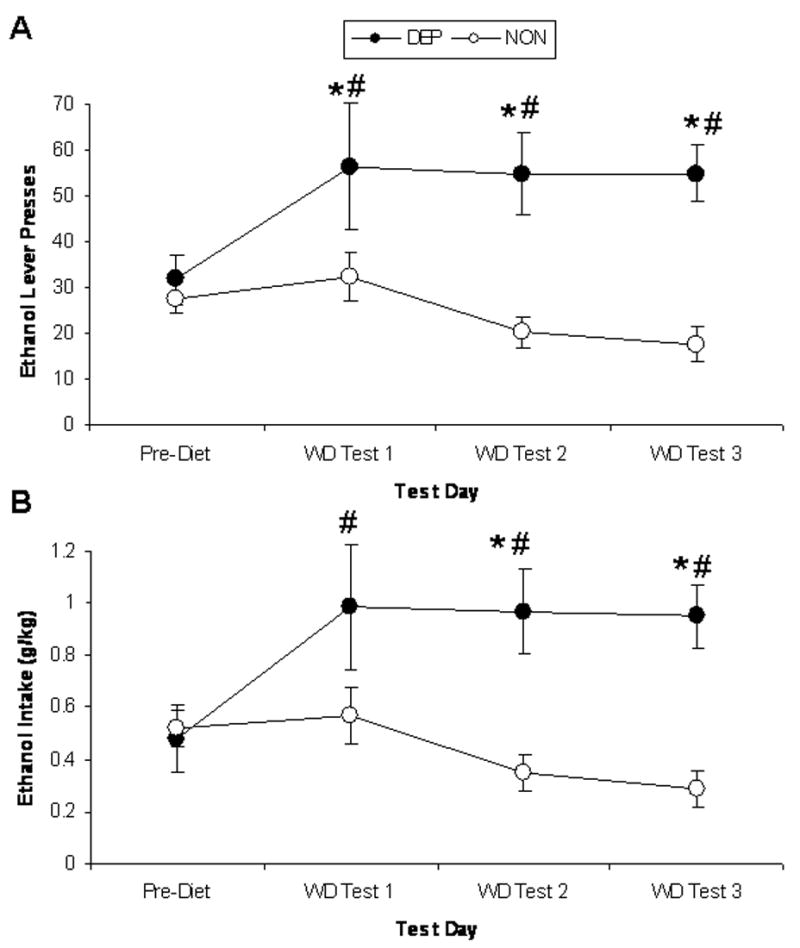
Operant lever presses for ethanol (A) and ethanol intake (g/kg; B) by rats consuming ethanol liquid diet (dependent group; closed circles) and rats consuming control liquid diet (non-dependent group; open circles). Pre-diet baseline represents an average of the six operant test sessions preceding start of liquid diet access. Withdrawal (WD) tests occurred six hours following the removal of alcohol liquid diet bottles from the home cage. * indicates p<0.05 significant difference from baseline; # indicates p<0.05 significant difference from non-dependent control rats.
Effects of NPY in the CeA on operant behavior during withdrawal
Figure 3 shows operant alcohol responding (Figure 3A) and alcohol consumption (g/kg; Figure 3B) by dependent and non-dependent rats following infusion of four NPY doses (0.0, 0.25, 0.5, 1.0 μg) and also during sham infusions. Dependent rats exhibited higher operant alcohol responding, F(1,102)=15.22, p=0.001, and higher alcohol intake (g/kg), F(1,102)=13.84, p=0.002, than non-dependent rats. There were also main effects of NPY on operant alcohol responding, F(6,102)=4.22, p<0.001, and alcohol intake (g/kg), F(6,102)=4.15, p<0.001. Finally, there were interaction effects of dependence history and NPY on operant alcohol responding, F(6,102)=3.33, p=0.005, and alcohol intake (g/kg), F(6,102)=3.21, p=0.006. Post-hoc comparisons indicated that dependent rats exhibited higher operant alcohol responding and alcohol intake (g/kg) than non-dependent rats following infusion of aCSF vehicle (p<0.05 in both cases). All doses of NPY (0.25, 0.5, 1.0 μg) abolished this elevated alcohol responding and consumption in dependent rats (no difference in responding or intake in the two groups; p>0.05 in all cases). Furthermore, all doses of NPY suppressed alcohol responding and consumption in dependent rats relative to their own vehicle baseline (p<0.05 in all cases). Analysis of data from rats that had inaccurate cannulae placements yielded a significant main effect of dependence history, F(1,16)=8.85, p=0.04, but no tendencies toward effects of NPY (p=0.84) or interaction effects (p=0.98), indicating some degree of anatomical specificity of the NPY effects.
