Abstract
Acidification of forest ecosystems leads to increased plant availability of the micronutrient manganese (Mn), which is toxic when taken up in excess. To investigate whether ectomycorrhizas protect against excessive Mn by improving plant growth and nutrition or by retention of excess Mn in the hyphal mantle, seedlings of two populations of Douglas fir (Pseudotsuga menziesii), two varieties, one being menziesii (DFM) and the other being glauca (DFG), were inoculated with the ectomycorrhizal fungus Rhizopogon subareolatus in sand cultures. Five months after inoculation, half of the inoculated and non-inoculated seedlings were exposed to excess Mn in the nutrient solution for further 5 months. At the end of this period, plant productivity, nutrient concentrations, Mn uptake and subcellular compartmentalisation were evaluated. Non-inoculated, non-stressed DFM plants produced about 2.5 times more biomass than similarly treated DFG. Excess Mn in the nutrient solution led to high accumulation of Mn in needles and roots but only to marginal loss in biomass. Colonisation with R. subareolatus slightly suppressed DFM growth but strongly reduced that of DFG (−50%) despite positive effects of mycorrhizas on plant phosphorus nutrition. Growth reductions of inoculated Douglas fir seedlings were unexpected since the degree of mycorrhization was not high, i.e. ca. 30% in DFM and 8% in DFG. Accumulation of high Mn was not prevented in inoculated seedlings. The hyphal mantle of mycorrhizal root tips accumulated divalent cations such as Ca, but not Mn, thus not providing a barrier against excessive Mn uptake into the plants associated with R. subareolatus.
Keywords: Manganese, Nutrition, Rhizopogon subareolatus, Stress, Subcellular localisation
Introduction
Mycorrhizal associations are generally characterised as affording mutual benefits to the symbiotic fungus and the host plant. The plant provides carbohydrates to the fungus, and the fungus improves the nutritional status of its host by supplying phosphorus, nitrogen and other mineral elements. Furthermore, mycorrhizal colonisation of roots was found to have positive effects on plant growth such as increased resistance to pathogens (Hampp et al. 1999), drought (Davis et al. 1992; Nilsen et al. 1998; Shi et al. 2002) or heavy metal stress (Kothari et al. 1991; Van Tichelen et al. 1996; Blaudez et al. 2000). It has been demonstrated that ectomycorrhizas protect plants against typical soil pollutants such as Cd, Pb, Cu, Mn, etc. by binding the metals to fungal cell wall components, e.g. chitin, cellulose, cellulose derivatives and melanins, leading to metal exclusion (Jentschke and Godbold 2000) or by the stimulation of plant-inherent tolerance through improved nutrient supply or activation of defence pathways including polyamines and glutathione (Galli et al. 1994; Zarb and Walters 1995, 1996; Schützendübel and Polle 2002).
Human activities have not only increased the burden of ecosystems with toxic metals but also influenced soil properties by causing increased acidification. The solubility and plant availability of some elements, especially that of manganese (Mn), strongly depends on soil pH and redox state (Marschner 1995). To date, amelioration of Mn stress has mainly been investigated in arbuscular mycorrhizal (AM) systems (Cardoso 1985; Bethlenfalvay and Franson 1989; Kothari et al. 1991; Posta et al. 1994). Posta et al. (1994) found that AM colonisation slightly decreased root dry mass production of maize and inhibited accumulation of Mn in shoots above control levels. Mycorrhiza decreased the number of Mn-reducing micro-organisms in the rhizosphere and increased Mn-solubilising root exudates. Lower uptake of Mn by mycorrhizal than by non-mycorrhizal plants has been found in several studies (Pacovsky 1986; Arines et al. 1989; Kothari et al. 1990, 1991). However, depending on fungal species, soil type and plant developmental stage, the effectiveness of the AM can vary leading from beneficial to adverse effects (Nogueira and Cardoso 2003). In soybean, ineffective interactions with AM increased Mn toxicity symptoms of the host plant, whereas in effective interactions, no Mn toxicity symptoms were detected in spite of increased Mn availability in the soil and increased Mn concentrations in plants (Nogueira and Cardoso 2003).
In contrast to AM fungi, much less in known about the ability of ectomycorrhizal fungi to modulate uptake of Mn in relation to that of other nutrient elements. This knowledge would be especially relevant for tree species suffering from symptoms of Mn toxicity. It was observed that the coastal variety of Douglas fir [Pseudotsuga menziesii var. menziesii (Mirbel) Franco (DFM)], when cultivated in Europe outside its natural range showed high growth rates and developed wood of good quality, whereas the interior variety [P. menziesii var. glauca (Beissn.) Franco (DFG)] displayed slower growth rates and was relatively susceptible to pathogens and other stress factors (Kleinschmit et al. 1974; Larsen 1978, 1981; Liesebach and Stephan 1995; Schober et al. 1983, 1984). On some sites in Rhineland-Palatine (Germany), DFG accumulated excess Mn in needles and bark and developed typical symptoms of Mn stress, such internal bark necrosis (Block 1997; Schöne 1992). In previous studies with non-mycorrhizal Douglas fir seedlings, we have identified variety-specific differences in Mn uptake and transport pointing to higher intrinsic Mn tolerance of DFM (Dučić et al. 2006; Dučić and Polle 2007). However, in natural ecosystems, Douglas fir roots are colonised by mycorrhizal fungi (Trappe 1977; Parladé et al. 1995; Outerbridge and Trofymow 2004). It is not known whether ectomycorrhizal interactions affect Mn uptake.
In the present study, Douglas fir seedlings of the interior and coastal varieties were grown under controlled conditions and inoculated with the ectomycorrhizal fungus Rhizopogon subareolatus. This fungal species was probably introduced with the host plant in early plantations of Douglas fir in Europe (Álvarez et al. 1993) and shows host specificity with Douglas fir (Molina and Trappe 1982). Rhizopogon species are common in disturbed forest soils (Molina and Trappe 1994; Luoma et al. 2006) and increase Douglas fir resistance against drought stress (Parke et al. 1983). R. subareolatus used to inoculate nursery trees has shown positive effects on rooting of Douglas fir cuttings, improved plant growth and stimulated height growth during the first 3 years of seedling establishment (Parladé et al. 1999; Pera et al. 1999). All previous studies have been conducted with DFM, which is commonly used for silviculture outside its natural range. However, nothing is known about the effect of R. subareolatus on the Douglas fir variety glauca.
The aim of the present study was to investigate the influence of mycorrhizal colonisation of DFM and DFG on growth and plant nutrition. We used the two varieties as indicators of potential genetic variation within the species and tested whether ectomycorrhizas would render the seedlings more Mn tolerant than non-mycorrhizal plants. To investigate these questions, Douglas fir seedlings were inoculated with R. subareolatus. Inoculated and non-inoculated plants were either exposed to excess Mn or maintained under regular fertilisation in sand cultures. Plant growth, biomass and nutrient element concentrations, as well as their subcellular distribution in hyphal mantle, Hartig net and root cells, were determined.
Materials and methods
Plant material and fungal inoculation
Seeds of P. menziesii (vars. menziesii and glauca) were obtained from Niedersachsen Forstamt (Oerrel, Munster-Oerrel, Germany) and Sheffield’s Seed Company (Locke, NY, USA), respectively. DFM seeds originated from Munster-Oerrel (Germany), lot: 853–04, Plantage Nonnenholz, elevation 310 m and DFG seeds from CO Rio Grande Source (Colorado, USA), lot: 980042, elevation 2.286–2.743 m. The Douglas fir variety menziesii, which we used in a series of experiments (Dučić et al. 2006; Dučić and Polle 2007), was previously named var. viridis and therefore abbreviated with DFV in those previous studies. The racial origin of seed lots was confirmed by isozyme analysis (Dučić et al. 2006).
Seeds of Douglas fir were surface-sterilised, germinated on water agar and transferred as 1-month-old seedlings to hydroponic culture using an Ingestad-based nutrient solution (Ingestad and Lund 1986) as described previously (Dučić et al. 2006). The seedlings were grown for 4 months in an acclimatised room with a day/night regime of 16 h/8 h (white light of 150 μmol m−1 s−1 photosynthetic photon flux, OSRAM L 18-W/21-840, Lumlux Pluseco, Germany) at 23°C/21°C air temperature before inoculation with the ectomycorrhizal fungus R. subareolatus.
Sporocarps of R. subareolatus, a Douglas-fir-specific fungus belonging to the Section Villosuli (Smith and Zeller 1966), were collected in a Douglas fir plantation in northern Spain. Isolation of strain 302 was made from sporocarp tissue plated in MMN agar medium (Molina and Palmer 1982). Voucher specimens of the sporocarps were deposited in the real Jardín Botánico de Madrid Herbarium (reference MA-Fungi 28351). Pure cultures of R. subareolatus, strain 302 (IRTA Culture Collection, Barcelona, Spain) were grown for 3 weeks on Petri dishes with agar and modified Melin–Norkrans fungal medium (1/2 MMN; Molina and Palmer 1982). To prepare inoculation substrate, 200-ml Erlenmeyer flasks were filled with 20 ml sieved peat (“Floratorf”, Oldenburg, Germany), 110 ml vermiculite (grade 3, Dämmstoffe, Sprockhövel, Germany), 70 ml 1/2 MMN medium containing 2.5 g L−1 glucose, mixed and autoclaved (Massicotte et al. 1994). Seven disks (d = 10 mm) from colonies of young, vigorously growing R. subareolatus fungi were buried in the peat/vermiculite mixture in the Erlenmeyer flasks. One Douglas fir seedling was planted into each flask. Control plants were planted into the same peat/vermiculite mixture without R. subareolatus.
