Abstract
Predatory mites locate herbivorous mites, their prey, by the aid of herbivore-induced plant volatiles (HIPV). These HIPV differ with plant and/or herbivore species, and it is not well understood how predators cope with this variation. We hypothesized that predators are attracted to specific compounds in HIPV, and that they can identify these compounds in odor mixtures not previously experienced. To test this, we assessed the olfactory response of Phytoseiulus persimilis, a predatory mite that preys on the highly polyphagous herbivore Tetranychus urticae. The responses of the predatory mite to a dilution series of each of 30 structurally different compounds were tested. They mites responded to most of these compounds, but usually in an aversive way. Individual HIPV were no more attractive (or less repellent) than out-group compounds, i.e., volatiles not induced in plants fed upon by spider-mites. Only three samples were significantly attractive to the mites: octan-1-ol, not involved in indirect defense, and cis-3-hexen-1-ol and methyl salicylate, which are both induced by herbivory, but not specific for the herbivore that infests the plant. Attraction to individual compounds was low compared to the full HIPV blend from Lima bean. These results indicate that individual HIPV have no a priori meaning to the mites. Hence, there is no reason why they could profit from an ability to identify individual compounds in odor mixtures. Subsequent experiments confirmed that naive predatory mites do not prefer tomato HIPV, which included the attractive compound methyl salicylate, over the odor of an uninfested bean. However, upon associating each of these odors with food over a period of 15 min, both are preferred. The memory to this association wanes within 24 hr. We conclude that P. persimilis possesses a limited ability to identify individual spider mite-induced plant volatiles in odor mixtures. We suggest that predatory mites instead learn to respond to prey-associated mixtures of volatiles and, thus, to odor blends as a whole.
Keywords: Herbivore-induced plant volatiles, Tritrophic system, Learning, Memory, Synthetic odor perception
Introduction
Natural plant odors are usually blends of many molecules. Upon infestation by herbivores, plants change the composition of the odors they emit (Arimura et al. 2005). This information is used by the third trophic level to locate and prey on the herbivores that feed on the plant (Sabelis et al. 2006). The induced odor thus constitutes a signal to predatory arthropods, but exactly which features of this induced odor are perceived as the signal? Predators may either respond to one or a few of the induced compounds that they recognize individually in the mixture. Alternatively, predators could respond to the new odor mixture as a whole. If herbivore species induce the same volatile compounds in the plants they feed on, and if predators possess the ability to identify specific compounds in odor mixtures, the population of predators could evolve a set genetically fixed olfactory responses to these specific compounds. Predators with such innate responses to specific prey-related odors can minimize the exploration of plants that are not infested with their prey by limiting their olfactory acuity to a subset, of specific, ecologically relevant compounds (Bernays 2001; Egan and Funk 2006). Alternatively, predators could maximize their olfactory acuity for a wide variety of odors and perceive the plant odor mixture as a whole, i.e., distinct from its components. Consequently, individual compounds have no a priori meaning to such predators. Although this forces the predators to cope with much more information, it will facilitate the ability to differentiate among odors with chemically overlapping compositions. This may, for example, help them learn the difference between the odors that emanate from plants harboring suitable prey and plants harboring unsuitable prey. Here, we ask, if we can find a preference for individual herbivore-induced plant compounds in a population of the predatory mite Phytoseiulus persimilis.
Phytoseiulus persimilis preferably preys on the two-spotted spider mite Tetranychus urticae Koch. The predatory mites are blind and rely on odors to locate distant prey patches (Sabelis and Van der Baan 1983; Sabelis et al. 1984a, b). By using olfactory cues that emanate from spider mite-infested plants, the predatory mites are able to discern plants with prey from plants without (Sabelis and Van der Baan 1983; Dicke and Sabelis 1988; Dicke et al. 1990a, 1998; Dicke 1994; Sabelis et al. 1999). The prey, T. urticae, is highly polyphagous and has been reported to feed on more than 900 species of plants in 124 genera (Bolland et al. 1998). Infestation by spider mites induces different host plant species to emit different blends (Van Den Boom et al. 2004).
Under natural conditions, the quantitative and qualitative odor emission from plants is not only affected by herbivory of a predator’s prey, but also by several other independent biotic and abiotic factors. Different herbivore species induce different blends of herbivore-induced plant volatiles (HIPV) in the same plant species (De Moraes et al. 1998; De Boer et al. 2004, 2005). The same herbivore induces different HIPV in different plant species (Van Den Boom et al. 2004). The HIPV composition changes with leaf age (Takabayashi et al. 1994) and during the onset of infestation (Kant et al. 2008). Genetic variation within herbivore and plant species also affects HIPV production (Degen et al. 2004; Kant et al. 2008). Biotic factors such as herbivore-vectored viruses (Eigenbrode et al. 2002; Jimenez-Martinez et al. 2004) and abiotic factors such as fertility of the soil, light, and temperature, all qualitatively and quantitatively, affect the odor composition of a plant (Gouinguene and Turlings 2002; Vallat et al. 2005). Under natural conditions, these factors together generate a wealth of olfactory cues that vary in time, space, and possibly also information (Sabelis et al. 2006).
Phytoseiulus persimilis is able to adjust its olfactory response based on experience. Rearing the predatory mites from egg to adulthood on different species of spide mite-infested plants induces a preference for these plant odors (Takabayashi and Dicke 1992; Krips et al. 1999). Prolonged feeding (24 hr or more) of adults in the presence of HIPV blends also induced an acquired olfactory preference for these HIPV (Krips et al. 1999; Drukker et al. 2000; de Boer and Dicke 2004a; De Boer et al. 2005). This ability to learn from experience may help dispersing predatory mites detect spider mite-infested plants that they are already familiar with. The polyphagous nature of the prey, however, makes it worthwhile to explore not previously experienced odors as well. How can the dispersing predatory mites determine which odor sources are worthwhile exploring and which are not? Predatory mites could generalize their preference from odors that have been previously experienced in association with their prey to similar odors not previously experienced. On the other hand, predatory mites could possess an innate preference for specific spider mite-induced compounds that they identify in odor mixtures not previously experienced. The literature offers evidence for and against both possibilities. Drukker et al. (2000) found that P. persimilis females reared in the absence of plant odors did not innately prefer the odor of spider mite-infested plants over that of uninfested plants. Results obtained by de Boer and Dicke (2004a) suggested that P. persimilis preferred methyl salicylate only if the mites had experience with a methyl salicylate containing odor-blend. Hence, an innate preference for methyl salicylate seems to be absent. This acquired attraction to methyl salicylate could indicate that P. persimilis generalizes the acquired preference from the mixture to the individual compound. This generalization could be facilitated by the fact that the few (about 20) olfactory receptor cells that P. persimilis possesses may perceive only a fraction of an odor mixture (Jackson 1974; Jagers op Akkerhuis et al. 1985; van Wijk et al. 2006). On the other hand, the acquired preference for methyl salicylate reported by de Boer and Dicke (2004a) could also indicate that P. persimilis possess the ability to identify the presence of this particular compound in a mixture, which would suggest that olfaction in P. persimilis is elemental. More evidence in support of elemental odor perception can be found in the reported attractiveness of several typical spider mite-induced plant compounds (Dicke et al. 1990b; de Boer and Dicke 2004a, b; Kappers et al. 2005). An innate preference for specific compounds in combination with the ability to identify these compounds in unfamiliar mixtures could explain how P. persimilis has been able to prefer, without prior experience, the odor of spider mite-infested plants over that of uninfested conspecifics for several plant species (van den Boom et al. 2002). Thus, some experiments with P. persimilis do not provide evidence for an innate preference for HIPV, whereas others indicate that individual compounds could represent attractants by themselves and as part of odor mixtures.
In this paper, we aim to elucidate whether individual compounds can represent a signal that is recognized as a distinct element of the odor mixture that spider mite-infested plants emit. Acceptance of this hypothesis requires two prerequisites: first, individual spider mite-induced compounds should be attractive, and second, the mites should be able to identify the attractive components in mixtures. To test the first prerequisite, we investigated if individual compounds that are typically induced by spider mites elicit a specific behavioral response that differs from the response elicited by compounds that—to the best of our knowledge—are not involved in indirect plant defense. We used the information about the olfactory preference for individual compounds gained in the first experiment to test the second prerequisite. In this experiment, we assessed whether the mites can utilize their preference for specific spider mite-induced plant compounds to select without prior experience the odor that emanates from a spider mite-infested plant, which emits attractive HIPV, over the odor of an uninfested plant. We further assessed whether the presence of HIPV affects the ability to learn about odors, and we asked how long the mites maintain this memory.