Figure 3.
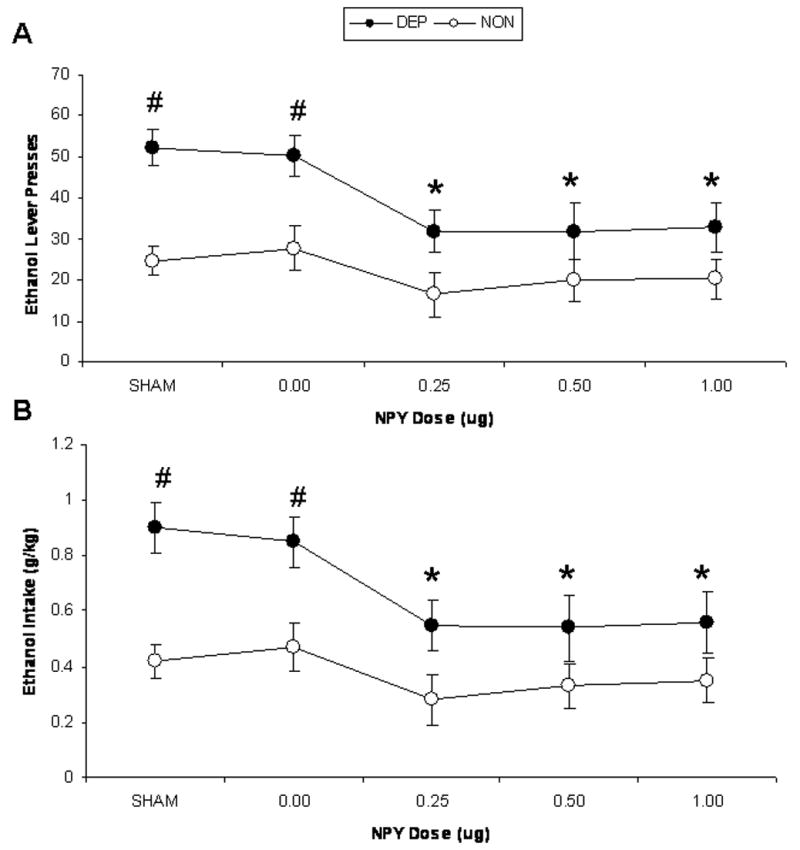
Operant lever presses for ethanol (A) and ethanol intake (g/kg; B) by alcohol-dependent (closed circles) and –non-dependent (open circles) Wistar rats following infusion of one of four NPY doses (0.0, 0.25, 0.5, 1.0 μg in 0.5 μl aCSF) and sham infusions (data point represents mean of three sham infusion days included in data analyses) six hours following the removal of alcohol liquid diet bottles from the home cage (i.e. 6 hrs withdrawal). * indicates p<0.05 significant difference from baseline; # indicates p<0.05 significant difference from non-dependent control rats.
Water response, alcohol preference, and total fluid intake data are displayed in Table 1. There were no effects of dependence history or NPY dose on water responding or alcohol preference. Dependent rats consumed more total fluid than non-dependent rats during operant test sessions, F(1,102)=9.52, p=0.007. There was also a main effect of NPY dose on total fluid intake F(6,102)=2.93, p=0.011, although there were no significant post-hoc pairwise comparisons. A separate one-way RM ANOVA yielded a significant effect of NPY injection on liquid diet intake (Table 1) by dependent animals (diet availability for non-dependent controls was not analyzed because it was yoked to intakes by dependent animals) during the 24 hrs following NPY infusion, F(6,69)=4.40, p=0.001, although there were no significant post-hoc differences between any NPY dose and vehicle, suggesting a non-specific injection effect.