The flasks were closed and kept under sterile conditions under white light 150 μmol s−1 m−2 of photosynthetic active radiation and an air temperature of 21°C (with a day/night regime of 16 h/8 h). The plants were maintained for 2 months under these conditions, and then 40 inoculated and 40 non-inoculated seedlings of each seed lot were planted individually in 650-ml cylinders (d = 50 mm, h = 410 mm with a perforated bottom), which contained a double-sterilised peat: sand (corn size 0.5–2 mm) mixture (50:50 v/v). Since visual inspection revealed only little mycorrhiza formation, inoculated Douglas fir seedlings were provided again with ten mycelium disks obtained from young R. subareolatus colonies. The cylinders were kept in a green house at an air temperature of 22 ± 2°C and air humidity of 40% with day/night regime of 16 h/8 h, achieved by additional light of 150 μmol m−1 s−1 photosynthetic active radiation (Osram, 400W HOL-R N528, Capelle a/d Ijssel, Belgium). The plants were irrigated with 10 ml of water twice a week and 10 ml of Ingestad-based nutrient solution once a week. After 3 months, half of the inoculated and non-inoculated plants of each seed lot were irrigated with 10 ml of 10 mM MnSO4 in the Ingestad-based nutrient solution once a week to induce Mn stress and with 10 ml water twice per week. This treatment was maintained for an additional 5 months. The nutrient solution supplied to controls contained 5 μM Mn. The seedlings were rearranged regularly during the experimental period to avoid positional effects.
Determination of plant growth and nutrient elements
Shoot heights of Douglas fir seedlings were recorded upon transfer into the growth cylinders and at the end of the experiment. At harvest, the plants were carefully removed from the growth cylinders, and the root systems were soaked in water to remove the peat/sand mixture without damage. Plants of each seed lot and experimental condition were separated into needles, stem, fine and coarse roots. The fresh mass was recorded, the tissues were dried at 60°C for 48 h and dry mass was recorded. Dry material was used for chemical analysis. Nutrient element concentrations were measured using ICP-AES (Spectro Analytic Instruments, Kleve, Germany) after wet ashing in 65% concentrated HNO3 at 170°C for 12 h (Heinrichs et al. 1986).
Determination of ectomycorrhizal abundance and anatomy
Three plants were analysed per treatment. To determine the degree of mycorrhizal colonisation, fine roots were cut into small pieces, and 100 root sections per plant were chosen to determine the number of ectomycorrhizal tips. The typical number of root tips per seedling was 601 ± 77 and 2,605 ± 202 for DFG and DFM, respectively. Only one mycorrhizal morphotype was observed. Mycorrhizas were photographed under a stereomicroscope with ×12 magnification (Stemi SV 11, Zeiss, Jena, Germany).
Samples of fine roots and mycorrhizal tips were cut and fixed in a solution of FAE (7% formaldehyde, 18% ethanol and 19.2% of acetic acid) for a minimum of 7 days. Samples were dehydrated by subsequent incubation in 70%, 80%, 90% and 96% ethanol for 15 min, 100% ethanol two times for 30 min, a mixture of 50% ethanol and 50% acetone (30 min) and two times in 100% acetone for 30 min. Samples were embedded in styrene–metacrylate (49–49%) and 2% dibenzoyl peroxide starting with acetone (30%) and styrene–metacrylate (70%) for 12 h and two times in the styrene–metacrylate mixture for another 12 and 24 h. Then, the samples from the styrene–metacrylate mixture were embedded in gelatine capsules and polymerised for 14 days at 30°C. Afterwards, 1-μm-thick cross sections were cut with an autocut microtome (Ultracut E, Reichert-Jung, Vienna, Austria). The cross sections were mounted on gelatinised microscopic slides, stretched in chloroform vapour and stained with 0.1% toluidine blue in 0.1% di-sodium tetra borate for 3 to 5 min. Photographs were taken with a Nikon Coolpix 4500 camera through the microscope (Stemi SV 11, Zeiss, Jena, Germany).
Subcellular element localisation by energy-dispersive X-ray microanalysis by transmission electron microscopy (EDX-TEM)
Sampling procedure
A set of three plants from each experimental variable was harvested for EDX analyses. To avoid delay, from each of these plants, several root tips of 5-mm length were rapidly frozen and further processed for microscopy. After cross-sectioning, it was checked that only mycorrhizal root tips from inoculated plants and non-mycorrhizal roots tips from non-inoculated plants were analysed. One cross section was analysed of each harvested plant. Among different cross sections of an individual plant, the most suitable, i.e. an entire, evenly trimmed cross section with no disruptures and in which the vascular system was not yet developed, was selected. In this cross section, the element concentrations were measured at six to nine positions of each of the analysed subcellular locations: outer epidermal wall, vacuole of the epidermal cells, cell wall of cortex cells, vacuoles of cortex cells, whole cells of the central cylinder. In mycorrhizal plants, measurements were also taken in the hyphal mantle and in the Hartig net. Since only cross sections of the root tip, where the vascular system was not yet developed, were analysed, the cells of the central cylinder showed no differentiation.
Sample preparation
Root tips were rapidly frozen in a mixture of propane–isopentane (2:1) cooled with liquid nitrogen to −196°C in an aluminium mesh. Samples were freeze-dried at −45°C for 3 days and stored at room temperature in a desiccator over silica gel. For transmission electron microscopy, freeze-dried samples were infiltrated with ether in a vacuum-pressure chamber and embedded in styrene–methacrylate using a technique specifically developed for analysis of diffusible elements (Fritz 1989). One-micrometre-thick sections were cut using glass knives, mounted on adhesive-coated 100-mesh hexagonal grits, coated with carbon and stored over silica gel. Details and testing of the method have been reported previously (Fritz 1989; Fritz and Jentschke 1994). The samples were analysed with a Philips EM 420 with the energy-dispersive system EDAX DX-4 (EDAX Inc., Mahwah, NJ, USA). The accelerating voltage was 120 kV, the take-off angle 25°, and counting time 30 live seconds.
Statistical analyses
Statistical analysis of the data was performed using the statistical package Statgraphics 2.1 (StatPoint, Inc., St Louis, MO, USA). Normal distribution of data was checked by the Shapiro–Wilk test at P ≤ 0.05. Where appropriate, data are indicated as means (±SD). Data were analysed by multifactorial analysis of variance using variety, Mn treatment and mycorrhizal inoculation as main factors. It must be noted, however, that the varieties DFM and DFG were not replicated and thus, served only to indicate the genetic variability between different P. menziesii seedlots. Differences among means were detected by LSD multiple range test and were considered to be significant if P ≤ 0.05.
For the EDX analyses, the Shapiro–Wilk test revealed that some data sets were not coming from a normal distribution. These results are, therefore, shown as box plots, with the box displaying the 10% to 90% range of the data, the full square of the mean and the horizontal line of the median. To compare differences between medians, the Mann–Whitney test was used.
Results
Growth, biomass and mycorrhizal colonisation
Douglas fir seedlings were pre-cultured for 2 months in flasks with R. subareolatus and subsequently for 3 months with one additional inoculation with R. subareolatus hyphae in sand culture before exposure to excess Mn. At the end of this 5-month-exposure phase, DFG seedlings grown in the presence of R. subareolatus had shorter shoots than those grown in the absence of this fungus (Table 1). At harvest of 10-month-old seedlings, the initial negative influence of R. subareolatus on shoot height had disappeared (Table 1). Exposure to excess Mn in the nutrient solution did not affect shoot heights (Table 1).
Table 1.
Shoot height (mm) of Douglas fir (Pseudotsuga menziesii) seedlings of the variety menziesii (DFM) and the variety glauca (DFG)
| DFM | DFG | |||
|---|---|---|---|---|
| 5-month-old | 10-month-old | 5-month-old | 10-month-old | |
| Non-inoculated | 85.7 ± 15.4c | 253.2 ± 38.7e | 41.2 ± 8.8b | 132.6 ± 3.1d |
| Rhizopogon | 65.6 ± 23.4bc | 265.5 ± 22.8e | 29.0 ± 7.9a | 103.9 ± 15.4d |
| Non-inoculated + Mn | na | 241.2 ± 23.6e | na | 119.8 ± 14.4d |
| Rhizopogon + Mn | na | 241.2 ± 23.7e | na | 115.8 ± 29.8d |
Seedlings were grown in the presence or absence of Rhizopogon subareolatus for 5 months, and subsequently, a subset of each treatment was irrigated with 10 mM Mn in the nutrient solution once a week for a further period of 5 months. Data indicate means (n = 10, ±SD). Different letters indicate significant differences at P ≤ 0.05.
na Not applicable
We observed furthermore for non-inoculated, non-stressed DFM plants that shoots were about twice as tall and biomass that was about 2.5 times higher than that of similarly treated DFG (DFM: 11.0 ± 2.4 g per plant and DFG: 4.4 ± 0.8 g per plant). Such growth differences have already been reported previously for seedlings of the two varieties grown in hydroponic solutions (Dučić et al. 2006).
