Abstract
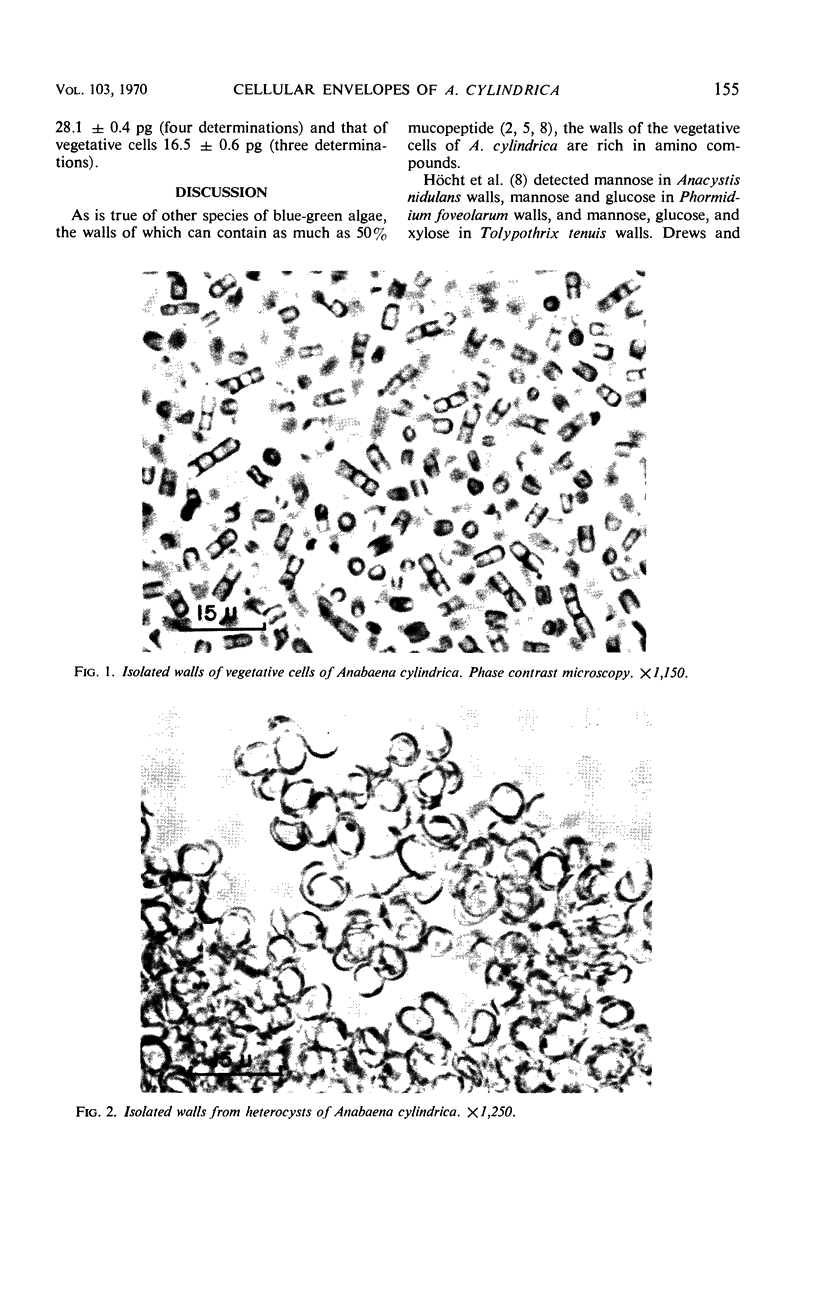
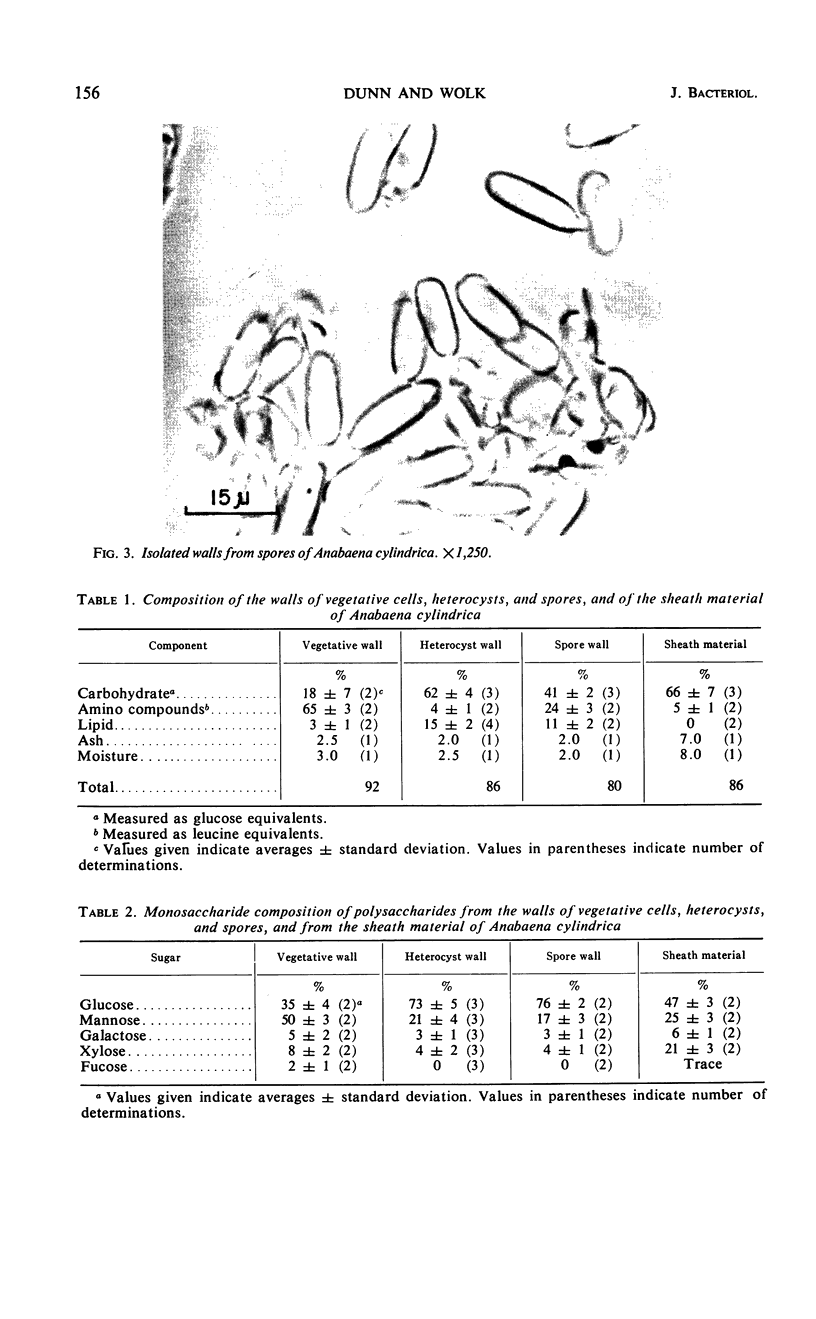
Comparative chemical analyses were made of the walls of vegetative cells, heterocysts, and spores, and of the mucilage of Anabaena cylindrica. The wall of the vegetative cell is composed predominantly of amino compounds, with a mannose-rich carbohydrate component comprising only 18% of the dry weight. In contrast, 62% of the heterocyst wall and 41% of the spore wall is carbohydrate. The carbohydrate moieties of the heterocyst wall and spore wall are similar in that the ratio of glucose, mannose, galactose, and xylose is approximately 75:20:3:4 in both walls. It appears that, during the differentiation of a vegetative cell into either a spore or a heterocyst, a glucose-rich wall polysaccharide is produced that is different from the polysaccharide component of the wall of the vegetative cell and of the sheath. In the case of the heterocyst, the wall was estimated to account for approximately 52% of the dry weight of the whole cell.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FRANK H., LEFORT M., MARTIN H. H. Chemical analysis of a mucopolymer component in cell walls of the blue-green alga Phormidium uncinatum. Biochem Biophys Res Commun. 1962 May 4;7:322–325. doi: 10.1016/0006-291x(62)90200-0. [DOI] [PubMed] [Google Scholar]
