Abstract
Cerebral arterial air embolism (CAAE) has been reported as a rare complication of medical intervention. There has been one reported case of CAAE after the use of an intraosseous infusion (IO) system. We report on a case of CAAE after tibial IO infusion in a 7-month-old girl during resuscitation.
Keywords: Tomography, Spiral computed, Embolism, Air, Intracranial embolism, Autopsy
Introduction
Cerebral arterial air embolism (CAAE) has been reported as a rare complication of medical intervention. We present a case of a 7-month-old girl who was admitted to our emergency department; post-mortem computed tomography (CT) showed a CAAE.
Case report
A 7-month-old girl was admitted to our emergency department. She was known in our hospital because of prematurity (gestational age, 30 weeks) and an omphalocele, for which initial non-operative treatment with epithelialisation was instituted, and delayed surgical correction was planned. Outpatient follow-up showed no other medical problems. Psychomotor development was normal. Vaccinations were given according to the Dutch vaccination program.
According to the mother, the child awoke at 3 ‘o clock at night and, as what had happened before, her mother bottle fed her. During feeding, she started to vomit and became unresponsive. A 911 call was placed, and a friend of the family was called. The latter was first to arrive and transported the girl and her mother to the emergency department of the Academic Medical Centre Amsterdam. They arrived in our hospital approximately 45 min after the 911 call. Upon physical examination, a non-responsive child without spontaneous respiration or cardiac output was seen. Resuscitation was immediately started. During resuscitation, significant amounts of food were aspirated from her mouth and endotracheal tube. Several attempts were made to insert a central venous and arterial catheter, however, to no avail. To gain venous access, an intraosseous infusion (IOI) needle was placed in the right tibia.
At 5:00 a.m., she was pronounced deceased. Although the clinical history and findings during resuscitation suggested food aspiration, questions regarding the cause of death remained. Therefore, according to our battered child protocol, the standard radiographs, following the guidelines of the American College of Radiography were performed [1]. In addition, a head CT, which was a standard in our hospital in children under the age of 2 years with unexplained trauma/death, was performed within 1h after death. The skeletal radiographs showed no abnormalities, except for the IO infusion in the proximal right tibia (Fig. 1). Neither fractures nor signs of malnutrition were noted. The head CT, however, showed a considerable amount of air within the arterial circulation (Fig. 2).
Fig. 1.
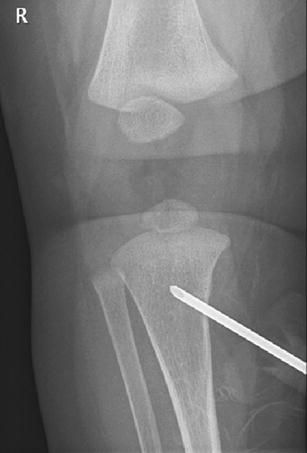
Intraosseous infusion needle correctly positioned within the proximal right tibial metaphysis
Fig. 2.
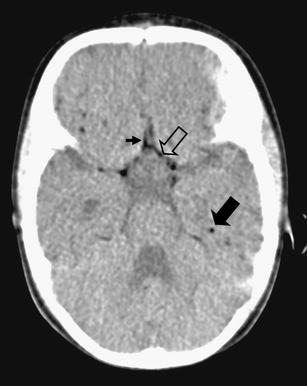
CT at the level of the circle of Willis showing air within the circle of Willis (open arrow) anterior cerebral artery (small arrow) and peripheral cerebral arteries (solid arrow)
A full judicial autopsy was performed in The Netherlands Forensic Institute, as is mandatory in children with a possible non-natural cause of death. The body showed normal measurements (length, 64 cm, and 6,900 grams, both p50) and a known omphalocele. Autopsy showed that the omphalocele contained a large segment of the right liver lobe and the ascending colon including the appendix, with adhesion to the abdominal wall (Fig. 3). The intestines showed no signs of ischemia. Inspection of the heart revealed only minor congenital abnormalities, which consisted of a defect in the interatrial septum, fossa ovalis type with deficient flap valve, and slight hypertrabeculation of the ventricles, particularly the right ventricle (Fig. 4). The latter findings, however, were clearly insufficient for a diagnosis of ventricular non-compaction. There was some pulmonary oedema, possibly due to the vigorous fluid resuscitation. The trachea was without abnormalities and, in contrast to the clinical history, showed no signs of food aspiration (Fig. 5). Additional toxicological, microbiological and biochemical analyses of body fluids and tissues were all inconclusive.