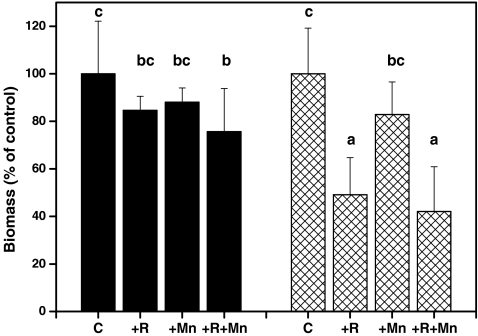
Because of the large differences in biomass between DFG and DFM, the relative changes were compared to elucidate treatment effects (Fig. 1). For this comparison, the final biomass of non-inoculated, non-stressed seedlings of each variety was set to 100%. The DFM seedlot showed a reduction in biomass (−25%) only when inoculated with R. subareolatus and treated with Mn (Fig. 1). In the DFG seedlot, inoculation with R. subareolatus decreased growth either in the presence or absence of excess Mn (−50%), but Mn had no effect on biomass (Fig. 1).
Fig. 1.
Relative changes in biomass in response to mycorrhization with R. subareolatus and exposure to Mn stress in Douglas fir seedlings (P. menziesii) of the variety menziesii (black) and glauca (hatched). C Non-inoculated control, R inoculated with R. subareolatus, Mn exposed to 10 mM Mn once a week for 5 months. Bars indicate means (±SD). Different letters indicate significant differences at P ≤ 0.05
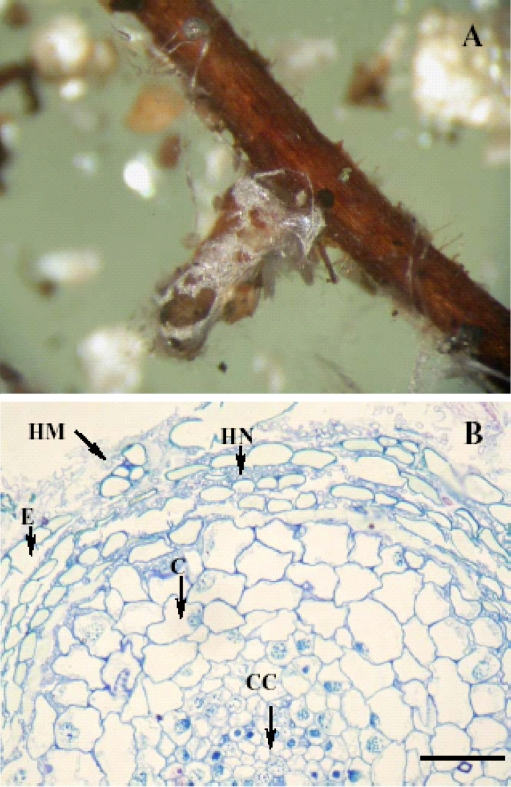
We suspected that high degrees of mycorrhization might have resulted in large carbon sinks, thereby affecting growth of the two Douglas varieties. In contrast to this assumption, analysis of mycorrhization rates showed that DFM, the variety with higher biomass production, exhibited significantly higher percentage of mycorrhizal colonisation of root tips than DFG (Table 2). Non-inoculated plants also showed low colonisation with ectomycorrhiza, indicating that unintended spreading of ectomycorrhiza was not completely avoided during long-term maintenance in sand culture (Table 2). Only one mycorrhizal morphotype was found on Douglas roots of all experimental treatments (Fig. 2a) and was classified as Rhizopogon sp. (Molina and Trappe 1994; Parladé et al. 1995). Cross sections of mycorrhizal root tips were also examined (Fig. 2b). A typical example, displaying a Hartig net characteristic for vital, functional mycorrhizas, is shown in Fig. 2b. Differences between the varieties or changes of the appearance of the mycorrhizas under the influence of excess Mn were not observed (not shown).
Table 2.
Mycorrhizal colonisation (% of root tips) of Douglas fir (Pseudotsuga menziesii) variety menziesii (DFM) and variety glauca (DFG)
| DFM | DFG | |
|---|---|---|
| Non-inoculated | 8.0 ± 0.8b | 0.7 ± 0.5a |
| Rhizopogon | 31.7 ± 4.5d | 8.0 ± 2.2b |
| Non-inoculated + Mn | 6.3 ± 2.6ab | 3.0 ± 1.4ab |
| Rhizopogon + Mn | 16.0 ± 5.4c | 6.7 ± 1.7ab |
Plants were cultured in the absence or presence of R. subareolatus for 10 months. Inoculated and non-inoculated plants were exposed to 10 mM Mn in the nutrient solution once week during 5 months. The whole root system of n = 3 plant per treatments was analysed. Different letters indicate significant differences at P ≤ 0.05.
Fig. 2.
Typical mycorrhizal morphotype found on both varieties of Douglas fir seedlings (P. menziesii) var. glauca and var. menziesii (a). Cross section of a mycorrhizal root tip of Douglas fir with R. subareolatus (b). HM Hyphae mantel, HN Hartig net, E epidermis cell, C cortex cell, CC central cylinder. Bar indicates 0.1 mm
Variety, inoculation with R. subareolatus and Mn stress significantly affected the mycorrhization rate (Table 2). The significant interaction of the factors variety × Mn stress (P = 0.001) and inoculation × Mn stress (P = 0.006) showed that exposure to excess Mn was associated with significantly less colonisation of root tips of DFM but not of DFG (Table 2).
Nutrient element concentrations and their subcellular localisation in roots
To investigate whether mycorrhiza and Mn stress affected the nutrient status of Douglas fir, element analyses were conducted in needles and roots (Table 3, see also Appendix 1). Exposure to excess Mn resulted in significant accumulation of Mn in roots and needles of the seedlings (Table 3). Mycorrhization with R. subareolatus did not prevent this increase (Table 3). The two varieties showed interesting differences in Mn-accumulation patterns: Under normal moderate Mn supply, DFG accumulated more Mn than DFM (P (variety) = 0.014), whereas the opposite was true under when the seedlings were exposed to excess Mn (P (variety × Mn stress) = 0.001).
Table 3.
Concentrations of Mn and P in roots and needles of Douglas fir (Pseudotsuga menziesii) variety menziesii (DFM) and variety glauca (DFG) after exposure to 10 mM for 5 months and plants inoculated with Rhizopogon subareolatus or non-inoculated plants
| Mn in roots (mg g−1 DM) | Mn in needles (mg g−1 DM) | P in roots (mg g−1 DM) | P in needles (mg g−1 DM) | |||||
|---|---|---|---|---|---|---|---|---|
| DFM | DFG | DFM | DFG | DFM | DFG | DFM | DFG | |
| Non-inoculated | 0.21 ± 0.13a | 0.46 ± 0.07a | 0.99 ± 0.14a | 1.50 ± 0.40a | 1.73 ± 1.23a | 8.11 ± 1.35ef | 6.63 ± 1.20a | 10.64 ± 1.68bc |
| Rhizopogon | 0.46 ± 0.23a | 0.61 ± 0.04ab | 0.78 ± 0.13a | 1.06 ± 0.07a | 4.37 ± 0.27bc | 9.63 ± 1.11f | 10.97 ± 0.83c | 9.82 ± 2.07abc |
| Non-inoculated + Mn | 2.58 ± 0.46d | 1.15 ± 0.24bc | 6.93 ± 1.60c | 2.53 ± 0.98ab | 2.75 ± 0.15ab | 7.32 ± 1.00de | 6.99 ± 1.54ab | 6.92 ± 1.73ab |
| Rhizopogon + Mn | 2.10 ± 0.30d | 1.28 ± 0.55c | 5.67 ± 1.40c | 3.55 ± 0.76b | 5.45 ± 0.68cd | 8.41 ± 1.59ef | 10.61 ± 1.26bc | 10.33 ± 3.31bc |
Data indicate means (n = 3 replicates per treatment ± SD). Each replicate is a pooled sample of tissue from 3 different plants. Different letters show significant differences at P ≤ 0.05.
Since many studies have shown that mycorrhization increases the phosphorus supply (Koide 1991), we focused on this nutrient. Other elements (S, Ca, K, Mg and Fe) were also determined (see Appendix 1) and did not reveal any nutrient deficiencies under any of our experimental treatments. The phosphorus concentrations in roots were much higher in DFG than in DFM in all treatments (P (variety) ≤ 0.001, Table 3). Seedlings of DFM inoculated with R. subareolatus contained higher P concentrations in roots and needles than non-inoculated control plants, whereas in DFG, the P concentrations remained unaffected (Table 3). Exposure to excess Mn did not influence P concentrations in any of the tissues studied (Table 3).