Methods and Materials
Plants and Mites
Lima bean plants (Phaseolus lunatus) were reared in a climate room (22°C, 60% RH, 16:8 LD) from seeds until they were 2 wk old. Next, they were infested with two-spotted spider mites, T. urticae. The predatory mites (P. persimilis) were reared in a climate room (25°C, 80% RH, 16:8 LD) on detached spider mite-infested Lima bean leaves. Every day (except on the weekend), the predatory mites received fresh spider-mite-infested Lima bean leaves, and the culture was harvested. The frequent harvesting of the mites ensured that the females used were one to a few days old since their final molt. The predatory mites were originally obtained in 2001 from field samples at various sites near the coast of Sicily, Italy. The predatory mites were collected from spider mite-infested plants, and the plants were from different genera in three different plant families (Convolvulaceae, Asteraceae, Euphorbiaceae). Before testing the olfactory response, all mites were brought in a hungry state, which mimics the conditions before and after dispersal (Sabelis and Afman 1994). To this end, adult female predatory mites were kept in Eppendorf tubes, deprived of water and food for 16–22 hr (24°C.).
Odors
The response to a panel of 30 odors was tested. Most were obtained from Fluka with the exception 2,3-dimethyl-pyrazine, which was obtained from Sigma-Aldrich. Octan-1-ol and butan-1-ol were obtained from Sigma, cis-3-hexenyl acetate, dodecyl-acetate, nerolidol, proionic acid were obtained from Aldrich, methyl salicylate from Sigma-Aldrich, and β-ocimene (70% E- and 30% Z- isomers) from R. C. Treatt & Co. (E)-4,8-dimethyl-1,3,7-nonatriene (DMNT) and (E,E)-4,8,12-trimethyl-1,3,7,11-tridecatetraene, (TMTT) were provided by Dr. W. Boland of the Max Planck Institute for Chemical Ecology, Jena, Germany.
Olfactory Response Tests
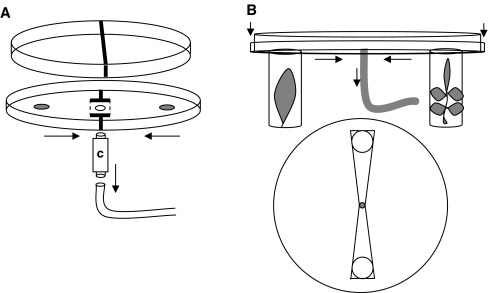
The response to each compound in the odor panel was tested by using a “choice arena”. The basic arena consisted of an upside-down Petri dish (diam 9 cm) (Fig. 1a). An opening at the bottom of the arena was connected to a vacuum pump (flow 0.42 l/min). Prior to the experiment, groups of about 20 female predatory mites were placed in a cartridge that could be fitted between the vacuum pump and the choice arena. For each replicate experiment, freshly made odor sources and a new cartridge with a new group of predatory mites were provided. Insect glue barriers divided the arena in two sides while leaving an opening at the center of the bottom of the arena (Fig. 1a). One side of the arena contained a filter paper (diam 1 cm) with the 0.5-μl odor (dissolved in hexane), while the alternative side contained a control filter paper treated with the solvent only (0.5 μl hexane). The odor sources were prepared in a fume, and the solvent was allowed to evaporate for exactly 1 min before the odor source was placed in the set-up. Mites were released from the cartridge, and after 3 min, the mites at each side of the arena were counted. Each odor was tested in five concentrations, covering a concentration range that spans five orders of magnitude (pure and diluted with a factor 10, 102, 103, and 104). The response to each concentration of each odor was tested in six replicate experiments, and each contained 20 predatory mites. Fresh arenas were used for each odor, and the choice arena was rotated between replicate experiments to correct for any unforeseen directional bias. To avoid contamination of the air that entered the choice arena, it was placed in a flow cabinet that was continuously supplied with clean air. Assuming individual mites make independent choices, the preference of the mites for either side of the arena was evaluated with a replicated G-test (Sokal and Rohlf 1995). In short, significant values of G t indicate an overall deviation from a 50:50 distribution. This statistic is broken down into two components that characterize/evaluate different aspects of the deviation; G h and G p. Significance of G h indicates heterogeneity among replicate experiments, while significance of G p indicates a deviation from an even distribution in the overall pooled result.
Fig. 1.
1A: The “choice arena” consists of an upside down Petri dish. At the center, the arena connects to a cartridge (c) that holds the predatory mites. The cartridge is connected to the vacuum pump. Arrows indicate the direction of the radial airflow in the arena. Odors are applied on filter paper (gray circles).The choice arena is divided in the two sides by a thin layer of insect glue (thick black lines). 1B: Modified version of 1 A that can be fitted with two veils that hold a tomato or a bean leaf placed on moist cotton wool. Gauze covered holes connect the veils to the choice arena. A single mite is released into the center and from 30 min the time it spends in each of the odor fields (triangles in the lower panel) is measured
To aid graphical display of the data, we calculated the following preference index to each odor-sample: [(mites at odor side—mites at control side)/total amount of mites) × 100]. In this way, repellent odors were assigned a negative preference index (−100 to 0), and attractive odors a positive preference index (0 to 100).
Natural Odors
In this set of experiments, individual mites were placed into an experimental arena. This consisted of an upside-down Petri dish (diam 14 cm) with two vials mounted just below an opening in the bottom (Fig. 1b). The vials held either an uninfested bean leaf (Phaseolus vulgaris) or an infested tomato leaf (Lycopersicon esculentum). Tomato plants were infested with T. urticae more than 1 wk before the onset of the experiment. Spider mite-infested tomato was selected because P. persimilis that originated from the same laboratory population are attracted to its odor when reared on spider mite-infested tomato leaves (Kant et al. 2004). To establish a radial airflow, a vacuum pump was connected to an opening in the center of the arena. Odor fields were defined as the triangular area that had a base just wide enough to encompass the opening above the vial that contained the odor source, while the tip encompassed the opening where the setup was mounted to the vacuum pump (Fig. 1b). The mites could freely move over the bottom of the arena, and the time spent in each odor field was continuously measured for 30 min. The mites were allowed to associate odors with the presence of food in an arena. To this end, washed spider mite eggs were placed on gauze that covered an odor source. To make sure that the odor sources were the only cues that the mites used during the behavioral observation in the post-experience phase, the mites were always observed in an identical, but different arena. The mites’ ability to learn about the absence of food in a particular odor field was assessed in two ways: first, by restricting the mites to one of the odor sources in the absence of food, and second by offering the same unrewarded odor at both sides, while the mites were not restricted to one of the odor sources and could leave the odor field.
Results
Validation of the Choice Arena
Several tests were conducted to validate the experimental setup. First, the airflow in the choice arena was visualized with smoke derived from droplets of chloric acid and ammonia. This revealed a steady radial airflow along the bottom of the choice arena. When the fumes were applied to one side of the arena, they never entered the other side, while gauze that covered the bottom of the cartridge in the vacuum entrance was progressively covered with NH4Cl salt starting from one side only. This indicated that the separation of the odor plumes extended to the bottom of the cartridge.
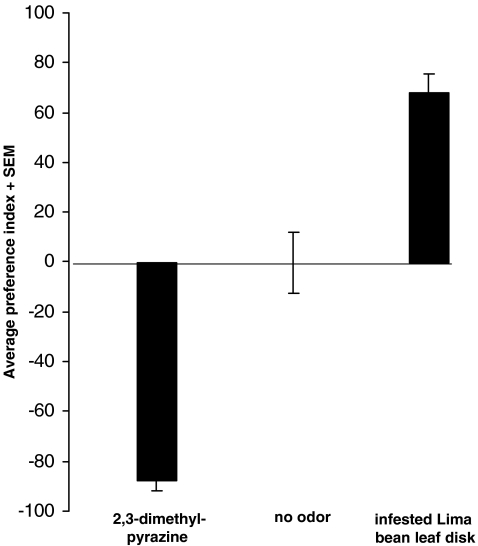
Unforeseen bias in the setup was investigated with a choice test in the absence of odor sources (Fig. 2). This resulted in an even distribution among both sides of the choice arena. The average preference index was −0.25. None of the six replicate experiments had a significant bias to either side, and the data were not anymore heterogeneous than expected (G p ns, G h ns, G t ns). Subsequently, two positive control experiments were conducted. In the first, an excised, spider mite-infested, lima bean leaf-disc (diam 1.5 cm) was placed on moist filter paper at one side, whereas only moist filter paper was placed at the other side. This resulted for each of the six replicate experiments in a significant attraction of starved P. persimilis, and the overall attraction was also significant (G p *, G h ns, G t *). The average preference index was 67.8. Finally, an odor that was a priori known to be highly repellent to the mites, 2,3-dimethyl pyrazine (0.5 μl), was offered at one side, while a control paper was placed at the other. This resulted for each of the six replicate experiments in a significant avoidance of the odor with an overall significant aversion (G p *, G h ns, G t *). The average preference index was −87.5.
Fig. 2.
Control experiments: 0.5 μl 2,3-dimethyl pyrazine is highly repellent and the mites easily avoid the side of the choice arena that contains this odor. In the absence of odors, the mites are evenly distributed along both sides of the choice arena. A small leaf disks excised from spider mite-infested lima bean leaf is attractive to the mites
Response to the Odor-Panel
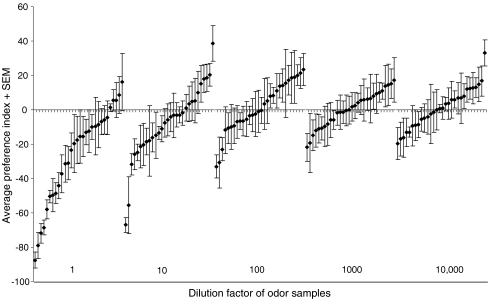
The overall response to odors changed as a function of the concentration (Fig. 3). High concentrations elicited on average the most aversive response. Also, the strength of the response increased with increasing concentration. This correlation between the (log) odor concentration and the strength of the response (R 2 = 0.77) was, however, present only for odor samples that were significantly repellent (G t *, G p *, and G h ns) (Table 1). For the nine moderately attractive samples (G p * and G h ns), such a correlation was absent (R 2 = 0.00002).