Table 1.
Mean ± SEM water responses, alcohol preference (Alcohol/Total), and total fluid intake (ml) for alcohol-dependent and – non-dependent rats during 30-min operant sessions following bilateral infusion of four NPY doses (0.0, 0.25, 0.5, 1.0 μg) into the CeA. Also presented are liquid diet intake (ml) data for the 24-hr period following the operant session. Data labeled “Mock Inf” represents the average of the 3 days of mock infusions included in statistical analyses. N/A indicates that non-dependent controls were given amounts of liquid diet that were yoked to ethanol liquid-diet intakes by dependent rats during previous 24-hr period.
| Water Lever Presses | Preference (A/T) | Total Fluid (ml) | Liquid Diet (ml) | ||
|---|---|---|---|---|---|
| Alcohol-Dependent | NPY Dose | ||||
| Mock Inf | 4.93±2.04 | 0.89±0.05 | 5.72±0.45 | 80.27±2.13 | |
| 0.0 μg | 5.30±2.99 | 0.91±0.05 | 5.57±0.50 | 69.40±6.56 | |
| 0.25 μg | 7.60±5.06 | 0.86±0.08 | 3.95±0.61 | 74.20±4.95 | |
| 0.5 μg | 9.80±6.43 | 0.83±0.07 | 4.17±0.94 | 77.44±4.31 | |
| 1.0 μg | 6.70±5.04 | 0.90±0.06 | 3.95±0.80 | 81.20±3.23 | |
|
|
|||||
| Alcohol Non-dependent | NPY Dose | ||||
| Mock Inf | 9.04±6.04 | 0.81±0.07 | 3.38±0.85 | N/A | |
| 0.0 μg | 9.89±4.01 | 0.76±0.09 | 3.77±0.59 | N/A | |
| 0.25 μg | 4.44±2.06 | 0.72±0.12 | 2.10±0.50 | N/A | |
| 0.5 μg | 5.89±2.73 | 0.76±0.09 | 2.58±0.59 | N/A | |
| 1.0 μg | 5.22±2.17 | 0.83±0.07 | 2.56±0.56 | N/A | |
Discussion
The present results show that neuropeptide Y infused bilaterally into the central nucleus of the amygdala abolishes elevations in operant alcohol responding produced by alcohol dependence. Following infusion of vehicle, alcohol-dependent rats exhibited higher operant alcohol responding and alcohol intake (g/kg) than non-dependent rats, and those differences were eliminated following infusion of all three NPY doses.
These results indicate that the CeA mediates the suppressive effects of whole-brain increases in NPY activity on ethanol drinking by Wistar rats. More specifically, alcohol drinking by dependent Wistar rats, but not non-dependent controls, is suppressed following ICV administration of NPY (Thorsell et al., 2005b) and BIIE0246, a Y2 autoreceptor-selective antagonist (Rimondini et al., 2005). ICV NPY has other behavioral effects (e.g., sedation and increased feeding) not specific to alcohol self-administration behavior, but those actions are thought to be mediated by the hypothalamus (Gilpin et al., 2004; Naveilhan et al., 2001; Stanley et al., 1985). In this context, the present results indicate that the CeA is involved specifically in the suppressive effects of NPY on alcohol drinking, and that those effects are not secondary to the orexigenic or sedative effects of NPY.