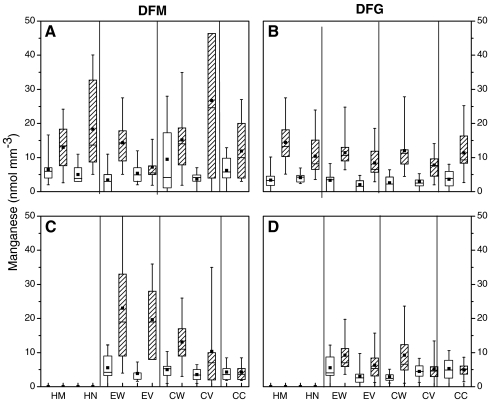
To find out whether seedlings from different seedlots differed in the subcellular localisation of Mn and other nutrients, we investigated cross sections of root tips employing energy-dispersive X-ray microanalyses (Fig. 3). Mycorrhizal non-stressed plants of DFM (Fig. 3a) and DFG (Fig. 3b) showed a relatively homogenous distribution of Mn across all tissues. There were neither pronounced differences between Mn concentrations in root cell walls or in vacuoles nor between these plant tissues and fungal tissues such as hyphal mantle or Hartig net. Non-mycorrhizal non-stressed plants of the seedlot DFG displayed higher Mn concentrations in the cortical vacuole than those of mycorrhizal seedlings (P = 0.016, Fig. 3b,d). Overall, there were no important differences between mycorrhizal and non-mycorrhizal tissues.
Fig. 3.
Box-and-whisker diagrams depicting the range of Mn concentrations in cell walls and vacuoles of different cell types in roots and fungal tissues of mycorrhizal and non-mycorrhizal Douglas fir plants (P. menziesii) of the varieties menziesii (DFM) and glauca (DFG). Mn stress: hatched boxes. DFM: Mycorrhizal (A) and non-mycorrhizal root tips (C). DFG: Mycorrhizal (B) and non-mycorrhizal root tips (D). HM Hyphal mantle, HN Hartig net, EW epidermal cell wall, EV epidermal cell vacuole, CW cortical cell wall, CV cortical cell vacuole, CC central cylinder, whole cells. (n = 15)
Exposure to excess Mn resulted in some tissue- and seedlot-specific effects. In DFM, the hyphal mantle (P ≤ 0.001), Hartig net (P ≤ 0.001), epidermal wall (P ≤ 0.001), cortical vacuole (P = 0.004) and central cylinder (P = 0.027) accumulated significantly higher Mn concentrations than these tissues of non-challenged plants (Fig. 3a). No Mn accumulation was found in the epidermal vacuoles of DFM exposed to Mn stress (P = 0.341), whereas the vacuoles of the cortex cells (P = 0.004) showed large variability in Mn content under these conditions (Fig. 3a). In mycorhizal plants of DFG exposed to elevated Mn, all subcellular locations showed increased Mn concentrations (P ≤ 0.001), and no compartment-specific effect were observed (Fig. 3b).
After exposure to Mn stress, non-mycorrhizal DFM roots showed a clear gradient of Mn concentrations from outside the root into the stele (Fig. 3c). The highest Mn concentrations were present in the epidermis (cell wall and vacuoles, P ≤ 0.001) and cortical cell walls (P ≤ 0.001), but a clear decrease to levels present in non-stressed roots was found in the central cylinder (P = 0.9840, Fig. 3c). In non-mycorrhizal root tips of DFG, Mn accumulation was found in epidermal vacuoles (P = 0.009) and cortical cell walls (P ≤ 0.001). However, compared with DFM, the extent of Mn accumulation was modest in DFG. Other tissues of DFG showed no increase in response to excess Mn (P = 0.8819, Fig. 3d).
The concentrations of other nutrients in different subcellular locations were not affected by exposure to excess Mn, with the exception of sulphur (Appendix 2). Sulphur concentrations were increased in most locations in roots of Mn-stressed DFG seedlings, an effect probably caused by using MnSO4 solutions to expose the plants to high Mn (Appendix 2).
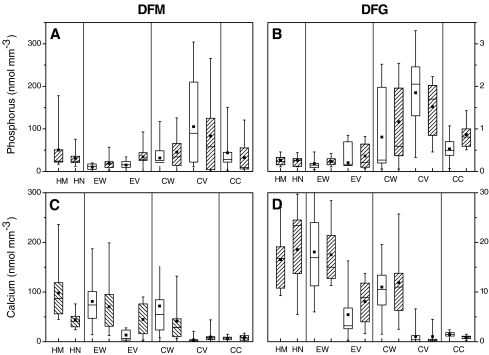
Elements like phosphorus and calcium showed seed lot- and cell type-specific patterns (Fig. 4). The P concentrations were barely affected by mycorrhization in DFG (Fig. 4b,d) but were increased in DFM epidermal walls and epidermal vacuoles of mycorrhizal compared with non-mycorrhizal plants (P = 0.013, P = 0.009, Fig. 4a,c). In DFM roots, phosphorus concentrations were higher in cortical locations and the developing vascular tissues than in the epidermis (P ≤ 0.001) and showed no clear gradient throughout fungal and root tissues (Fig. 4a). In DFG, phosphorus concentrations were higher than in DFM and increased towards the endodermal barrier (P ≤ 0.001), especially in the vacuoles of the cortex cells (Fig. 4b).
Fig. 4.
Box-and-whisker diagrams depicting the range of phosphorus (A, B) and calcium concentrations (C, D) in cell walls and vacuoles of different cell types in roots and fungal tissues of mycorrhizal and non-mycorrhizal Douglas fir (P. menziesii) plants of the varieties menziesii (DFM: A, C) and glauca (DFG: B, D). Hatched boxes indicate mycorrhizal and open boxes non-mycorrhizal root tips. HM Hyphal mantle, HN Hartig net, EW epidermal cell wall, EV epidermal cell vacuole, CW cortical cell wall, CV cortical cell vacuole, CC central cylinder, whole cells. (n = 15)
In contrast to phosphorus or manganese, calcium showed a clear gradient in DFM roots with decreasing concentrations from the hyphal mantle to the epidermis (P = 0.014), the cortical tissues (P ≤ 0.001) and the vascular tissue (P = 0.035, Fig. 4c). In DFG, accumulation of Ca in cell walls was higher than in DFM (P ≤ 0.001), and a pronounced decrease from outside to inside the root was also observed (P (fungal tissue–epidermis) = 0.015, P (epidermis–cortex) = ≤0.001, P (cortex–vascular system) = 0.044, Fig. 4d).
In most cases, cell-type-specific element concentrations were unaffected by mycorrhization (Appendix 2). The only exception was iron in DFM roots, which accumulated in the hyphal mantle and was significantly enriched in the epidermal vacuole (Appendix 2).
Discussion
Effect of mycorrhiza on Douglas fir growth and nutrition
A main result of this study was that the ectomycorrhiza-forming fungus R. subareolatus did not stimulate growth and biomass production in young Douglas fir seedlings of any of the two seedlots of DFG and DFM studied here (Table 1, Fig. 1). Since earlier investigations demonstrated positive effects of mycorrhization on Douglas fir with R. subareolatus and other Rhizopogon species (Castellano 1996; Parladé et al. 1999; Pera et al. 1999), the detrimental effect of the fungus on DFG in our experiment was unexpected. Growth depressions associated with mycorrhizal development have generally been attributed to the carbohydrate drain to the mycorrhizal fungus, while positive growth effects of mycorrhiza are thought to occur when the benefits of increased nutrient uptake exceed the carbon cost of the association (Schroeder and Janos 2004; Thomson et al. 1994). Mycorrhizal establishment leads to an increase in the demand for carbohydrates for fungal maintenance and growth (Dosskey et al. 1990; Hampp et al. 1999; Reid et al. 1983). For example, it was shown that Pisolithus tinctorius acted as a strong C sink during the early stages of the symbiosis (Cairney and Chambers 1997). Therefore, plants may react differently to ectomycorrhiza formation depending on their age and their initial nutritional status (Corrêa et al. 2006). In fact, ectomycorrhizal symbioses can enhance plant growth (Burgess et al. 1994; Lu et al. 1998), have no effect (Bâ et al. 1999; Thomson et al. 1994) or can even reduce growth, especially that of seedlings (Colpaert et al. 1992; Eltrop and Marschner 1996).
It was nevertheless surprising that the two seed lots belonging to Douglas fir varieties DFG and DFM differed so strongly in their growth response to inoculation with R. subareolatus. The relatively slow growth of DFG and its increased sensitivity to R. subareolatus were not caused by nutrient deficits (Table 4, Appendix 1). By contrast, most of the nutrient elements showed higher concentrations in DFG than in DFM, and inoculation with the symbiotic fungus had either no effect or even increased the concentrations of nutrients. Whether this positive effect was caused by improved fungal supply of nutrients to its host is not clear. Another reason could be the decreased plant-internal demand for nutrients because of growth suppression. In this case, lacking “dilution” of nutrients by growth and continued uptake would lead to increasing tissue concentrations. This explanation appears likely, at least for DFG, which showed only a low degree of ectomycorrhiza but strong growth inhibition and is furthermore supported by the observation that major P accumulation sites in DFG were the vacuoles of the cortex cells where this compound would be ineffective as nutrient (Fig. 4).