Fig. 3.
The response that odors elicit from P. persimilis as a function of the odor concentration. Along the x-axis, the odors are sorted from repellent to attractive for each tested concentration in decreasing order. The strength of the response decreases with decreasing odor concentration. The number of repellent samples is correlated strongly with increasing odor concentration, whereas this correlation is absent for the occurrence of attractive odor samples. At the lowest concentration, most odors do not elicit a significant response
Table 1.
The table contains the response of the mites to all 30 tested compounds at each of the five concentrations
| Compound | Dilution | Significant G values | Average preference index | SEM | P value G test | Spider mite induced | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| P Gt | P Gp | P Gh | |||||||
| 1-trans-2-hexenol | 0 | Gp Gt | −50.43 | 11.380 | 0.000 | 0.000 | 0.923 | SIPV | van den Boom et al. 2004 |
| 1- trans-2-hexenol | 1:10 | Gp Gt | −31.77 | 17.738 | 0.028 | 0.001 | 0.829 | ||
| 1- trans-2-hexenol | 1:100 | −0.91 | 32.439 | 0.213 | 0.922 | 0.138 | |||
| 1- trans-2-hexenol | 1:1000 | −1.29 | 18.691 | 0.787 | 0.866 | 0.678 | |||
| 1- trans-2-hexenol | 1:10000 | Gp | −19.61 | 25.070 | 0.053 | 0.016 | 0.249 | ||
| 2,3-dimethyl-pyrazine | 0 | Gp Gt | −87.48 | 13.640 | 0.000 | 0.000 | 0.325 | ||
| 2,3-dimethyl-pyrazine | 1:10 | Gp Gh Gt | −18.04 | 52.455 | 0.000 | 0.046 | 0.000 | ||
| 2,3-dimethyl-pyrazine | 1:100 | −3.37 | 15.433 | 0.948 | 0.698 | 0.912 | |||
| 2,3-dimethyl-pyrazine | 1:1000 | −1.73 | 19.997 | 0.793 | 0.655 | 0.711 | |||
| 2,3-dimethyl-pyrazine | 1:10000 | 3.34 | 12.014 | 0.961 | 0.601 | 0.944 | |||
| 2-benzyl-ethanol | 0 | Gp Gt | −48.54 | 17.166 | 0.000 | 0.000 | 0.617 | ||
| 2-benzyl-ethanol | 1:10 | −18.49 | 21.150 | 0.340 | 0.063 | 0.649 | |||
| 2-benzyl-ethanol | 1:100 | 8.38 | 13.680 | 0.923 | 0.431 | 0.931 | |||
| 2-benzyl-ethanol | 1:1000 | −5.98 | 27.394 | 0.349 | 0.574 | 0.270 | |||
| 2-benzyl-ethanol | 1:10000 | 1.07 | 18.213 | 0.857 | 0.866 | 0.766 | |||
| 3-octanone | 0 | Gp Gh Gt | −79.00 | 21.178 | 0.000 | 0.000 | 0.015 | ||
| 3-octanone | 1:10 | Gp Gh Gt | −55.50 | 42.991 | 0.000 | 0.000 | 0.000 | ||
| 3-octanone | 1:100 | Gp | −22.98 | 19.095 | 0.090 | 0.007 | 0.599 | ||
| 3-octanone | 1:1000 | 5.53 | 23.156 | 0.502 | 0.508 | 0.429 | |||
| 3-octanone | 1:10000 | −9.37 | 30.917 | 0.163 | 0.541 | 0.117 | |||
| acetic acid | 0 | Gp Gt | −44.25 | 21.021 | 0.000 | 0.000 | 0.457 | ||
| acetic acid | 1:10 | −5.88 | 25.027 | 0.495 | 0.864 | 0.374 | |||
| acetic acid | 1:100 | Gh | −2.30 | 34.405 | 0.057 | 0.571 | 0.036 | ||
| acetic acid | 1:1000 | −1.42 | 26.981 | 0.257 | 1.000 | 0.171 | |||
| acetic acid | 1:10000 | Gh Gt | −2.30 | 44.558 | 0.002 | 0.931 | 0.001 | ||
| acetone | 0 | 5.38 | 17.702 | 0.727 | 0.343 | 0.742 | |||
| acetone | 1:10 | −4.23 | 23.147 | 0.372 | 0.825 | 0.267 | |||
| acetone | 1:100 | Gh Gt | 3.38 | 38.938 | 0.037 | 0.867 | 0.020 | ||
| acetone | 1:1000 | 10.92 | 13.954 | 0.825 | 0.223 | 0.927 | |||
| acetone | 1:10000 | 14.80 | 27.238 | 0.144 | 0.133 | 0.198 | |||
| α-humulene | 0 | −4.64 | 26.174 | 0.243 | 0.516 | 0.186 | SIPV | van den Boom et al. 2004, Maeda and Takabayashi 2001 | |
| α-humulene | 1:10 | −2.97 | 19.575 | 0.664 | 0.690 | 0.558 | |||
| α-humulene | 1:100 | −6.65 | 14.264 | 0.879 | 0.470 | 0.865 | |||
| α-humulene | 1:1000 | −0.33 | 24.073 | 0.474 | 0.930 | 0.352 | |||
| α-humulene | 1:10000 | −12.94 | 9.272 | 0.904 | 0.189 | 0.994 | |||
| α-pinene | 0 | Gh Gt | −8.48 | 35.317 | 0.034 | 0.739 | 0.019 | ||
| α-pinene | 1:10 | Gh Gt | 5.29 | 33.930 | 0.039 | 0.283 | 0.033 | ||
| α-pinene | 1:100 | −5.45 | 27.387 | 0.364 | 0.609 | 0.279 | |||
| α-pinene | 1:1000 | −9.67 | 31.294 | 0.065 | 0.327 | 0.053 | |||
| α-pinene | 1:10000 | 0.71 | 8.678 | 0.997 | 0.874 | 0.991 | |||
| α-terpinene | 0 | Gp Gt | −49.70 | 25.792 | 0.000 | 0.000 | 0.126 | ||
| α-terpinene | 1:10 | −1.89 | 19.436 | 0.748 | 0.690 | 0.652 | |||
| α-terpinene | 1:100 | Gp Gh Gt | 19.04 | 39.852 | 0.008 | 0.035 | 0.026 | ||
| α-terpinene | 1:1000 | −10.78 | 18.878 | 0.420 | 0.141 | 0.570 | |||
| α-terpinene | 1:10000 | 12.59 | 24.948 | 0.139 | 0.111 | 0.211 | |||
| benzyl benzoate | 0 | Gp Gh Gt | −17.59 | 35.880 | 0.007 | 0.039 | 0.019 | ||
| benzyl benzoate | 1:10 | −2.94 | 14.248 | 0.943 | 0.609 | 0.917 | |||
| benzyl benzoate | 1:100 | Gp | 23.45 | 25.618 | 0.174 | 0.030 | 0.509 | ||
| benzyl benzoate | 1:1000 | Gh Gt | −19.35 | 44.371 | 0.000 | 0.057 | 0.000 | ||
| benzyl benzoate | 1:10000 | −0.13 | 31.128 | 0.126 | 0.796 | 0.078 | |||
| β-farnesene | 0 | Gp Gh Gt | −15.58 | 40.683 | 0.011 | 0.049 | 0.026 | SIPV | van den Boom et al. 2004 |
| β-farnesene | 1:10 | 15.23 | 28.867 | 0.105 | 0.095 | 0.173 | |||
| β-farnesene | 1:100 | −7.02 | 25.401 | 0.312 | 0.292 | 0.307 | |||
| β-farnesene | 1:1000 | Gh Gt | −8.42 | 32.653 | 0.049 | 0.280 | 0.043 | ||
| β-farnesene | 1:10000 | Gp | 16.75 | 24.337 | 0.097 | 0.046 | 0.241 | ||
| butan-1-ol | 0 | Gp Gt | −31.42 | 28.407 | 0.001 | 0.000 | 0.168 | SIPV | Krips et al. 1999 |
| butan-1-ol | 1:10 | −16.05 | 23.296 | 0.353 | 0.166 | 0.447 | |||
| butan-1-ol | 1:100 | −9.28 | 27.612 | 0.142 | 0.227 | 0.148 | |||
| butan-1-ol | 1:1000 | 1.79 | 22.515 | 0.566 | 1.000 | 0.437 | |||
| butan-1-ol | 1:10000 | 11.90 | 20.433 | 0.597 | 0.300 | 0.621 | |||
| cis-3-hexen-1-ol | 0 | Gp | −23.59 | 27.464 | 0.069 | 0.018 | 0.296 | SIPV | van den Boom et al. 2004 |
| cis-3-hexen-1-ol | 1:10 | 3.57 | 27.695 | 0.159 | 0.881 | 0.100 | |||
| cis-3-hexen-1-ol | 1:100 | Gp Gt | 21.41 | 24.232 | 0.049 | 0.009 | 0.328 | ||
| cis-3-hexen-1-ol | 1:1000 | 10.12 | 16.646 | 0.415 | 0.116 | 0.608 | |||
| cis-3-hexen-1-ol | 1:10000 | Gh Gt | 6.85 | 38.427 | 0.020 | 0.479 | 0.012 | ||
| cis-3-hexenyl-acetate | 0 | Gp Gt | −68.57 | 13.092 | 0.000 | 0.000 | 0.614 | SIPV | Arimura et al. 2000, Horiuchi et al. 2003 |
| cis-3-hexenyl-acetate | 1:10 | Gp Gt | −24.70 | 14.241 | 0.031 | 0.001 | 0.777 | ||
| cis-3-hexenyl-acetate | 1:100 | Gp Gh Gt | −10.07 | 34.414 | 0.007 | 0.037 | 0.021 | ||
| cis-3-hexenyl-acetate | 1:1000 | −5.87 | 15.581 | 0.805 | 0.503 | 0.764 | |||
| cis-3-hexenyl-acetate | 1:10000 | Gp Gh Gt | −16.16 | 30.116 | 0.006 | 0.013 | 0.034 | ||
| decan-1-ol | 0 | 5.63 | 27.480 | 0.271 | 0.737 | 0.188 | |||
| decan-1-ol | 1:10 | Gp | 20.39 | 18.612 | 0.273 | 0.032 | 0.706 | ||