Fig. 3.
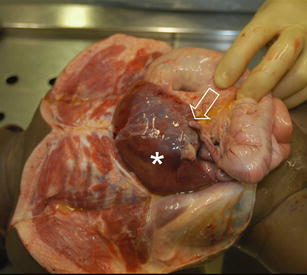
Omphalocele containing a large section of the right liver lobe (asterisk) and the ascending colon including the appendix (arrow)
Fig. 4.
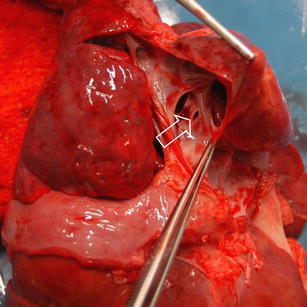
View of the atrial septum showing a persistent foramen ovale (arrow) and a fenestration (arrowhead)
Fig. 5.
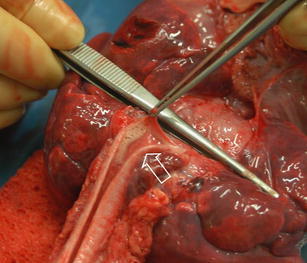
View of the trachea devoid of food remnants, a small amount of saliva is seen (arrow)
As no distinct cause of death could be established, the cause of death remains undetermined.
Discussion
CAAE is a rare finding; it has been described after medical instrumentation in trauma patients, after mechanical ventilation or resuscitation [2–8]. In case of vascular access procedures, CAAE can occur as a result from backflow, a right to left shunt, or shunting through the pulmonary capillary bed [9–11]. Backflow can be a result of manipulation of central lines (not necessarily IOI) and has been described in literature [12, 13]. Right to left shunting can be caused by an atrial septum defect (estimated incidence in adult patients 0.2–0.7 in 1, 000), a ventricular septum defect (incidence at birth ranging from 2–5%; however, 85–90% of these defects will close spontaneously by 1 year of age) and persistent foramen ovale (this has been reported to be present in up to 27% of autopsies) [14–16].
A special category are divers who suffer from decompression sickness after diving accidents [17, 18]. In severe cases, CAAE can develop, which is reported to be the second most common cause of death in divers [19]. Recently, an animal study with sheep was performed to test the hypothesis that artifacts caused by postmortem off gassing is at least partly responsible for the presence of gas within the vascular system and tissues of the cadaver after death associated with compressed air diving [20]. None of the control animals showed intravascular air on CT, respectively, 1 and 8 h after death; only after 24 h after the time of death, relatively small amounts of gas were seen. Although it is unknown how well sheep resemble 7-month-old infants, we assume that decay rates will not differ that much and certainly not to the extent that it can explain the amount of air visible on the CT scan.
In our case, the only vascular access had been IO infusion, which is a widely implemented technique and is part of the standard protocols, such as the Advanced Paediatric Life Support textbook. The advantage of IO infusion over peripheral vascular access is that it provides a rapid and reliable access to the systemic venous circulation in children [21–23]. It can be used in children of all ages, with the smallest child reported in literature weighing 800 g [24]. This has led to IO infusion having almost completely replaced saphenous cut down procedures in critically ill children in emergency situations. IO infusion has been used to introduce fluids, medication and even contrast media [25]. It is generally considered to be a safe technique with a reported complication rate of 1% [26]. Several complications resulting from the use of IO infusion have been reported; these consisted of extravasation in some cases leading to compartment syndrome (in rare cases leading to limb amputation), osteomyelitis, fracture, skin necrosis and fat embolism [24, 27–32].
Recently, investigators from the Berne Institute of Forensic Medicine (Switzerland) reported on a case of air embolism after resuscitation and IOI in a 4-month-old boy [33]. In their case, resuscitation was considered as the cause for CAAE; however, whole-body CT showed air in the lower extremity, which was used for IOI. As in our case, IO infusion was the only vascular access. Given the patent foramen ovale and atrial septal defect, leading to a right to left shunt, we feel that the only logical explanation for the CAAE is that air is introduced into the bloodstream via this route. To the best of our knowledge, our case is the second reported case of an air embolism after the use of an IO infusion.