The finding that DFG with the strongest growth reduction showed much lower mycorrhization rates than DFM, whose growth was less affected by interaction with R. subareolatus, seems to contradict the idea that a higher degree of ectomycorrhizal colonisation acts as a stronger carbon sink to its host than a lower degree of ectomycorrhiza. It is possible that this seed lot DFG is a poor host for this fungal species. In this case, infection with R. subareolatus may activate defence reactions, which in turn would consume substantial amounts of assimilates and, thus, lead to the observed growth reductions. Although this is a valid possibility, we have no evidence for such defence reactions. For example, interaction of Populus × canescens with an incompetent strain of Paxillus involutus resulted in cell wall thickenings of epidermal cells, clearly indicating the formation of barriers against the invading fungus (Gafur et al. 2004). However, such formations were not observed in DFG–R. subareolatus interactions (Fig. 2). Indeed, earlier field studies indicated lower degrees of ectomycorrhizal colonisation of DFG than those of DFM trees (Linnemann 1960). Therefore, differences in mycorrhiza formation of populations of Douglas fir, represented by two varieties, appear innate. But the reasons for such differences remain unknown. The present data suggest that the presence of ectomycorrhiza-forming fungi in the soil may already constitute a carbon sink, even if a functional mycorrhiza has not yet been developed.
Effect of mycorrhiza on the response of Douglas fir to excess manganese
In this study, we confirmed that Douglas fir like other conifers is relatively Mn tolerant (St Clair and Lynch 2004; Dučić and Polle 2007). The concentrations of Mn accumulated under the present experiment conditions in needles of DFM (about 7 mg g−1 dry mass) and DFG (about 3.5 mg g−1 dry mass) were close to those found in needles of injured Douglas firs suffering from internal bark necrosis on field sites (about 8 mg g−1 dry mass, Schöne 1992), whereas such symptoms did not occur in the present study. An important result of this study was that ectomycorrhizas formed with R. subareolatus did not prevent Mn accumulation in any of the two varieties. This might have been expected since the mycorrhization rates were low, especially those of DFG (Table 3). Thus, the operation of mechanisms affording protection from heavy metals such as extracellular metal precipitation or complexation, sorption to cell walls or extracellular polysaccharides and decreased transport (Gadd 1992; Jentschke and Godbold 2000) would have been limited to a small percentage of root tips. Moreover, X-ray microanalysis did not provide any evidence that ectomycorrhizas of R. subareolatus might provide a barrier against Mn uptake. Our results suggest that this interaction might even facilitate transport of Mn into the root tissue since the Mn concentrations were highest in cortical vacuoles of mycorrhizal roots, whereas in non-mycorrhizal roots, highest Mn concentrations were found in epidermal cell walls and vacuoles (Fig. 3). Still, other cations, i.e. Ca and Fe, were enriched in R. subareolatus hyphae compared with cell walls or other locations in roots cells, indicating that a potential for cation accumulation is present in the fungus (Fig. 4, Appendix 2).
In previous studies on other species, X-ray microanalysis of hyphae showed the presence of soluble polyphosphate in the vacuole and formation of polyphosphate granules after exposure to Ni, Zn, Cd, Pb and Cr, a process leading to sequestration of these heavy metals (Kunst and Roomans 1985; Turnau et al. 1993; Bücking and Heyser 2000). Similar detoxification mechanisms were found for Mn in non-mycorrhizal Douglas fir roots (Dučić and Polle 2007). In the mycorrhizal roots of DFM and DFG studied here, Mn did not accumulate together with elevated P levels. However, the overall vacuolar P concentrations were always higher than those of Mn and thus, may have contributed to Mn complexation.
In conclusion, DFM and DFG showed population related differences in colonisation with the ectomycorrhizal fungus R. subareolatus. However, during the first year of seedling growth, higher mycorrhization rates were neither positive for plant growth nor protected from excess Mn in this study.
Acknowledgements
We are grateful to the German Science Foundation (DFG) for financial support (Po362/14). We thank Theres Riemekasten and Mariane Smiatacz for excellent technical assistance. We are grateful to two anonymous reviewers for helpful comments.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Appendix 1
Table 4.
Element concentrations in roots and needles in Douglas fir (Pseudotsuga menziesii) variety menziesii and variety glauca after inoculation with Rhizopogon subareolatus and treatment with 10 mM manganese and after inoculation with R. subareolatus
| Concentration in root (mg g−1) | Concentration in needles (mg g−1) | |||
|---|---|---|---|---|
| DFM | DFG | DFM | DFG | |
| Ca | ||||
| Non-inoculated | 1.49 ± 0.95 | 2.58 ± 0.19 | 3.21 ± 0.17 | 2.95 ± 0.46 |
| Rhizopogon | 1.91 ± 0.17 | 1.60 ± 0.14 | 3.05 ± 0.80 | 2.58 ± 0.24 |
| Non-inoculated + Mn | 3.25 ± 0.90 | 4.01 ± 1.43 | 4.34 ± 0.42 | 2.79 ± 0.45 |
| Rhizopogon + Mn | 1.97 ± 0.21 | 1.96 ± 0.34 | 4.14 ± 0.72 | 3.39 ± 0.14 |
| P (main effect) | ||||
| Variety | 0.292 | 0.006 | ||
| Inoculation | 0.014 | 0.890 | ||
| Mn stress | 0.022 | 0.009 | ||
| P (interaction) | ||||
| Variety × inoculation | 0.145 | 0.550 | ||
| Variety × Mn stress | 0.987 | 0.122 | ||
| Inoculation × Mn stress | 0.067 | 0.343 | ||
| All factors | 0.661 | 0.300 | ||
| S | ||||
| Non-inoculated | 1.33 ± 0.81 | 2.18 ± 0.45 | 2.90 ± 0.37 | 1.86 ± 0.35 |
| Rhizopogon | 4.38 ± 0.50 | 3.62 ± 1.61 | 8.66 ± 0.96 | 3.37 ± 0.80 |
| Non-inoculated + Mn | 1.65 ± 0.11 | 1.61 ± 0.19 | 1.99 ± 0.39 | 1.67 ± 0.16 |
| Rhizopogon + Mn | 3.04 ± 0.43 | 2.05 ± 0.37 | 7.72 ± 0.78 | 3.70 ± 0.33 |
| P (main effect) | ||||
| Variety | 0.524 | 0.000 | ||
| Inoculation | 0.042 | 0.163 | ||
| Mn stress | 0.000 | 0.000 | ||
| P (interaction) | ||||
| Variety × inoculation | 0.439 | 0.106 | ||
| Variety × Mn stress | 0.930 | 0.000 | ||
| Inoculation × Mn stress | 0.081 | 0.696 | ||
| All factors | 0.654 | 0.642 | ||
| K | ||||
| Non-inoculated | 7.24 ± 4.38 | 12.57 ± 4.39 | 13.46 ± 0.59 | 9.03 ± 0.48 |
| Rhizopogon | 10.60 ± 1.13 | 13.22 ± 3.29 | 14.36 ± 2.14 | 8.68 ± 1.02 |
| Non-inoculated + Mn | 10.05 ± 0.74 | 12.39 ± 1.77 | 13.90 ± 0.88 | 9.06 ± 1.51 |
| Rhizopogon + Mn | 10.18 ± 0.30 | 11.25 ± 2.69 | 14.92 ± 2.68 | 10.53 ± 1.97 |
| P (main effect) | ||||
| Variety | 0.057 | 0.000 | ||
| Inoculation | 0.964 | 0.379 | ||
| Mn stress | 0.597 | 0.354 | ||
| P (interaction) | ||||
| Variety × inoculation | 0.425 | 0.784 | ||
| Variety × Mn stress | 0.484 | 0.808 | ||
| Inoculation × Mn stress | 0.380 | 0.551 | ||
| All factors | 0.796 | 0.605 | ||
| Mg | ||||
| Non-inoculated | 0.76 ± 0.50 | 1.86 ± 0.67 | 2.78 ± 0.61 | 3.16 ± 0.71 |
| Rhizopogon | 1.26 ± 0.08 | 1.34 ± 0.60 | 3.72 ± 0.32 | 2.70 ± 0.20 |
| Non-inoculated + Mn | 2.09 ± 0.36 | 2.25 ± 1.07 | 3.54 ± 0.26 | 3.21 ± 0.47 |
| Rhizopogon + Mn | 1.20 ± 0.28 | 1.82 ± 0.84 | 4.67 ± 0.76 | 3.81 ± 0.58 |
| P (main effect) | ||||
| Variety | 0.135 | 0.104 | ||
| Inoculation | 0.105 | 0.015 | ||
| Mn stress | 0.296 | 0.052 | ||
| P (interaction) | ||||
| Variety × inoculation | 0.752 | 0.607 | ||
| Variety × Mn stress | 0.654 | 0.087 | ||
| Inoculation × Mn stress | 0.310 | 0.258 | ||
| All factors | 0.255 | 0.424 | ||
| Fe | ||||
| Non-inoculated | 0.25 ± 0.17 | 0.58 ± 0.18 | 0.05 ± 0.01 | 0.04 ± 0.00 |
| Rhizopogon | 0.49 ± 0.13 | 0.69 ± 0.36 | 0.03 ± 0.00 | 0.04 ± 0.00 |
| Non-inoculated + Mn | 0.64 ± 0.16 | 0.90 ± 0.32 | 0.05 ± 0.00 | 0.05 ± 0.01 |
| Rhizopogon + Mn | 0.37 ± 0.04 | 0.82 ± 0.36 | 0.04 ± 0.01 | 0.04 ± 0.00 |
| P (main effect) | ||||
| Variety | 0.022 | 0.314 | ||
| Inoculation | 0.164 | 0.086 | ||
| Mn stress | 0.989 | 0.002 | ||
| P (interaction) | ||||
| Variety × inoculation | 0.732 | 0.857 | ||
| Variety × Mn stress | 0.914 | 0.405 | ||
| Inoculation × Mn stress | 0.179 | 0.764 | ||
| All factors | 0.522 | 0.132 | ||
P values of the ANOVAs show differences between varieties, mycorrhiza and Mn treatment and their interactions n = 3 (±SD).