| decan-1-ol | 1:100 | Gp Gh Gt | 18.64 | 38.983 | 0.002 | 0.014 | 0.013 | ||
| decan-1-ol | 1:1000 | 9.29 | 30.110 | 0.166 | 0.508 | 0.122 | |||
| decan-1-ol | 1:10000 | 6.79 | 24.671 | 0.264 | 0.374 | 0.230 | |||
| (E)-DMNT | 0 | Gh Gt | −9.50 | 43.609 | 0.003 | 0.165 | 0.003 | SIPV | Arimura et al. 2001, Horiuchi et al. 2003 |
| (E)-DMNT | 1:10 | 1.06 | 21.437 | 0.597 | 0.876 | 0.471 | |||
| (E)-DMNT | 1:100 | Gh Gt | 15.42 | 51.064 | 0.000 | 0.065 | 0.000 | ||
| (E)-DMNT | 1:1000 | 15.16 | 23.818 | 0.182 | 0.070 | 0.350 | |||
| (E)-DMNT | 1:10000 | 7.91 | 20.072 | 0.493 | 0.245 | 0.542 | |||
| dodecyl-acetate | 0 | −12.93 | 21.021 | 0.905 | 0.479 | 0.895 | |||
| dodecyl-acetate | 1:10 | 10.09 | 32.449 | 0.436 | 0.170 | 0.549 | |||
| dodecyl-acetate | 1:100 | Gp | 17.11 | 23.460 | 0.115 | 0.245 | 0.114 | ||
| dodecyl-acetate | 1:1000 | 6.20 | 18.163 | 0.075 | 0.038 | 0.209 | |||
| dodecyl-acetate | 1:10000 | 5.97 | 14.803 | 0.542 | 0.329 | 0.540 | |||
| farnesol | 0 | Gp Gt | −15.55 | 31.118 | 0.022 | 0.023 | 0.085 | ||
| farnesol | 1:10 | Gp Gt | −25.87 | 24.490 | 0.034 | 0.004 | 0.382 | ||
| farnesol | 1:100 | −3.17 | 26.252 | 0.316 | 0.631 | 0.234 | |||
| farnesol | 1:1000 | Gt | 13.93 | 32.012 | 0.042 | 0.105 | 0.063 | ||
| farnesol | 1:10000 | 12.55 | 17.671 | 0.494 | 0.088 | 0.778 | |||
| hexan-1-ol | 0 | Gp Gt | −37.31 | 29.375 | 0.000 | 0.000 | 0.088 | ||
| hexan-1-ol | 1:10 | Gh Gt | −14.87 | 35.386 | 0.017 | 0.214 | 0.017 | ||
| hexan-1-ol | 1:100 | −11.90 | 35.857 | 0.070 | 0.393 | 0.053 | |||
| hexan-1-ol | 1:1000 | −0.03 | 22.357 | 0.625 | 1.000 | 0.496 | |||
| hexan-1-ol | 1:10000 | −8.93 | 24.573 | 0.247 | 0.458 | 0.198 | |||
| hexyl-acetate | 0 | Gp Gh Gt | −19.55 | 43.790 | 0.000 | 0.004 | 0.001 | SIPV | van den Boom et al. 2004 |
| hexyl-acetate | 1:10 | −13.43 | 12.028 | 0.746 | 0.151 | 0.923 | |||
| hexyl-acetate | 1:100 | 8.03 | 19.609 | 0.762 | 0.514 | 0.710 | |||
| hexyl-acetate | 1:1000 | −10.95 | 22.756 | 0.367 | 0.163 | 0.470 | |||
| hexyl-acetate | 1:10000 | −17.02 | 26.315 | 0.159 | 0.074 | 0.298 | |||
| (+/-) linalool | 0 | −6.97 | 21.019 | 0.626 | 0.369 | 0.613 | SIPV | van den Boom et al. 2004, Krips et al. 1999 Kant et al. 2004 | |
| (+/-) linalool | 1:10 | −3.26 | 33.782 | 0.160 | 0.922 | 0.100 | |||
| (+/-) linalool | 1:100 | −10.72 | 24.657 | 0.434 | 0.344 | 0.415 | |||
| (+/-) linalool | 1:1000 | 4.18 | 16.197 | 0.828 | 0.516 | 0.787 | |||
| (+/-) linalool | 1:10000 | −13.01 | 22.873 | 0.192 | 0.116 | 0.286 | |||
| MeSA | 0 | Gp Gt | −71.81 | 16.270 | 0.000 | 0.000 | 0.188 | SIPV | van den Boom et al. 2004, Agrawal et al. 2002, Meada and Takabayashi 2001, Kant et al. 2004, Arimura et al. 2000, Arimura et al. 2001 |
| MeSA | 1:10 | Gp Gt | −67.05 | 12.818 | 0.000 | 0.000 | 0.769 | ||
| MeSA | 1:100 | Gp Gt | −32.96 | 18.756 | 0.000 | 0.000 | 0.609 | ||
| MeSA | 1:1000 | Gh Gt | 5.85 | 32.439 | 0.034 | 0.324 | 0.027 | ||
| MeSA | 1:10000 | Gp Gt | 33.19 | 21.047 | 0.000 | 0.000 | 0.324 | ||
| Nerolidol | 0 | 1.26 | 7.664 | 0.998 | 0.941 | 0.994 | SIPV | Kant et al. 2004 | |
| Nerolidol | 1:10 | −21.29 | 33.040 | 0.805 | 0.503 | 0.764 | |||
| Nerolidol | 1:100 | −6.54 | 23.245 | 0.805 | 0.503 | 0.764 | |||
| Nerolidol | 1:1000 | Gp Gt | −21.89 | 22.061 | 0.019 | 0.003 | 0.262 | ||
| Nerolidol | 1:10000 | −5.46 | 19.499 | 0.671 | 0.547 | 0.597 | |||
| octan-1-ol | 0 | 8.51 | 28.581 | 0.217 | 0.321 | 0.199 | |||
| octan-1-ol | 1:10 | Gp Gt | 38.61 | 27.403 | 0.000 | 0.000 | 0.206 | ||
| octan-1-ol | 1:100 | 10.95 | 26.854 | 0.278 | 0.215 | 0.310 | |||
| octan-1-ol | 1:1000 | 2.33 | 21.224 | 0.717 | 0.785 | 0.604 | |||
| octan-1-ol | 1:10000 | −8.58 | 20.986 | 0.467 | 0.265 | 0.496 | |||
| propan-1-ol | 0 | −9.83 | 20.185 | 0.379 | 0.177 | 0.468 | |||
| propan-1-ol | 1:10 | −7.64 | 22.954 | 0.475 | 0.431 | 0.424 | |||
| propan-1-ol | 1:100 | 13.93 | 19.908 | 0.390 | 0.136 | 0.538 | |||
| propan-1-ol | 1:1000 | Gt | 14.41 | 32.185 | 0.039 | 0.057 | 0.085 | ||
| propan-1-ol | 1:10000 | 3.75 | 24.897 | 0.332 | 0.586 | 0.253 | |||
| propionic acid | 0 | Gp Gt | −57.94 | 15.977 | 0.000 | 0.000 | 0.742 | ||
| propionic acid | 1:10 | Gp | 17.96 | 22.287 | 0.133 | 0.039 | 0.352 | ||
| propionic acid | 1:100 | Gp | 20.14 | 17.428 | 0.158 | 0.011 | 0.732 | ||
| propionic acid | 1:1000 | Gp Gh Gt | 17.38 | 34.147 | 0.002 | 0.023 | 0.009 | ||
| propionic acid | 1:10000 | −4.21 | 26.868 | 0.519 | 0.577 | 0.430 | |||
| (S)-(-)-limonene | 0 | Gp Gt | −31.01 | 25.992 | 0.000 | 0.000 | 0.087 | SIPV | van den Boom et al. 2004, Arimura et al. 2000 |
| (S)-(-)-limonene | 1:10 | Gh | 4.98 | 29.693 | 0.072 | 0.649 | 0.045 | ||
| (S)-(-)-limonene | 1:100 | 14.16 | 26.283 | 0.110 | 0.089 | 0.187 | |||
| (S)-(-)-limonene | 1:1000 | Gp | −14.89 | 24.379 | 0.140 | 0.037 | 0.377 | ||
| (S)-(-)-limonene | 1:10000 | 5.92 | 28.631 | 0.130 | 0.519 | 0.092 | |||
| ( E,E) TMTT | 0 | Gp Gh Gt | 16.22 | 43.235 | 0.000 | 0.037 | 0.001 | SIPV | van den Boom et al. 2004, Krips et al. 1999, Meada and Takabayashi 2001, Arimura et al. 2001 |
| ( E,E) TMTT | 1:10 | 18.46 | 21.878 | 0.348 | 0.063 | 0.660 | |||
| ( E,E) TMTT | 1:100 | Gh Gt | 5.20 | 34.916 | 0.015 | 0.787 | 0.008 | ||
| ( E,E) TMTT | 1:1000 | 6.51 | 37.286 | 0.086 | 0.564 | 0.057 | |||
| ( E,E) TMTT | 1:10000 | −4.87 | 22.878 | 0.304 | 0.768 | 0.214 | |||
| trans-β-ocimene | 0 | Gp Gt | −12.30 | 32.780 | 0.022 | 0.023 | 0.089 | SIPV | Horiuchi et al. 2003, van den Boom et al. 2004, Agrawal et al. 2002, Krips et al. 1999, Kant et al. 2004, Arimura et al. 2000, Arimura et al. 2001 |
| trans-β-ocimene | 1:10 | Gp Gt | −20.17 | 32.144 | 0.009 | 0.006 | 0.090 | ||
| trans-β-ocimene | 1:100 | Gp Gh Gt | −30.60 | 38.632 | 0.000 | 0.000 | 0.002 | ||
| trans-β-ocimene | 1:1000 | 8.04 | 21.053 | 0.672 | 0.438 | 0.633 | |||
| trans-β-ocimene | 1:10000 | 13.02 | 16.434 | 0.444 | 0.073 | 0.761 | |||
| (-) trans-caryophyllene | 0 | −5.97 | 22.730 | 0.385 | 0.435 | 0.332 | SIPV | Krips et al. 1999, Meada and Takabayashi 2001, van de Boom et al. 2004 | |
| (-) trans-caryophyllene | 1:10 | −11.03 | 21.033 | 0.449 | 0.219 | 0.512 | |||
| (-) trans-caryophyllene | 1:100 | −0.24 | 21.158 | 0.670 | 0.871 | 0.546 | |||
| (-) trans-caryophyllene | 1:1000 | −11.97 | 26.011 | 0.188 | 0.117 | 0.280 | |||
| (-) trans-caryophyllene | 1:10000 | −1.47 | 31.029 | 0.161 | 0.732 | 0.105 | |||