- FREY-WYSSLING A., STECHER H. Uber den feinbau des Nostoc-schleimes. Z Zellforsch Mikrosk Anat. 1954;39(5):515–519. [PubMed] [Google Scholar]
- Leak L. V. Fine structure of the mucilaginous sheath of Anabaena sp. J Ultrastruct Res. 1967 Nov;21(1):61–74. doi: 10.1016/s0022-5320(67)80006-6. [DOI] [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]
- ROSEN H. A modified ninhydrin colorimetric analysis for amino acids. Arch Biochem Biophys. 1957 Mar;67(1):10–15. doi: 10.1016/0003-9861(57)90241-2. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Wolk C. P. Control of sporulation in a blue-green alga. Dev Biol. 1965 Aug;12(1):15–35. doi: 10.1016/0012-1606(65)90018-7. [DOI] [PubMed] [Google Scholar]
- Wolk C. P. Movement of carbon from vegetative cells to heterocysts in Anabaena cylindrica. J Bacteriol. 1968 Dec;96(6):2138–2143. doi: 10.1128/jb.96.6.2138-2143.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk C. P. Physiological basis of the pattern of vegetative growth of a blue-green alga. Proc Natl Acad Sci U S A. 1967 May;57(5):1246–1251. doi: 10.1073/pnas.57.5.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]