Bold letters indicate significant P-values
Appendix 2
Table 5.
Element concentrations in different subcellular locations in mycorrhizal and non-mycorrhizal root tips of Douglas fir (Pseudotsuga menziesii) variety menziesii (DFM) and variety glauca (DFG)
| Sulfur | Potassium | Magnesium | Iron | ||||||
|---|---|---|---|---|---|---|---|---|---|
| DFM | DFG | DFM | DFG | DFM | DFG | DFM | DFG | ||
| Non-inoculated | Epidermis CW | 18.1 ± 1.3 | 19.0 ± 1.8 | 17.6 ± 8.5 | 15.2 ± 3.0 | 26.6 ± 2.0 | 31.4 ± 7.8 | 4.8 ± 1.4 | 8.0 ± 2.4 |
| Epidermis vacuole | 28.3 ± 2.2 | 13.9 ± 7.5 | 216.5 ± 100.2 | 7.2 ± 2.6 | 21.5 ± 4.0 | 19.6 ± 9.0 | 4.5 ± 1.2 | 5.8 ± 2.2 | |
| Cortex CW | 16.7 ± 1.9 | 18.1 ± 3.9 | 154.6 ± 47.0 | 64.7 ± 30.0 | 10.5 ± 2.4 | 20.4 ± 6.9 | 4.6 ± 1.9 | 6.4 ± 1.2 | |
| Cortex vacuole | 65.2 ± 39.4 | 3.5 ± 1.8 | 188.4 ± 44.0 | 110.7 ± 42.8 | 15.9 ± 3.7 | 15.7 ± 9.5 | 6.3 ± 0.8 | 2.3 ± 0.4 | |
| Central cylinder | 8.3 ± 3.2 | 19.2 ± 3.0 | 36.1 ± 27.2 | 79.0 ± 16.8 | 7.8 ± 2.2 | 17.3 ± 2.5 | 3.2 ± 1.3 | 2.6 ± 0.2 | |
| Rhizopogon | Hyphae mantel | 57.8 ± 6.5 | 37.7 ± 3.6 | 27.3 ± 20.5 | 98.2 ± 58.4 | 37.6 ± 3.1 | 28.8 ± 3.7 | 16.3 ± 7.0 | 8.2 ± 3.0 |
| Hartig net | 44.9 ± 4.8 | 47.1 ± 5.9 | 14.9 ± 6.3 | 115.3 ± 72.2 | 30.1 ± 8.2 | 37.0 ± 6.4 | 9.8 ± 3.3 | 8.7 ± 2.7 | |
| Epidermis CW | 19.6 ± 5.8 | 22.0 ± 1.6 | 28.3 ± 20.6 | 126.5 ± 82.9 | 23.9 ± 11.9 | 34.9 ± 0.2 | 6.4 ± 2.4 | 4.6 ± 1.4 | |
| Epidermis vacuole | 38.1 ± 7.8 | 29.1 ± 8.8 | 91.3 ± 87.2 | 106.3 ± 79.5 | 21.4 ± 3.7 | 24.1 ± 4.1 | 16.6 ± 7.4 | 5.8 ± 0.3 | |
| Cortex CW | 32.1 ± 1.9 | 17.6 ± 10.0 | 126.4 ± 82.6 | 23.5 ± 13.8 | 17.2 ± 7.4 | 22.7 ± 12.4 | 5.4 ± 2.2 | 6.7 ± 3.2 | |
| Cortex vacuole | 40.0 ± 11.1 | 14.7 ± 12.0 | 103.2 ± 59.4 | 138.4 ± 24.4 | 6.6 ± 3.7 | 24.5 ± 12.2 | 6.5 ± 2.5 | 4.0 ± 1.9 | |
| Central cylinder | 13.8 ± 2.9 | 17.6 ± 14.4 | 57.0 ± 28.3 | 99.4 ± 41.6 | 6.6 ± 3.4 | 16.3 ± 2.0 | 5.1 ± 2.0 | 3.5 ± 0.0 | |
| Non-inoculated + Mn | Epidermis CW | 42.2 ± 12.4 | 23.1 ± 6.3 | 36.0 ± 22.3 | 38.9 ± 16.1 | 19.1 ± 7.8 | 18.4 ± 4.9 | 5.8 ± 2.5 | 25.3 ± 17.7 |
| Epidermis vacuole | 42.5 ± 18.7 | 49.9 ± 26.4 | 71.7 ± 44.5 | 338.7 ± 333.2 | 17.4 ± 7.0 | 21.9 ± 7.1 | 3.7 ± 1.9 | 14.1 ± 8.6 | |
| Cortex CW | 54.6 ± 24.1 | 34.3 ± 7.5 | 174.3 ± 96.0 | 110.8 ± 80.5 | 18.2 ± 6.9 | 17.2 ± 4.4 | 7.6 ± 5.3 | 4.2 ± 1.1 | |
| Cortex vacuole | 32.4 ± 6.7 | 56.0 ± 34.3 | 96.2 ± 28.6 | 102.6 ± 82.2 | 6.0 ± 1.7 | 11.7 ± 2.9 | 2.9 ± 0.9 | 5.6 ± 1.0 | |
| Central cylinder | 34.9 ± 12.5 | 26.7 ± 4.1 | 111.0 ± 52.6 | 17.2 ± 9.7 | 11.9 ± 3.1 | 9.1 ± 0.8 | 1.9 ± 0.4 | 9.5 ± 5.9 | |
| Rhizopogon + Mn | Hyphae mantel | 35.7 ± 3.8 | 44.5 ± 15.2 | 10.3 ± 1.5 | 19.1 ± 12.2 | 19.9 ± 7.6 | 29.2 ± 11.8 | 13.6 ± 3.7 | 15.6 ± 4.7 |
| Hartig net | 38.0 ± 5.0 | 42.7 ± 18.0 | 12.5 ± 3.5 | 26.7 ± 19.6 | 36.0 ± 8.1 | 26.1 ± 11.9 | 31.8 ± 20.2 | 16.0 ± 7.8 | |
| Epidermis CW | 24.5 ± 7.3 | 22.0 ± 3.0 | 20.1 ± 10.2 | 13.9 ± 4.1 | 29.7 ± 10.7 | 16.5 ± 3.1 | 14.3 ± 1.7 | 7.7 ± 1.0 | |
| Epidermis vacuole | 25.9 ± 7.4 | 42.5 ± 13.0 | 22.8 ± 18.1 | 7.5 ± 2.5 | 17.9 ± 5.6 | 26.8 ± 8.2 | 5.8 ± 0.9 | 10.3 ± 1.8 | |
| Cortex CW | 24.3 ± 10.3 | 23.7 ± 4.7 | 90.9 ± 31.8 | 24.1 ± 8.7 | 24.3 ± 8.8 | 17.8 ± 4.5 | 17.2 ± 11.0 | 7.9 ± 2.0 | |
| Cortex vacuole | 14.9 ± 8.9 | 45.0 ± 14.0 | 132.1 ± 2.1 | 76.7 ± 33.9 | 8.2 ± 2.2 | 29.0 ± 11.7 | 2.7 ± 0.6 | 4.6 ± 1.7 | |
| Central cylinder | 35.1 ± 19.4 | 74.3 ± 26.6 | 121.0 ± 66.0 | 69.3 ± 27.9 | 12.3 ± 7.0 | 23.6 ± 10.4 | 4.2 ± 2.2 | 4.3 ± 1.1 | |
| Statistic—main effect | |||||||||
| Variety | 0.7021 | 0.7091 | 0.1408 | 0.8748 | |||||
| Main effects | |||||||||
| A: Cell type | 0.5764 | 0.6418 | 0.0402 | 0.7936 | 0.0105 | 0.5410 | 0.2158 | 0.3168 | |
| B: Rhizopogon | 0.2372 | 0.5051 | 0.2069 | 0.6381 | 0.6694 | 0.1240 | 0.0225 | 0.3243 | |
| C: Mn stress | 0.4203 | 0.0020 | 0.5555 | 0.9460 | 0.8230 | 0.3062 | 0.8732 | 0.0800 | |
| Interactions | |||||||||
| AB | 0.8172 | 0.7090 | 0.6670 | 0.7144 | 0.8646 | 0.7874 | 0.6788 | 0.5424 | |
| AC | 0.1025 | 0.3598 | 0.2416 | 0.7025 | 0.6808 | 0.4714 | 0.0742 | 0.6816 | |
| BC | 0.1626 | 0.8792 | 0.6668 | 0.1407 | 0.3979 | 0.6120 | 0.7311 | 0.3417 | |
| ABC | 0.7244 | 0.4239 | 0.7618 | 0.4665 | 0.9190 | 0.8705 | 0.3941 | 0.8460 | |
The seedlings were analysed 10 months after inoculation with Rhizopogon subareolatus and 5 months after the start of Mn stress. Cross sections were analysed by transmission electron microscopy coupled with energy-dispersive X-ray microanalyses (EDX). The concentrations obtained by EDX refer to volume units of the embedded specimen (nmol mm−3). n = 18–27 (±SE). Results of statistical analysis for cell-type-specific effects of mycorrhization and Mn stress are shown. Multivariate analysis of variance was conducted for cell type, inoculation with R. subareolatus and Mn stress as main effects in each variety separately. Two-sample comparison and t test was employed to compare the varieties.