Each sample was tested with six replicate experiments that each contained about 20 starved mites. Negative average preference indices correspond to cases where the majority of the mites avoided the odor side, whereas positive preference indices correspond to cases where the majority of the mites moved toward the odor source. Average preference indices marked by gray cells correspond to nine moderately attractive samples (Gp* and Gh ns). Just 3 of these nine odors additionally include significance of the total G statistic (Gt *), octan-1-ol, methyl salicylate, and cis-3-hexen-1-ol. SIPV indicates that the compound has been reported as a spider-mite-induced plant volatile in de references in the adjacent column.
Thus, the predatory mites perceived most odors in the panel. Out of 30 odors, 24 elicited a response (e.g., (P (G p , df = 1) < 0.05)) at one or more of the tested concentrations (Table 1). The six that did not elicit a response were acetone, α-humulene, α-pinene, linalool, propan-1-ol, and trans-caryophylene. Overall, 27 out of 150 choice tests were more heterogeneous than expected under the null hypothesis (P (G h , df = 5) < 0.05) (Table 1). These heterogeneous results were more prevalent at the higher concentration range (pure to 100× diluted odor samples) than in the lower concentration range (1,000 and 10,000× diluted samples). Based on a significance of G p, 20 out of 30 odors at the highest concentration elicited a response, of which 19 were repellent and only one was attractive. The 10 and 100× diluted samples each contained 10 odors that elicited a response, but here, at the10× dilution, six were attractive whereas only four were repellent. At the 100× dilution, seven odors were repellent and only three were attractive. At the lowest concentrations, the 1,000x dilution yielded only two repellent and one attractive odor, whereas the 10.000× dilution only yielded two repellent and two attractive odors.
Out of 150 samples tested, 18% were significantly more heterogeneous than expected, and sometimes the same set of six replicate experiments revealed statistically significant heterogeneity, whereas the pooled results deviated significantly from an even distribution. In such cases, the pooled significance may depend on one or two extreme replicate experiments. A better indication of the olfactory preference of the population of predatory mites is, thus, found by using more stringent statistical criteria of the replicated G test for goodness of fit (Sokal and Rohlf, 1995). By using significance of G t and G p and non-significance of G h as the criteria for choice, 13 repellent compounds at the highest concentration remain; these are 1-trans-2-hexenol, 2,3-dimethyl-pyrazine, 2-benzyl-ethanol, acetic acid, α-terpinene, butan-1-ol, cis-3-hexenyl acetate, farnesol, hexan-1-ol, methyl salicylate, propionic acid, (S)-(-)-limonene, and β-ocimene. At the 10× diluted samples, only five repellent compounds remain; these are 1-trans-2-hexenol, cis-3-hexenyl acetate, farnesol, methyl salicylate, and trans-β-ocimene. The lowest concentration (10,000×) no longer contains repellent compounds under these statistical criteria, whereas the 100× and 1,000× diluted samples contain only one repellent compound each; these are methyl salicylate and nerolidol. In total, just three samples are attractive based on significance of G t and G p and non-significance of G h , and each at just one of the five tested concentrations. Octan-1-ol was attractive at dilution 10×, cis-3-hexen-1-ol at dilution 100×, and methyl salicylate at a dilution of 10,000×.
We also investigated whether typical spider mite-induced compounds are more attractive than other compounds. The set of attractive odor samples, whose preference index does not exceed 39, is only weakly to moderately attractive compared to the repellent samples that often have a preference index smaller than −50, and to the control experiment with a spider mite-infested leaf disc that has a preference index of 68 (Fig. 2). If the statistical criteria for a choice are reduced to the significance of G p, non-significance of G h and no restrictions on the significance of G t are applied, nine out of 150 (30 odors at five concentrations) samples were attractive (indicated by marked preference index in Table 1). This set contains eight out of 30 odors tested (propionic acid was present twice at 10× and 100x dilution). These represent a variety of structurally very different molecules. At the same time, the other set of “non-attractive” odors contains molecules that are structurally similar to some attractive odors. For example, propionic acid was attractive, whereas acetic acid was not; octan-1ol was attractive, whereas 3-octanone was not; cis-3-hexen-1-ol was attractive, whereas hexen-1-ol and cis-3-hexenyl acetate were not; and octan-1-ol and decan-1-ol were attractive, whereas hexan-1-ol was not. Finally, the attractiveness was highly concentration-dependent. Only propionic acid was attractive at two concentrations and all others at only one. Only three out of these eight attractive odors have been implicated in HIPV produced after spider mite infestation, i.e., methyl salicylate, cis-3-hexen-1-ol, and β-farnesene. The other five compounds (propionic acid, octan-1-ol, benzyl benzoate, decan-1-ol, and dodecyl-acetate) have, to the best of our knowledge, never been reported as part of a spider mite-induced odor blend. Thus, starved females of P. persimilis are not specifically more attracted by individual spider mite-induced plant volatiles than by volatiles that are not associated with their prey.
Natural Odor Sources
If P. persimilis does not specifically respond to typical spider mite-induced plant compounds, one may wonder if the mites are able to detect individual HIPV in complex mixtures for which they have no prior experience. This was investigated by using natural plant odors. In this experiment, we were interested not only in the initial choice of the predatory mites, but also in the time spent in the exploration of odor sources that could be associated with prey. To this end, the time that an individual mite spend in each of two odor fields that contained the odor of spider mite-infested tomato (Lycopersicon esculentum) (HIPV) or the odor of uninfested bean (Phaseolus vulgaris) (no HIPV), was continuously measured for 30 min.