Given the incidence of right-to-left shunts in the general population, this is a serious complication of IOI of which clinicians should be aware.
Acknowledgments
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Slovis TL, Smith W, Kushner DC, et al. Imaging the child with suspected physical abuse. American College of Radiology. ACR Appropriateness Criteria. Radiology. 2000;215(Suppl:805–9):805–809. [PubMed] [Google Scholar]
- 2.Yang CW, Yang BP. Massive cerebral arterial air embolism following arterial catheterization. Neuroradiology. 2005;47:892–894. doi: 10.1007/s00234-005-1437-x. [DOI] [PubMed] [Google Scholar]
- 3.Nayagam J, Ho KM, Liang J. Fatal systemic air embolism during endoscopic retrograde cholangio-pancreatography. Anaesth Intensive Care. 2004;32:260–264. doi: 10.1177/0310057X0403200217. [DOI] [PubMed] [Google Scholar]
- 4.Dube L, Soltner C, Daenen S, Lemariee J, Asfar P, Alquier P. Gas embolism: an exceptional complication of radial arterial catheterization. Acta Anaesthesiol Scand. 2004;48:1208–1210. doi: 10.1111/j.1399-6576.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez RA, Belway D. Comparison of two different extracorporeal circuits on cerebral embolization during cardiopulmonary bypass in children. Perfusion. 2006;21:247–253. doi: 10.1177/0267659106074764. [DOI] [PubMed] [Google Scholar]
- 6.Banagale RC. Massive intracranieal air embolism: complication of mechanical ventilation. Am J Dis Child. 1980;134:799–800. doi: 10.1001/archpedi.1980.02130200067023. [DOI] [PubMed] [Google Scholar]
- 7.Brown ZA, Clark JM, Jung AL. Systemic gas embolus: a discussion of its pathogenesis in the neonate, with a review of the literature. Am J Dis Child. 1977;131:984–985. doi: 10.1001/archpedi.1977.02120220050008. [DOI] [PubMed] [Google Scholar]
- 8.Yamaki T, Ando S, Ohta K, Kawasaki K, Hirama M. Demonstration of massive cerebral air embolism from pulmonary barotrauma due to cardiopulmonary resuscitation. J Comput Assist Tomogr. 1989;13:313–315. doi: 10.1097/00004728-198903000-00024. [DOI] [PubMed] [Google Scholar]
- 9.Sowell MW, Lovelady CL, Brogdon BG, Wecht CH. Infant death due to air embolus from peripheral venous infusion. J Forensic Sci. 2007;52:183–188. doi: 10.1111/j.1556-4029.2006.00307.x. [DOI] [PubMed] [Google Scholar]
- 10.Wald M, Kirchner L, Lawrenz K, Amann G. Fatal air embolism in an extremely low birth weight infant: can it be caused by intravenous injections during resuscitation? Intensive Care Med. 2003;29:630–633. doi: 10.1007/s00134-003-1681-7. [DOI] [PubMed] [Google Scholar]
- 11.Murphy GS, Szokol JW, Marymont JH, Avram MJ, Vender JS, Kubasiak J. Retrograde blood flow in the brachial and axillary arteries during routine radial arterial catheter flushing. Anesthesiology. 2006;105:492–497. doi: 10.1097/00000542-200609000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Morello FP, Donaldson JS, Saker MC, Norman JT. Air embolism during tunneled central catheter placement performed without general anesthesia in children: a potentially serious complication. J Vasc Interv Radiol. 1999;10:781–784. doi: 10.1016/S1051-0443(99)70114-4. [DOI] [PubMed] [Google Scholar]
- 13.Smith J, Els I. Intracardiac air—the ‘hospital killer’ identified? Case reports and review of the literature. S Afr Med J. 2003;93:922–927. [PubMed] [Google Scholar]
- 14.Hara H, Virmani R, Ladich E. Patent foramen ovale: current pathology, pathophysiology, and clinical status. J Am Coll Cardiol. 2005;46:1768–1776. doi: 10.1016/j.jacc.2005.08.038. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman JL, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;19:1890–1900. doi: 10.1016/S0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 16.Konstantides S, Geibel A, Kasper W, Just H. The natural course of atrial septal defect in adults—a still unsettled issue. Klin Wochenschr. 1991;69:506–510. doi: 10.1007/BF01649286. [DOI] [PubMed] [Google Scholar]