Bold letters indicate significant P-values
References
- Álvarez IF, Parladé J, Trappe JM, Castellano MA. Hypogeous mycorrhizal fungi of Spain. Mycotaxon. 1993;47:201–217. [Google Scholar]
- Arines J, Vilarino A, Sainz M. Effect of different inocula of vesicular–arbuscular mycorrhizal fungi on manganese content and concentration in red-clover (Trifolium Pratense L) plants. New Phytol. 1989;112:215–219. doi: 10.1111/j.1469-8137.1989.tb02376.x. [DOI] [Google Scholar]
- Bâ AM, Sanon KB, Duponnois R, Dexheim J. Growth response of Afzelia africana Sm. seedlings to ectomycorrhizal inoculation in a nutrient-deficient soil. Mycorrhiza. 1999;9:91–95. doi: 10.1007/s005720050292. [DOI] [Google Scholar]
- Bethlenfalvay GJ, Franson RL. Manganese toxicity alleviated by mycorrhizae in soybean. J Plant Nutr. 1989;12:953–970. [Google Scholar]
- Blaudez D, Jacob C, Turnau K, Colpaert JV, Ahonen-Jonnarth U, Finlay R, Botton B, Chalot M. Differential responses of ectomycorrhizal fungi to heavy metals in vitro. Mycol Res. 2000;104:1366–1371. doi: 10.1017/S0953756200003166. [DOI] [Google Scholar]
- Block J (1997) Schadsituation der Douglasie in Rheinland-Pfalz. Stand der Ursachenforschung zu Douglasienschäden-derzeitige Empfehlungen für die Praxis. Mitteilungen der Forstlichen Forschungsanstalt Rheinland-Pfalz 41, Trippstadt, pp 46–75
- Bücking H, Heyser W. Subcellular compartmentation of elements in non-mycorrhizal and mycorrhizal roots of Pinus sylvestris: an X-ray microanalytical study. I. The distribution of phosphate. New Phytol. 2000;145:311–320. doi: 10.1046/j.1469-8137.2000.00572.x. [DOI] [Google Scholar]
- Burgess T, Dell B, Malajczuk N. Variation in mycorrhizal development and growth stimulation of 20 Pisolithus isolates inoculated onto Eucalyptus grandis W. Hill ex Maiden. New Phytol. 1994;127:731–739. doi: 10.1111/j.1469-8137.1994.tb02977.x. [DOI] [PubMed] [Google Scholar]
- Cairney JWG, Chambers SM. Interaction between Pisolithus tinctoriusits hosts: a review of current knowledge. Mycorrhiza. 1997;7:117–131. doi: 10.1007/s005720050172. [DOI] [Google Scholar]
- Cardoso EJBN. Effect of vesicular arbuscular mycorrhiza and rock phosphate on the soybean–Rhizobium symbiosis. Rev Bras Ciênc Solo. 1985;9:125–130. [Google Scholar]
- Castellano MA. Outplanting performance of mycorrhizal inoculated seedlings. In: Mukerji KG, editor. Concepts in mycorrhizal research. Dordrecht, The Netherlands: Kluwer Academic; 1996. pp. 223–302. [Google Scholar]
- Colpaert JV, Van Assche JA, Luijtens K. The growth of the extramatrical mycelium of ectomycorrhizal fungi and the growth response of Pinus sylvestris L. New Phytol. 1992;120:127–135. doi: 10.1111/j.1469-8137.1992.tb01065.x. [DOI] [Google Scholar]
- Corrêa A, Strasser RJ, Martins-Loucao MA. Are mycorrhiza always beneficial? Plant Soil. 2006;279:65–73. doi: 10.1007/s11104-005-7460-1. [DOI] [Google Scholar]
- Davis FT, Potter JR, Linderman RG. Mycorrhiza and repeated drought exposure affect drought resistance and extraradical hyphae development of pepper plants independent of plant size and nutrient. J Plant Physiol. 1992;139:289–294. [Google Scholar]
- Dosskey M, Boersma L, Linderman RG. Role for photosynthate demand for ectomycorrhizas in the response of Douglas-fir seedlings to drying soil. New Phytol. 1990;117:327–334. doi: 10.1111/j.1469-8137.1991.tb04914.x. [DOI] [PubMed] [Google Scholar]
- Dučić T, Polle A. Manganese toxicity in two varieties of Douglas fir (Pseudotsuga menziesii var. viridis and glauca) seedlings as affected by phosphorus supply. Funct Plant Biol. 2007;34:31–40. doi: 10.1071/FP06157. [DOI] [PubMed] [Google Scholar]
- Dučić T, Leinemann L, Finkeldey R, Polle A. Uptake and translocation of manganese in seedlings of two varieties of Douglas fir (Pseudotsuga menziesii var. viridis and glauca) New Phytol. 2006;170:11–20. doi: 10.1111/j.1469-8137.2006.01666.x. [DOI] [PubMed] [Google Scholar]
- Eltrop L, Marschner H. Growth and mineral nutrition of non-mycorrhizal and mycorrhizal Norway spruce (Picea abies) seedlings grown in semi-hydroponic sand culture.1. Growth and mineral nutrient uptake in plants supplied with different forms of nitrogen. New Phytol. 1996;133:469–478. doi: 10.1111/j.1469-8137.1996.tb01914.x. [DOI] [Google Scholar]
- Fritz E. X-ray-microanalysis of diffusible elements in plant cells after freeze-drying, pressure-infiltration with ether and embedding in plastic. Scanning Microsc. 1989;3:517–526. [Google Scholar]
- Fritz E, Jentschke G. Agar standards for quantitative X-ray-microanalysis of resin-embedded plant-tissues. J Microsc. 1994;174:47–50. [Google Scholar]
- Gadd GM. Interaction of fungi with toxic metals. New Phytol. 1992;124:25–60. doi: 10.1111/j.1469-8137.1993.tb03796.x. [DOI] [Google Scholar]
- Gafur A, Schutzendubel A, Langenfeld-Heyser R, Fritz E, Polle A. Compatible and incompetent Paxillus involutus isolates for ectomycorrhiza formation in vitro with poplar (Populus × canescens) differ in H2O2 production. Plant Biol. 2004;6:91–99. doi: 10.1055/s-2003-44718. [DOI] [PubMed] [Google Scholar]
- Galli U, Schuepp H, Brunhold C. Heavy metal binding by mycorrhizal fungi. Physiol Plant. 1994;92:364–368. doi: 10.1111/j.1399-3054.1994.tb05349.x. [DOI] [Google Scholar]
- Hampp R, Wiese J, Mikolajewski S, Nehls U. Biochemical and molecular aspects of C/N interaction in ectomycorrhizal plants: an update. Plant Soil. 1999;215:103–113. doi: 10.1023/A:1004650324646. [DOI] [Google Scholar]
- Heinrichs H, Brumsack HJ, Loftfield N, Konig N. Improved pressure digestion system for biological and anorganic materials. Z Pflanzenernähr Bodenkd. 1986;149:350–353. doi: 10.1002/jpln.19861490313. [DOI] [Google Scholar]
- Ingestad T, Lund AB. Theory and techniques for steady state mineral nutrition and growth of plants. Scand J For Res. 1986;1:439–453. [Google Scholar]
- Jentschke G, Godbold DL. Metal toxicity and ectomycorrhizas. Physiol Plant. 2000;109:107–116. doi: 10.1034/j.1399-3054.2000.100201.x. [DOI] [Google Scholar]
- Kleinschmit J, Racz J, Weisgerber H, Dietze W, Dieterich H, Dimpflmeier R. Ergebnisse aus dem internationalen Douglasien-Herkunftsversuch von 1970 in der Bundesrepublik Deutschland. Silvae Genet. 1974;23:167–176. [Google Scholar]
- Koide RT. Nutrient supply, nutrient demand and plant response to mycorrhizal infection. New Phytol. 1991;117:365–386. doi: 10.1111/j.1469-8137.1991.tb00001.x. [DOI] [PubMed] [Google Scholar]
- Kothari SK, Marschner H, Römheld V. Direct and indirect effects of VA mycorrhizal fungi and rhizosphere microorganisms on acquisition of mineral nutrients by maize (Zea mays L) in a calcareous soil. New Phytol. 1990;116:637–645. doi: 10.1111/j.1469-8137.1990.tb00549.x. [DOI] [Google Scholar]