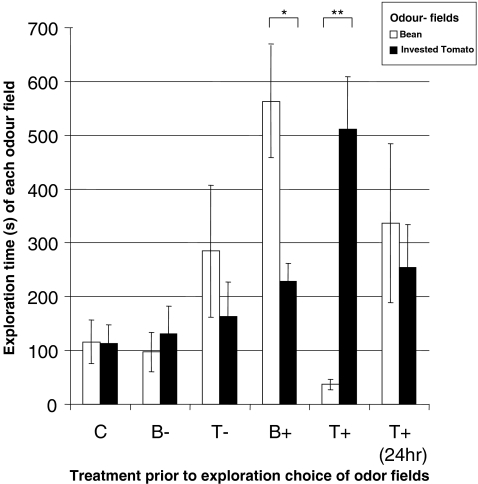
In the absence of experience, the mites invested an equal amount of time in exploring both odor sources (Fig. 4). Subsequently, we experimentally mimicked conditions in which predatory mites explore an uninfested plant for a prolonged time. To this end, the mites were not starved in an Eppendorf tube outside the setup, but instead the starvation took place inside the setup with two odor sources that each contained an uninfested bean leaf. Twenty-four hours later, the mites were transferred to a fresh, identical arena that contained an uninfested bean leaf in one vial, and an infested tomato leaf in the alternative vial. As in the first experiment with naïve individuals, these mites did not spend more time in one of the two fields (Fig. 4). To assess whether the mites were able to associate the odors with food, washed spider mite eggs were offered above one of the odor sources. However, because feeding in the presence of an odor also results in the arrestment of predatory mites in the presence of the odor, we first conducted a control experiment to assess the role of this potential confounding effect. In this control experiment, starved individuals were forced to stay for 15 min in the area above the infested tomato odor (Fig. 4). This treatment did not significantly affect the subsequent olfactory response of the mites, and they still did not prefer one odor over the other. Next, the predatory mites were allowed to feed for 15 min on washed spider mite eggs while experiencing the infested tomato leaf odor or the uninfested bean leaf odor (Fig. 4). This short period of feeding and contact with their food induced a strong behavioral change. After placing the predatory mites in an identical arena without food, they now spent much more time in the odor fields of the odors that were associated with a reward. The mites were able to learn both odors, but they seemed to have slightly more trouble ignoring the infested tomato odor field than the uninfested bean odor field as is evident from the difference in the time spent in both unrewarded odor fields (t test: P < 0.05) (Fig. 4). To analyze the role of memory, we used this acquired response to assess if a prolonged experience with a reward-associated and a non-reward-associated odor would influence the olfactory response of the mites 24 hr after this experience. To this end, the predatory mites were first starved 24 hr, subsequently allowed to forage in the arena for 24 hr where food was available above the infested tomato leaf, and the area above the uninfested bean leaf yielded no food. After this experience, the predatory mites were again starved for 24 hr and subsequently, the time spent in each odor field was measured. After this experience, the mites again spent an equal amount of time in both odor fields (Fig. 4). Hence, we conclude that the type of learning involved in our setup induces only a short-term memory.
Fig. 4.
Innate and acquired preference for spider mite-infested- and uninfested plant odors for which mites have no prior experience. Bars represent the time mites spent in each odor field (during 30 min). Black represents spider mite-infested tomato (HIPV source), white represents uninfested bean (no HIPV source). Significance was tested with a paired sample t-test (* P < 0.05, ** P < 0.001). C: without prior experience, predatory mites do not invest more time in the exploration of the HIPV source than in the alternative (N = 11). B−: mites were starved in the arena for 24 hr, while both sides contained bean leaves. There was no evidence of an acquired aversion (N = 10). T−: Starved mites were restrained for 15 min above the infested tomato field without food (N = 8). There is no evidence of a non-associative acquired response as a result of this treatment. B + and T + : Starved mites were allowed to feed for 15 min in the presence of either odor, the mites associate the odor with the reward (N = 15 and N = 16). T + (24 hr): Mites were first starved, subsequently allowed to forage in the arena, while the tomato patch contained food and the bean patch was unrewarded. Subsequently, the mites were starved for 24 hr until tested (N = 12). The result suggests that the memory was lost within 24 hr
Discussion
Several authors have reported on the olfactory responses of P. persimilis to individual spider mite-induced plant volatiles (Dicke et al. 1990b; De Boer and Dicke 2004b; De Boer et al. 2004; Kappers et al. 2005). Dicke et al. (1990b) reported on responses elicited by linalool, methyl salicylate, (E,E)- and (Z,E) TMTT, cis-3-hexen-1-ol, cis-3 hexen-1-yl acetate, octan-3-ol, and both (Z)- and (E)-β-ocimene. In contrast to the experiments presented here, the predatory mites in the experiments by Dicke at al. (1990b) were satiated and thus in a physiological condition that is different from that during long-distance dispersal. Dicke et al. (1990b) reported that, as in our study, methyl salicylate was attractive, but in contrast to our results these authors also reported that (E,E)-TMTT, linalool, and (E)-β-ocimene were attractive. They also assessed the effect of mixing (E)- and (Z)-β-ocimene, and found only (E)-β-ocimene to be attractive, whereas the chemotactic response attenuated when (Z)-β-ocimene was added to (E)-β-ocimene. We used a racemic mixture of 70% (E)-β-ocimene and 30% (Z)-β-ocimene, and as in Dicke et al. (1990b) this blend was not attractive. De Boer and Dicke (2004a, 2004b) also reported on the attractiveness of methyl salicylate and additionally showed that it is attractive to both starved and satiated mites. In a second study, de Boer and Dicke (2004b) reported on the attractiveness of 2-butanone, a compound not tested by us. In addition, they found that (E,E)-TMTT and (E)-DMNT did not elicit a significant response from starved P. persimilis, although as in Dicke et al. (1990), (E)-DMNT was attractive to satiated mites while (E,E)-TMTT bordered significance. Similarly, these two compounds did not elicit a significant response (G p *, G h ns, G t *) from the starved predatory mites in our study, whereas the attractiveness bordered significance at some concentrations. Finally, Kappers et al. (2005) reported on the attractiveness of (E)-nerolidol to starved (24 hr) P. persimilis, whereas we observed no attraction to this compound.
Behavioral studies are sometimes difficult to compare because starvation times (Dicke et al. 1998; Shimoda and Dicke 2000), wind speeds, the genetic background of the population (Margolies et al. 1997), rearing condition, and previous experience (Takabayashi and Dicke 1992; Krips et al. 1999; Drukker et al. 2000; de Boer and Dicke 2004a, 2005; De Boer et al. 2005) might all vary among experiments from different authors. In this study, we report a comprehensive study on the olfactory responses to a range of individual spider mite-induced plant volatiles in starved P. persimilis females. To avoid reporting on the particular preference of a particular strain of P. persimilis, a large laboratory population was founded with mites that were collected at various locations along the coast of Sicily (Italy). The results should, thus, be interpreted as responses at the population level. The olfactory response among individual mites could differ as a result of genetic factors (Margolies et al. 1997), and might explain some of the heterogeneity observed among replicate experiments.
The predatory mites responded to a wide range of structurally different molecules. There also was no clearly observable pattern of chemical motifs that elicited a specific response from the mites. It appears that the olfactory system of the mites is not specifically sensitive to a few ecologically relevant compounds. The olfactory system is rather more likely to identify a wide range of chemical motifs. This is not self-evident, especially if we do not take the simplicity of the olfactory system into account (Jagers op Akkerhuis et al. 1985; van Wijk et al. 2006). These results are, however, consistent with observations in insects and vertebrates where olfactory receptor cells possess a broader molecular receptive range, and respond to several, similar odors, particularly at higher concentrations (de Bruyne et al. 2001; Abaffy et al. 2006; Pelz et al. 2006). Moreover, the strength of the response, which odors elicit in predatory mites, increases with increasing concentration. Close examination of the data, however, revealed that this only applies to significantly repellent (G t *, G p *, and G h ns) samples. For the nine attractive samples (G p * and G h ns), such a correlation does not exist. Repellence of individual compounds is, thus, largely explained by odor quantity, whereas attraction appears to depend on unique combinations of odor quality and quantity.
The most remarkable result is the low number of significantly attractive compounds among spider mite-induced plant volatiles. Under the most stringent statistical criteria (G t *, G p *, and G h ns) just three samples were attractive, i.e., octan-1-ol, cis-3-hexen-1-ol, and methyl salicylate. These odors are only moderately attractive compared to the control experiment that involved spider mite-infested Lima bean (Fig. 1), and the results indicate that individual compounds hardly induce a chemotactic response. Octan-1-ol is—to the best of our knowledge — not involved in indirect plant defense. Cis-3-hexen-1-ol is known as a green leaf volatile, and is induced in many plants upon spider mite infestation (Van Den Boom et al. 2004), but also in direct response to wounding of plant tissue (Arimura et al. 2001). Like cis-3-hexen-1-ol, methyl salicylate is often induced by spider-mite feeding (e.g., Ament et al. 2004; De Boer et al. 2004; Kant et al. 2004; Van Den Boom et al. 2004). Methyl salicylate is, however, already induced in plants when only aspects of the mechanical damage caused by chewing insects are simulated (Mithofer et al. 2005). Thus, it is by no means a spider mite-specific signal.