- 17.Ozdoba C, Weis J, Plattner T, Dirnhofer R, Yen K. Fatal scuba diving incident with massive gas embolism in cerebral and spinal arteries. Neuroradiology. 2005;47:411–416. doi: 10.1007/s00234-004-1322-z. [DOI] [PubMed] [Google Scholar]
- 18.Smerz RW. Concomitant cerebral and coronary arterial gas emboli in a sport diver: a case report. Hawaii Med J. 2005;64:12–13. [PubMed] [Google Scholar]
- 19.Kizer KW. Dysbaric cerebral air embolism in Hawaii. Emerg Med. 1987;535:25–41. doi: 10.1016/s0196-0644(87)80679-0. [DOI] [PubMed] [Google Scholar]
- 20.Cole J, Griffiths S, Lavender S, Summers P, Rich K. Relevance of postmortem radiology to the diagnosis of fatal cerebral gas embolism from compressed air diving. J Clin Pathol. 2006;59:489–491. doi: 10.1136/jcp.2005.031708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith R, Davis N, Bouamra O, Lecky F. The utilisation of intraosseous infusion in the resuscitation of paediatric major trauma patients. Injury. 2005;36:1034–1038. doi: 10.1016/j.injury.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Haas NA. Clinical review: vascular access for fluid infusion in children. Crit Care. 2004;8:478–484. doi: 10.1186/cc2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guy J, Haley K, Zuspan SJ. Use of intraosseous infusion in the pediatric trauma patient. J Pediatr Surg. 1993;28:158–161. doi: 10.1016/S0022-3468(05)80263-5. [DOI] [PubMed] [Google Scholar]
- 24.Ramet J, Clybouw C, Benatar A, Hachimi-Idrissi S, Corne L. Successful use of an intraosseous infusion in an 800 grams preterm infant. Eur J Emerg Med. 1998;5:327–328. doi: 10.1097/00063110-199809000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Geller E, Crisci KL. Intraosseous infusion of iodinated contrast in an abused child. Ped Emerg Care. 1999;15:328–329. doi: 10.1097/00006565-199910000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Hoelzer M. Recent advances in intravenous therapy. Emerg Med Clin North Am. 1986;4:487–495. [PubMed] [Google Scholar]
- 27.Stoll E, Golej J, Burda G, Hermon M, Boigner H, Tritterwein G. Osteomyelitis at the injection site of adrenalin through an intraosseous needle in a 3-month-old infant. Resuscitation. 2002;53:315–318. doi: 10.1016/S0300-9572(02)00039-4. [DOI] [PubMed] [Google Scholar]
- 28.Byrick RJ. Pulmonary fat embolism and intraosseous infusion. Ped Crit Care Med. 2001;2:184–185. doi: 10.1097/00130478-200104000-00016. [DOI] [PubMed] [Google Scholar]
- 29.Katz DS, Wojtowycz AR. Tibial fracture: a complication of intraosseous infusion. Am J Emerg Med. 1994;12:258–259. doi: 10.1016/0735-6757(94)90261-5. [DOI] [PubMed] [Google Scholar]
- 30.Rosovsky M, Fitzpatrick M, Goldfarb CR, Finestone H. Bilateral osteomyelitis due to intraosseous infusion: case report and review of the English-language literature. Pediatr Radiol. 1994;24:72–73. doi: 10.1007/BF02017671. [DOI] [PubMed] [Google Scholar]
- 31.Moscati R, Moore GP. Compartment syndrome with resultant amputation following intraosseous infusion. Am J Emerg Med. 1990;8:470–471. doi: 10.1016/0735-6757(90)90247-W. [DOI] [PubMed] [Google Scholar]
- 32.Christensen DW, Vernon DD, Banner W, Jr, Dean JM. Skin necrosis complicating intraosseous infusion. Pediatr Emerg Care. 1991;7:289–290. doi: 10.1097/00006565-199110000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Hillewig E, Aghayev E, Jackowski C, Christe A, Plattner T, Thali MJ. Gas embolism following intraosseous medication application proven by post-mortem multislice computed tomography and autopsy. Resuscitation. 2007;72:149–153. doi: 10.1016/j.resuscitation.2006.06.023. [DOI] [PubMed] [Google Scholar]