The present results are also consistent with several studies that have shown the ability of NPY to suppress alcohol drinking by rat subpopulations characterized by an increased propensity for high alcohol-drinking behavior. Infusion into the CeA of a viral vector encoding for the NPY precursor reduces alcohol drinking by “anxious,” but not “nonanxious” Long Evans rats, as determined by behavior in an elevated plus-maze (Primeaux et al. 2006). In Wistar rats with a history of dependence that have endured multiple abstinence periods, viral vector-induced amygdala NPY overexpression reduced anxiety-like behavior and produced long-term suppressions of alcohol drinking (Thorsell et al., 2007). A seemingly contradictory finding showed that infusion of a Y1 receptor antagonist into the CeA reduces operant responding for alcohol by Wistar rats in limited-access operant sessions, but those rats were not dependent on alcohol or divided according to innate phenotypic profiles (Schroeder et al., 2003). Alcohol-preferring P rats, selectively bred for high alcohol preference, consumed less alcohol following NPY infusion into the CeA, and also following cAMP-responsive element-binding protein (CREB) –induced increases in NPY activity in the CeA (Pandey et al. 2005). A more recent study indicated that the ability of intra-amygdalar NPY injections to suppress alcohol drinking by P rats is augmented following a period of imposed alcohol abstinence (Gilpin et al., 2008). Therefore, increased vulnerability due to genetic manipulations, phenotypic selection, alcohol dependence, and/or cycles of abstinence facilitates the ability of NPY to suppress alcohol drinking, indicating that brain NPY systems are recruited during the development of high alcohol-drinking behavior under these conditions.
It has been suggested that the negative reinforcing effects of alcohol may be mediated by NPY brain systems involved in regulating anxiety-like behavior (i.e., amygdala; Valdez & Koob, 2004). The negative reinforcing properties of alcohol are typically more predominant in the alcohol-dependent organism (Koob, 2003) and are partially driven by the negative affective state (i.e. anxiety) that manifests in the absence of the drug. Because the anxiolytic effects of NPY are mediated by the amygdala (Heilig et al., 1993; Sajdyk et al., 2002), it is reasonable to hypothesize that NPY activity in that brain region is more heavily involved in the regulation of alcohol-drinking behavior following the development of dependence. The results of the present investigation are consistent with that hypothesis and suggest that NPY in the CeA may suppress alcohol drinking in alcohol-dependent animals via opposition of the high-anxiety state produced by the absence of alcohol itself.
The hypothesis that NPY suppresses alcohol drinking via its anxiolytic effects generalizes beyond withdrawal-induced drinking by dependent animals and is not limited to the anxiety state produced by the absence of alcohol. As described above, NPY effectively suppressed alcohol drinking by non-dependent rats shown to have an “anxious” phenotype on the elevated plus-maze (Primeaux et al., 2006). Furthermore, alcohol-naïve P rats exhibited higher anxiety-like behavior than their non-preferring counterparts as measured by the elevated plus-maze test (Stewart et al., 1993), and chronic NPY infusions into the CeA suppressed alcohol drinking by non-dependent P rats and also decreased anxiety-like behavior of P rats on the elevated plus-maze (Pandey et al., 2005). Therefore, innate differences between animals contribute to the ability of NPY to affect alcohol drinking, and a history of alcohol dependence does not necessarily determine the presence of NPY effects, but rather the magnitude of those effects on alcohol drinking.
The mechanism by which NPY in the CeA suppresses alcohol drinking is not known, but possible mechanisms can be discussed in light of what is known about corticotropin-releasing factor (CRF), a peptide that exhibits behavioral effects that are opposite those of NPY (Valdez and Koob, 2004). Acute ethanol enhances GABA transmission in the CeA (Roberto et al., 2003), an effect that is replicated by CRF (Nie et al., 2004), and augmented in alcohol-dependent animals (Roberto et al., 2004). Furthermore, CRF enhances and NPY inhibits GABA transmission in the bed nucleus of the stria terminalis (Kash and Winder, 2006), another brain region thought to be important in the motivational aspects of alcoholism (Koob, 2003). Because NPY and GABA are colocalized in many brain regions, including multiple amygdaloid subnuclei (Hendry et al., 1984; Kohler et al., 1986; McDonald and Pearson, 1989; Oberto et al., 2001; Pu et al., 1999), it can be hypothesized that NPY in the CeA suppresses alcohol drinking in dependent animals by inhibiting GABA transmission, possibly via intrinsic interneurons, in the same brain region.
In summary, the present investigation showed that NPY infused into the CeA suppressed alcohol drinking by rats. This effect was augmented following a history of alcohol dependence, such that dependence-induced increases in operant alcohol responding were abolished by intra-CeA infusion of NPY. These results are consistent with previous findings and implicate the recruitment of limbic NPY systems in the motivational drive to consume alcohol following the transition to dependence. These results suggest a key role for NPY on alcohol drinking in this and other subpopulations especially vulnerable to the development of high alcohol-drinking behavior.