The absence of a strong chemotactic response to individual typical HIPV indicates that these stimuli have no a priori meaning to predatory mites. This suggests that the ability to perceive the individual compounds in mixtures will be of little survival value. Consequently, mites are unlikely to possess the ability to discriminate between odors from herbivore-infested and clean plants on the basis of one or a few attractive compounds when they have never experienced these odors before. The results indicate that naïve mites are not able to discern infested tomato odor from uninfested bean odor (Fig. 4). The infested tomato is known to produce several typical spider mite-induced volatiles, among which is methyl salicylate (Ament et al. 2004; Kant et al. 2004), a compound that is attractive to the mites. With experience, the predatory mites were capable of differentiating between these odors. These data are, thus, not consistent with the hypothesis that mites possess an elemental odor perception that enables them to respond to the presence of specific HIPV that they identify in mixtures. The results also suggest that mites do not possess an innate preference for a combination of HIPV that could help them to select without prior experience the infested tomato leaf. Similar results were reported by Drukker et al. (2000) who found that P. persimilis reared in the absence of plant odors did not prefer the odors of infested plants over those of uninfested plants. These predatory mites only acquired a preference after prolonged (24 hr) feeding in the presence of either the uninfested- or infested plant odor. In a similar way, the mites in our experiment were able to associate both odors, irrespective of the HIPV contents of the blend, with the presence of food (Fig. 4).
The predatory mites require only a short learning period of less than 15 min to acquire a preference for an odor, a period much shorter than previously reported for the mites. Prolonged experience with odors that were not associated with food (24 hr) did not induce avoidance, in contrast to the results reported by Drukker et al. (2000). This result, however, is similar to those reported by de Boer et al. (2004), who also found no evidence of an acquired aversion as a result of starvation in the presence of odors. With respect to learning and memory, we conclude that the mites were able to associate odors with the presence of prey after a brief learning experience of less than 15 min, but the same learning experience of 24 hrs was insufficient to induce a long-term memory.
In summary, the mites responded to a wide range of structurally different molecules, suggesting that their small olfactory system is not sensitive to a few specific ecologically relevant compounds. This is further supported by the fact that the mites were not more attracted to spider mite-induced plant volatiles than to volatiles that are not associated with spider mites. This indicates that these individual spider mite-induced plant volatiles have no a priori meaning and that the ability to identify these compounds in mixtures is of little use to the mites. As a consequence, mites are not expected to use the presence of these compounds in odor mixtures, to select a spider mite-infested plant, for which they have no prior experience. Results of our study confirm that the predatory mites were not able to innately identify a spider mite-infested plant odor, even though it emits methyl salicylate, a compound that was attractive to P. persimilis. We, thus, conclude that P. persimilis females possess a limited ability to identify individual spider mite-induced plant volatiles in odor mixtures. Instead, the mites learn to respond to prey-associated odor mixtures.
Acknowledgments
We thank Dr. W. Boland of the Max Planck Institute for Chemical Ecology, Jena, Germany for providing us with (E)-4,8-dimethyl-1,3,7-nonatriene (DMNT) and (E,E)-4,8,12-trimethyl-1,3,7,11-tridecatetraene, (TMTT).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Abaffy T., Matsunami H., Luetje C. W. Functional analysis of a mammalian odorant receptor subfamily. J. Neurochem. 2006;97:1506–1518. doi: 10.1111/j.1471-4159.2006.03859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A. A., Janssen A., Bruin J., Posthumus M. A., Sabelis M. W. An ecological cost of plant defense: attractiveness of bitter cucumber plants to natural enemies of herbivores. Ecol. Lett. 2002;5:377–385. doi: 10.1046/j.1461-0248.2002.00325.x. [DOI] [Google Scholar]
- Ament K., Kant M. R., Sabelis M. W., Haring M. A., Schuurink R. C. Jasmonic acid is a key regulator of spider mite-induced volatile terpenoid and methyl salicylate emission in tomato. Plant. Physiol. 2004;135:2025–2037. doi: 10.1104/pp.104.048694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura G., Kost C., Boland W. Herbivore-induced, indirect plant defenses. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2005;1734:91–111. doi: 10.1016/j.bbalip.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Arimura G., Ozawa R., Horiuchi J., Nishioka T., Takabayashi J. Plant–plant interactions mediated by volatiles emitted from plants infested by spider mites. Biochem. Syst. Ecol. 2001;29:1049–1061. doi: 10.1016/S0305-1978(01)00049-7. [DOI] [Google Scholar]
- Arimura G., Ozawa R., Shimoda T., Nishioka T., Boland W., Takabyashi J. Herbivory-induced volatiles elicit defense genes in lima bean leaves. Nature. 2000;406(6795):512–515. doi: 10.1038/35020072. [DOI] [PubMed] [Google Scholar]
- Bernays E. A. Neural limitations in phytophagous insects: Implications for diet breadth and evolution of host affiliation. Annu. Rev. Entomol. 2001;46:703–727. doi: 10.1146/annurev.ento.46.1.703. [DOI] [PubMed] [Google Scholar]
- Bolland H. R., Gutierrez J., Flechtmann C. H. W. World catalogue of the spider mite family. Leiden, The Netherlands: Brill; 1998. [Google Scholar]
- De Boer J. G., Dicke M. Experience with methyl salicylate affects behavioral responses of a predatory mite to blends of herbivore-induced plant volatiles. Entomol. Exp. Appl. 2004;110:181–189. doi: 10.1111/j.0013-8703.2004.00133.x. [DOI] [Google Scholar]
- De Boer J. G., Dicke M. The role of methyl salicylate in prey searching behavior of the predatory mite Phytoseiulus persimilis. J. Chem. Ecol. 2004;30:255–271. doi: 10.1023/B:JOEC.0000017976.60630.8c. [DOI] [PubMed] [Google Scholar]
- De Boer J. G., Dicke M. Information use by the predatory mite Phytoseiulus persimilis (Acari: Phytoseiidae), a specialised natural enemy of herbivorous spider mites. Appl. Entomol. Zool. 2005;40:1–12. doi: 10.1303/aez.2005.1. [DOI] [Google Scholar]
- De Boer J. G., Posthumus M. A., Dicke M. Identification of volatiles that are used in discrimination between plants infested with prey or nonprey herbivores by a predatory mite. J. Chem. Ecol. 2004;30:2215–2230. doi: 10.1023/B:JOEC.0000048784.79031.5e. [DOI] [PubMed] [Google Scholar]
- De Boer J. G., Snoeren T. A. L., Dicke M. Predatory mites learn to discriminate between plant volatiles induced by prey and nonprey herbivores. Anim. Behav. 2005;69:869–879. doi: 10.1016/j.anbehav.2004.07.010. [DOI] [Google Scholar]
- De Bruyne M., Foster K., Carlson J. R. Odor coding in the Drosophila antenna. Neuron. 2001;30:537–552. doi: 10.1016/S0896-6273(01)00289-6. [DOI] [PubMed] [Google Scholar]
- De Moraes C. M., Lewis W. J., Pare P. W., Alborn H. T., Tumlinson J. H. Herbivore-infested plants selectively attract parasitoids. Nature. 1998;393:570–573. doi: 10.1038/31219. [DOI] [Google Scholar]
- Degen T., Dillmann C., Marion-Poll F., Turlings T. C. J. High genetic variability of herbivore-induced volatile emission within a broad range of maize inbred lines. Plant Physiol. 2004;135:1928–1938. doi: 10.1104/pp.104.039891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicke M. Local and systemic production of volatile herbivore-induced terpenoids—their role in plant–carnivore mutualism. J. Plant Physiol. 1994;143:465–472. [Google Scholar]
- Dicke M., Sabelis M. W. How plants obtain predatory mites as bodyguards. Neth. J. Zool. 1988;38:148–165. [Google Scholar]
- Dicke M., Sabelis M. W., Takabayashi J., Bruin J., Posthumus M. A. Plant strategies of manipulating predator–prey interactions through allelochemicals—prospects for application in pest-control. J. Chem. Ecol. 1990;16:3091–3118. doi: 10.1007/BF00979614. [DOI] [PubMed] [Google Scholar]
- Dicke M., Takabayashi J., Posthumus M. A., Schutte C., Krips O. E. Plant–phytoseiid interactions mediated by herbivore-induced plant volatiles: variation in production of cues and in responses of predatory mites. Exp. Appl. Acarol. 1998;22:311–333. doi: 10.1023/A:1024528507803. [DOI] [Google Scholar]