Acknowledgments
The authors thank Mike Arends for his excellent editorial assistance and Ben Isakson and Lisa Zazworsky for their skilled technical assistance. This is manuscript number 19213 from The Scripps Research Institute. This work was supported by the Pearson Center for Alcoholism and Addiction Research and NIAAA grants AA06420 and AA08459.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Badia-Elder NE, Stewart RB, Powrozek TA, Murphy JM, Li TK. Effects of neuropeptide Y on sucrose and ethanol intake and on the elevated plus maze test of anxiety in high alcohol drinking (HAD) and low alcohol drinking (LAD) rats. Alcohol Clin Exp Res. 2003;27:894–9. doi: 10.1097/01.ALC.0000071929.17974.DA. [DOI] [PubMed] [Google Scholar]
- Badia-Elder NE, Stewart RB, Powrozek TA, Roy KF, Murphy JM, Li TK. Effect of neuropeptide Y (NPY) on oral ethanol intake in Wistar, alcohol-preferring (P), and –nonpreferring (NP) rats. Alcohol Clin Exp Res. 2001;25:386–90. [PubMed] [Google Scholar]
- Britton KT, Southerland S, Van Uden E, Kirby D, Rivier J, Koob G. Anxiolytic activity of NPY receptor agonists in the conflict test. Psychopharmacol. 1997;132:6–13. doi: 10.1007/s002130050313. [DOI] [PubMed] [Google Scholar]
- Broqua P, Wettstein JG, Rocher MN, Gauthier-Martin B, Junien JL. Behavioral effects of neuropeptide receptor agonists in the elevated plus-maze and fear-potentiated startle procedure. Behav Pharmacol. 1995;6:215–22. [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Stewart RB, Badia-Elder NE. Neuropeptide Y administration into the amydala suppresses ethanol drinking in alcohol-preferring (P) rats following imposed ethanol abstinence. Pharmacol Biochem Behav. 2008 doi: 10.1016/j.pbb.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Stewart RB, Murphy JM, Badia-Elder NE. Neuropeptide Y in the paraventricular nucleus of the hypothalamus increases ethanol intake in high- and low-alcohol-drinking rats. Alcohol Clin Exp Res. 2004;28:1492–8. doi: 10.1097/01.alc.0000141813.27875.d5. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Stewart RB, Murphy JM, Badia-Elder NE. Sensitized effects of neuropeptide Y on multiple ingestive behaviors in P rats following ethanol abstinence. Pharmacol Biochem Behav. 2005;81:740–9. doi: 10.1016/j.pbb.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Stewart RB, Murphy JM, Li TK, Badia-Elder NE. Neuropeptide Y reduces oral ethanol intake in alcohol-preferring (P) rats following a period of imposed ethanol abstinence. Alcohol Clin Exp Res. 2003;27:787–94. doi: 10.1097/01.ALC.0000065723.93234.1D. [DOI] [PubMed] [Google Scholar]
- Heilig M, McLeod S, Brot M, Heinrichs SC, Menzaghi F, Koob GF, Britton KT. Anxiolytic-like action of neuropeptide Y: mediation by Y1 receptors in amygdala, and dissociation from food intake effects. Neuropsychopharmacol. 1993;8:357–63. doi: 10.1038/npp.1993.35. [DOI] [PubMed] [Google Scholar]
- Heilig M, McLeod S, Koob GF, Britton KT. Anxiolytic-like effect of neuropeptide Y (NPY), but not other peptides in an operant conflict test. Regulat Pept. 1992;41:61–9. doi: 10.1016/0167-0115(92)90514-u. [DOI] [PubMed] [Google Scholar]
- Heilig M, Söderpalm B, Engel JA, Widerlöv E. Centrally administered neuropeptide Y (NPY) produces anxiolytic-like effects in animal anxiety models. Psychopharmacol. 1989;98:524–9. doi: 10.1007/BF00441953. [DOI] [PubMed] [Google Scholar]