- Dicke M., Vanbeek T. A., Posthumus M. A., Bendom N., Vanbokhoven H., Degroot A. E. Isolation and identification of volatile kairomone that affects acarine predator–prey interactions—involvement of host plant in its production. J. Chem. Ecol. 1990;16:381–396. doi: 10.1007/BF01021772. [DOI] [PubMed] [Google Scholar]
- Drukker B., Bruin J., Jacobs G., Kroon A., Sabelis M. W. How predatory mites learn to cope with variability in volatile plant signals in the environment of their herbivorous prey. Exp. Appl. Acarol. 2000;24(12):881–895. doi: 10.1023/A:1010645720829. [DOI] [PubMed] [Google Scholar]
- Egan S. P., Funk D. J. Individual advantages to ecological specialization: insights on cognitive constraints from three conspecific taxa. Proc. R. Soc. Lond. B. Biol. Sci. 2006;273:843–848. doi: 10.1098/rspb.2005.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigenbrode S. D., Ding H., Shiel P., Berger P. H. Volatiles from potato plants infected with potato leafroll virus attract and arrest the virus vector, Myzus persicae (Homoptera: Aphididae) (vol 269, pg 455, 2002) Proc. R. Soc. Lond. B. Biol. Sci. 2002;269:2603–2603. doi: 10.1098/rspb.2001.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouinguene S. P., Turlings T. C. J. The effects of abiotic factors on induced volatile emissions in corn plants. Plant Physiol. 2002;129:1296–1307. doi: 10.1104/pp.001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi J., Arimura G., Ozawa R., Shimoda T., Takabayashi J., Nishioka T. A comparison of the responses of Tetranychus urticae (Acari: Tetranychidae) and Phytoseiulus persimilis (Acari: Phytoseiidae) to volatiles emitted from lima bean leaves with different levels of damage made by T-urticae or Spodoptera exigua (Lepidoptera: Noctuidae) Appl. Entomol. Zool. 2003;38:109–116. doi: 10.1303/aez.2003.109. [DOI] [Google Scholar]
- Jackson G. J. Chaetotaxy and setal morphology of the palps and first tarsi of Phytoseiulus persimilis A. -H (Acarina: Phytoseiidae) Acarologia. 1974;16:583–594. [Google Scholar]
- Jagers Op Akkerhuis G., Sabelis M. W., Tjallingii W. F. Ultrastructure of chemical receptors on the pedipalps and first tarsi of Phytoseiulus persimilis. Ex.p Appl. Acarol. 1985;1:235–251. doi: 10.1007/BF01198521. [DOI] [Google Scholar]
- Jimenez-Martinez E. S., Bosque-Perez N. A., Berger P. H., Zemetra R., Ding H. J., Eigenbrode S. D. Volatile cues influence the response of Rhopalosiphum padi (Homoptera: Aphididae) to barley yellow dwarf virus-infected transgenic and untransformed wheat. Environ. Entomol. 2004;33:1207–1216. [Google Scholar]
- Kant M. R., Ament K., Sabelis M. W., Haring M. A., Schuurink R. C. Differential timing of spider mite-induced direct and indirect defenses in tomato plants. Plant Physiol. 2004;135:483–495. doi: 10.1104/pp.103.038315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant M. R., Sabelis M. W., Haring M. A., Schuurink R. C. Intrapsecific variation in a generalist herbivore accounts for differential induction and impact of host plant defenses. Proc. R. Soc., B. 2008;275:443–452. doi: 10.1098/rspb.2007.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappers I. F., Aharoni A., Van Herpen T., Luckerhoff L. L. P., Dicke M., Bouwmeester H. J. Genetic engineering of terpenoid metabolism attracts, bodyguards to Arabidopsis. Science. 2005;309:2070–2072. doi: 10.1126/science.1116232. [DOI] [PubMed] [Google Scholar]
- Krips O. E., Willems P. E. L., Gols R., Posthumus M. A., Dicke M. The response of Phytoseiulus persimilis to spider mite-induced volatiles from gerbera: influence of starvation and experience. J. Chem. Ecol. 1999;25:2623–2641. doi: 10.1023/A:1020887104771. [DOI] [Google Scholar]
- Maeda T., Takabayashi J. Production of herbivore-induced plant volatiles and their attractiveness to Phytoseius persimilis (Acari: Phytoseiidae) with changes of Tetranychus urticae (Acari: Tetranychidae) density on a plant. Appl. Entomol. Zool. 2001;36:47–52. doi: 10.1303/aez.2001.47. [DOI] [Google Scholar]
- Margolies D. C., Sabelis M. W., Boyer J. E. Response of a Phytoseiid predator to herbivore-induced plant volatiles: selection on attraction and effect on prey exploitation. J. Insect Behav. 1997;10:695–709. doi: 10.1007/BF02765387. [DOI] [Google Scholar]
- Mithofer A., Wanner G., Boland W. Effects of feeding Spodoptera littoralis on lima bean leaves. II. Continuous mechanical wounding resembling insect feeding is sufficient to elicit herbivory-related volatile emission. Plant Physiol. 2005;137:1160–1168. doi: 10.1104/pp.104.054460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelz D., Roeske T., Syed Z., De Bruyne M., Galizia C. G. The molecular receptive range of an olfactory receptor in vivo (Drosophila melanogaster Or22a) J. Neurobiol. 2006;66:1544–1563. doi: 10.1002/neu.20333. [DOI] [PubMed] [Google Scholar]
- Sabelis M. W., Afman B. P. Synomone-induced suppression of take-off in the phytoseiid mite Phytoseiulus persimilis Athias-Henriot. Exp. Appl. Acarol. 1994;18:711–721. doi: 10.1007/BF00114171. [DOI] [Google Scholar]
- Sabelis M. W., Afman B. P., Slim P. J. Location of distant spider-mite colonies by Phytoseiulus persimilis: Localisation and extraction of a kairomone . In: Griffiths D. A., Bowman C. E., editors. Acarology VI, vol. 1. New York: John Wiley and Sons; 1984. pp. 431–440. [Google Scholar]
- Sabelis, M. W., Janssen, A., Pallini, A., Venzon, M., Bruin, J., Drukker, B., and Scutareanu, P. 1999. Behavioral responses of predatory and herbivorous arthropods to induced plant volatiles: From evolutionary ecology to agricultural applications, in A. Agrawal, S. Tuzun and E. Bent (eds.). Induced Plant Defenses against Pathogens and Herbivores. The American Phytopathological Society (APS) Press, St. Paul, Minnesota.
- Sabelis, M. W., Takabayashi, J., Janssen, A., Kant, M. R., Van Wijk, M., Sznajder, B., Aratchige, N., Lesna, I., Belliure, B., and Schuurink, R. C. 2006. Ecology meets plant physiology: Herbivore-induced plant responses and their indirect effects on arthropod communities pp. 188–217, in T. Ohgushi, T. P. Craig and P. W. Price. (eds.). Ecological Communities: Plant Mediation in Indirect Interaction Webs. Cambridge University Press, Cambridge.
- Sabelis M. W., Van Der Baan H. E. Location of distant spider mite colonies by phytoseiid predators: demonstration of specific kairomones emitted by Tetranychus urticae and Panonychus ulmi. Exp. Appl. Acarol. 1983;33:303–314. [Google Scholar]
- Sabelis M. W., Vermaat J. E., Groeneveld A. Arrestment responses of the predatory mite, Phytoseiulus persimilis, to steep odor gradients of a kairomone. Physiol. Entomol. 1984;9:437–446.. doi: 10.1111/j.1365-3032.1984.tb00786.x. [DOI] [Google Scholar]
- Shimoda T., Dicke M. Attraction of a predator to chemical information related to nonprey: when can it be adaptive. Behav. Ecol. 2000;11:606–613. doi: 10.1093/beheco/11.6.606. [DOI] [Google Scholar]
- Sokal R. R., Rohlf F. J. Biometry. New York: W. H. Freedman and Company; 1995. [Google Scholar]
- Takabayashi J., Dicke M. Response of predatory mites with different rearing histories to volatiles of uninfested plants. Entomol. Exp. Appl. 1992;64:187–193. [Google Scholar]
- Takabayashi J., Dicke M., Takahashi S., Posthumus M. A., Vanbeek T. A. Leaf age affects composition of herbivore-induced synomones and attraction of predatory mites. J. Chem. Ecol. 1994;20:373–386. doi: 10.1007/BF02064444. [DOI] [PubMed] [Google Scholar]
- Vallat A., Gu H. N., Dorn S. How rainfall, relative humidity and temperature influence volatile emissions from apple trees in situ. Phytochemistry. 2005;66:1540–1550. doi: 10.1016/j.phytochem.2005.04.038. [DOI] [PubMed] [Google Scholar]
- Van Den Boom C. E. M., Van Beek T. A., Dicke M. Attraction of Phytoseiulus persimilis (Acari: Phytoseiidae) towards volatiles from various Tetranychus urticae-infested plant species. Bull. Entomol. Res. 2002;92:539–546. doi: 10.1079/BER2002193. [DOI] [PubMed] [Google Scholar]
- Van Den Boom C. E. M., Van Beek T. A., Posthumus M. A., De Groot A., Dicke M. Qualitative and quantitative variation among volatile profiles induced by Tetranychus urticae feeding on plants from various families. J. Chem. Ecol. 2004;30:69–89. doi: 10.1023/B:JOEC.0000013183.72915.99. [DOI] [PubMed] [Google Scholar]
- Van Wijk M., Wadman W. J., Sabelis M. W. Morphology of the olfactory system in the predatory mite Phytoseiulus persimilis. Exp. Appl. Acarol. 2006;40:217–229. doi: 10.1007/s10493-006-9038-x. [DOI] [PubMed] [Google Scholar]