- Hendry SH, Jones EG, DeFelipe J, Schmechel D, Brandon C, Emson PC. Neuropeptide-containing neurons of the cerebral cortex are also GABAergic. Proc Natl Acad Sci USA. 1984;81:6526–30. doi: 10.1073/pnas.81.20.6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash TL, Winder DG. Neuropeptide Y and corticotropin-releasing factor bi-directionally modulate inhibitory synaptic transmission in the bed nucleus of the stria terminalis. Neuropsychopharmacol. 2006;51:1013–22. doi: 10.1016/j.neuropharm.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Katner SN, Slawecki CJ, Ehlers CL. Neuropeptide Y administration into the third ventricle does not increase sucrose or ethanol self-administration but does affect the cortical EEG and increases food intake. Psychopharmacol. 2002a;160:146–54. doi: 10.1007/s00213-001-0950-9. [DOI] [PubMed] [Google Scholar]
- Katner SN, Slawecki CJ, Ehlers CL. Neuropeptide Y administration into the amygdala does not affect ethanol consumption. Alcohol. 2002b;28:29–38. doi: 10.1016/s0741-8329(02)00235-5. [DOI] [PubMed] [Google Scholar]
- Kohler C, Eriksson L, Davies S, Chan-Palay V. Neuropeptide Y innervation of the hippocampal region in the rat and monkey brain. J Comp Neurol. 1986;244:384–400. doi: 10.1002/cne.902440310. [DOI] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–43. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Majchrowicz E. Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacologia. 1975;43:245–254. doi: 10.1007/BF00429258. [DOI] [PubMed] [Google Scholar]
- McBride WJ. Central nucleus of the amygdala and the effects of alcohol and alcohol-drinking behavior in rodents. Pharmacol Biochem Behav. 2002;71:509–15. doi: 10.1016/s0091-3057(01)00680-3. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Pearson JC. Coexistence of GABA and peptide immunoreactivity in non-pyramidal neurons of the basolateral amygdala. Neurosci Letters. 1989;100:53–8. doi: 10.1016/0304-3940(89)90659-9. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. Washington D.C.: National Research Council; 1996. [Google Scholar]
- Naveilhan P, Canals JM, Valjakka A, Vartiainen J, Arenas E, Ernfors P. Neuropeptide Y alters sedation through a hypothalamic Y1-mediated mechanism. Eur J Neurosci. 2001;13:2241–6. doi: 10.1046/j.0953-816x.2001.01601.x. [DOI] [PubMed] [Google Scholar]
- Nie Z, Schweitzer P, Roberts AJ, Madamba SG, Moore SD, Siggins GR. Ethanol augments GABAergic transmission in the central amygdala via CRF1 receptors. Science. 2004;303:1512–4. doi: 10.1126/science.1092550. [DOI] [PubMed] [Google Scholar]
- Oberto A, Panzica GC, Altruda F, Eva C. GABAergic and NPY-Y1 network in the medial amygdala: a neuroanatomical basis for their functional interaction. Neuropharmacol. 2001;41:639–42. doi: 10.1016/s0028-3908(01)00109-5. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Similar anxiety-like responses in male and female rats exposed to repeated withdrawals from ethanol. Pharmacology, Biochemistry & Behavior. 2004;78:459–463. doi: 10.1016/j.pbb.2004.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Roy A, Xu T. Deficits in amygdaloid cAMP-responsive element-binding protein signaling play a role in genetic predisposition to anxiety and alcoholism. J Clin Invest. 2005;115:2762–73. doi: 10.1172/JCI24381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th. Academic Press; San Diego, CA: 1998. [Google Scholar]
- Primeaux SD, Wilson SP, Bray GA, York DA, Wilson MA. Overexpression of neuropeptide Y in the central nucleus of the amygdala decreases ethanol self administration in “anxious” rats. Alcohol Clin Exp Res. 2006;30:791–801. doi: 10.1111/j.1530-0277.2006.00092.x. [DOI] [PubMed] [Google Scholar]
- Pu S, Jain MR, Horvath TL, Diano S, Kalra PS, Kalra SP. Interactions between neuropeptide Y and γ-aminobutyric acid in stimulation of feeding: a morphological and pharmacological analysis. Endocrinol. 1999;140:933–40. doi: 10.1210/endo.140.2.6495. [DOI] [PubMed] [Google Scholar]
- Rimondini R, Thorsell A, Heilig M. Suppression of ethanol self-administration by the neuropeptide Y (NPY) Y2 receptor antagonist BIIE0246: evidence for sensitization in rats with a history of dependence. Neurosci Letters. 2005;375:129–33. doi: 10.1016/j.neulet.2004.10.084. [DOI] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci U S A. 2003;100:2053–8. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Schweitzer P, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Acute and chronic ethanol alter glutamatergic transmission in rat central amygdala: an in vitro and in vivo analysis. J Neurosci. 2004;24:1594–603. doi: 10.1523/JNEUROSCI.5077-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdyk TJ, Schober DA, Gehlert DR. Neuropeptide Y receptor subtypes in the basolateral nucleus of the amygdala modulate anxiogenic responses in rats. Neuropharmacol. 2002;43:1165–72. doi: 10.1016/s0028-3908(02)00234-4. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Vandergriff MG, Gehlert DR. Amygdalar neuropeptide Y Y1 receptors mediate the anxiolytic-like actions of neuropeptide Y in the social interaction test. Eur J Pharmacol. 1999;368:143–7. doi: 10.1016/s0014-2999(99)00018-7. [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Olive F, Koenig H, Hodge CW. Intra-amygdala infusion of the NPY Y1 receptor antagonist BIBP 3226 attenuates operant ethanol self-administration. Alcohol Clin Exp Res. 2003;27:1884–91. doi: 10.1097/01.ALC.0000098875.95923.69. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Betancourt M, Walpole T, Ehlers CL. Increases in sucrose consumption, but not ethanol consumption, following ICV NPY administration. Pharmacol Biochem Behav. 2000;66:591–4. doi: 10.1016/s0091-3057(00)00215-x. [DOI] [PubMed] [Google Scholar]
- Stanley BG, Chin AS, Leibowitz SF. Feeding and drinking elicited by central injection of neuropeptide Y: evidence for a hypothalamic site(s) of action. Brain Res Bull. 1985;14:521–4. doi: 10.1016/0361-9230(85)90100-5. [DOI] [PubMed] [Google Scholar]
- Stewart RB, Gatto GJ, Lumeng L, Li TK, Murphy JM. Comparison of alcohol-preferring (P) and nonpreferring (NP) rats on tests of anxiety and for the anxiolytic effects of ethanol. Alcohol. 1993;10:1–10. doi: 10.1016/0741-8329(93)90046-q. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Repunte-Canonigo V, O'Dell LE, Chen SA, King AR, Lekic D, et al. Viral vector-induced amygdala NPY overexpression reverses increased alcohol intake caused by repeated deprivations in Wistar rats. Brain. 2007;130:1330–7. doi: 10.1093/brain/awm033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsell A, Slawecki CJ, Ehlers CL. Effects of neuropeptide Y on appetitive and consummatory behaviors associated with alcohol drinking in Wistar rats with a history of ethanol exposure. Alcohol Clin Exp Res. 2005a;29:584–90. doi: 10.1097/01.alc.0000160084.13148.02. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Slawecki CJ, Ehlers CL. Effects of neuropeptide Y and corticotropin-releasing factor on ethanol intake in Wistar rats: interaction with chronic ethanol exposure. Behav Brain Res. 2005b;161:133–40. doi: 10.1016/j.bbr.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Koob GF. Allostasis and dysregulation of corticotropin-releasing factor and neuropeptide Y systems: implications for the development of alcoholism. Pharmacol Biochem Behav. 2004;79:671–89. doi: 10.1016/j.pbb.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Valenstein ES, Cox VC, Kakolewski JW. Polydipsia elicited by the synergistic action of a saccharin and glucose solution. Science. 1967;157:552–4. doi: 10.1126/science.157.3788.552. [DOI] [PubMed] [Google Scholar]