- Kothari SK, Marschner H, Römheld V. Contribution of the VA mycorrhizal hyphae in acquisition of phosphorus and zinc by maize grown in a calcareous soil. Plant Soil. 1991;131:177–185. doi: 10.1007/BF00009447. [DOI] [Google Scholar]
- Kunst L, Roomans GM. Intracellular localization of heavy metals in yeast Saccharomyces cerevisae by X-ray microanalysis. Scan Electron Micros. 1985;1:191–199. [Google Scholar]
- Larsen JB. Investigations on significance of potassium and nitrogen supply for drought hardiness in Douglas fir (Pseudotsuga menziesii) in winter. Flora. 1978;167:197–207. [Google Scholar]
- Larsen JB. Geographic variation in winter drought resistance of Douglas fir (Pseudotsuga menziesii Mirb. Franco) Silvae Genet. 1981;30:109–114. [Google Scholar]
- Liesebach M, Stephan BR. Growth performance and reaction to biotic and abiotic factors of Douglas fir progenies (Pseudotsuga menziesii [Mirb] Franco) Silvae Genet. 1995;44:303–311. [Google Scholar]
- Linnemann G. Rassenunterschiede bei Pseudotsuga taxifolia hinsichtlich der Mycorrhiza. Allg Forst Jagdztg. 1960;131:41–48. [Google Scholar]
- Luoma DL, Stockdale CA, Molina R, Eberhart JL. The spatial influence of Douglas-fir retention trees on ectomycorrhiza diversity. Can J For Res. 2006;36:2561–2573. doi: 10.1139/X06-143. [DOI] [Google Scholar]
- Lu X, Malajczuk N, Dell B. Mycorrhiza formation and growth of Eglobulus seedlings inoculated with spores of various ectomycorrhizal fungi. Mycorrhiza. 1998;8:81–86. doi: 10.1007/s005720050216. [DOI] [Google Scholar]
- Marschner H. Mineral nutrition of higher plants. London: Academic; 1995. [Google Scholar]
- Massicotte HB, Molina R, Luoma DL, Smith JE. Biology of the ectomycorrhizal genus, Rhizopogon: II. Patterns of host-fungus specificity following spore inoculation of diverse hosts grown in monoculture and dual culture. New Phytol. 1994;126:677–690. doi: 10.1111/j.1469-8137.1994.tb02962.x. [DOI] [Google Scholar]
- Molina R, Palmer JG. Isolation, maintance, and pure culture manipulation of ectomycorrhizal fungi. In: Schenck NC, editor. Methods and principles of mycorrhizal research. St Paul, Minnesota: The American Phytopathological Society; 1982. pp. 115–129. [Google Scholar]
- Molina R, Trappe JM. Patterns of ectomycorrhizal host specificity and potential among Pacific northwest conifers and fungi. Forest Sci. 1982;28:423–458. [Google Scholar]
- Molina R, Trappe JM. Biology of the ectomycorrhizal genus, Rhizopogon. 1. Host association, host specificity and pure culture synthesis. New Phytol. 1994;126:653–675. doi: 10.1111/j.1469-8137.1994.tb02961.x. [DOI] [Google Scholar]
- Nilsen P, Borja I, Knutsen H, Brean R. Nitrogen and drought effects on ectomycorrhizae of Norway spruce [Picea abies L. (Karst.)] Plant Soil. 1998;198:179–184. doi: 10.1023/A:1004399303192. [DOI] [Google Scholar]
- Nogueira MA, Cardoso EJ. Microbial interactions on manganese availability and uptake by soybean. Pesqui Agropecu Bras. 2003;37:1605–1612. [Google Scholar]
- Outerbridge RA, Trofymow JA. Diversity of ectomycorrhizae on experimentally planted Douglas-fir seedlings in variable retention forestry sites on southern Vancouver Island. Can J Bot. 2004;82:1671–1681. doi: 10.1139/b04-134. [DOI] [Google Scholar]
- Pacovsky RS. Micronutrient uptake and distribution in mycorrhizal or phosphorus-fertilized soybeans. Plant Soil. 1986;95:379–388. doi: 10.1007/BF02374618. [DOI] [Google Scholar]
- Parke JL, Linderman RG, Trappe JM. Effects of forest litter on mycorrhiza development and growth of Douglas-fir and Western Red Cedar seedlings. Can J For Res. 1983;13:666–671. doi: 10.1139/x83-095. [DOI] [Google Scholar]
- Parladé J, Álvarez IF, Pera J. Ability of native ectomycorrhizal fungi from northern Spain to colonize Douglas-fir and other introduced conifers. Mycorrhiza. 1995;6:51–55. doi: 10.1007/s005720050105. [DOI] [Google Scholar]
- Parladé J, Pera J, Álvarez IF, Bouchard D, Genere B, Le Tacon F. Effect of inoculation and substrate disinfection method on rooting and ectomycorrhiza formation of Douglas fir cuttings. Ann For Sci. 1999;56:35–40. doi: 10.1051/forest:19990105. [DOI] [Google Scholar]
- Pera J, Álvarez IF, Rincón A, Parladé J. Field performance in northern Spain of Douglas-fir seedlings inoculated with ectomycorrhizal fungi. Mycorrhiza. 1999;9:77–84. [Google Scholar]
- Posta K, Marschner H, Römheld V. Manganese reduction in the rhizosphere of mycorrhizal and nonmycorrhizal maize. Mycorrhiza. 1994;5:119–124. doi: 10.1007/BF00202343. [DOI] [Google Scholar]
- Reid CPP, Kidd FA, Ekwebalam SA. Nitrogen nutrition, photosynthesis and carbon allocation in ectomycorrhizal pine. Plant Soil. 1983;71:415–532. doi: 10.1007/BF02182683. [DOI] [Google Scholar]
- Schober R, Kleinschmit J, Svolba J. Results of the Douglas-fir provenances experiment of 1958 in Northern Germany. 1. Allg Forst Jagdztg. 1983;154:209–236. [Google Scholar]
- Schober R, Kleinschmit J, Svolba J. Results of the Douglas-fir provenances experiment of 1958 in Northern Germany. 2. Allg Forst Jagdztg. 1984;155:53–80. [Google Scholar]
- Schöne D. Site- and acid-rain induced nutritional disorders of Douglas-fir in Southwestern Germany. Allg Forst Jagdztg. 1992;163:53–59. [Google Scholar]
- Schroeder MS, Janos DP. Phosphorus and intraspecific density alter plant responses to arbuscular mycorrhizas. Plant Soil. 2004;264:335–348. doi: 10.1023/B:PLSO.0000047765.28663.49. [DOI] [Google Scholar]
- Schützendübel A, Polle A. Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J Exp Bot. 2002;53:1351–1365. doi: 10.1093/jexbot/53.372.1351. [DOI] [PubMed] [Google Scholar]
- Shi LB, Guttenberger M, Kottke I, Hampp R. The effect of drought on mycorrhizas of beech (Fagus sylvatica L.): changes in community structure, and the content of carbohydrates and nitrogen storage bodies of the fungi. Mycorrhiza. 2002;12:303–311. doi: 10.1007/s00572-002-0197-2. [DOI] [PubMed] [Google Scholar]
- Smith HA, Zeller M. A preliminary account of the North American species of Rhizopogon. Mem N Y Bot Gard. 1966;14:1–178. [Google Scholar]
- St Clair SB, Lynch JP. Photosynthetic and antioxidant enzyme responses of sugar maple and red maple seedlings to excess manganese in contrasting light environments. Funct Plant Biol. 2004;31:1005–1014. doi: 10.1071/FP04049. [DOI] [PubMed] [Google Scholar]
- Thomson BD, Grove TS, Malajczuk N, StJ. Hardy G. The effectiveness of ectomycorrhizal fungi in increasing the growth of Eucalyptus globulus Labill in relation to root colonization and hyphal development in soil. New Phytol. 1994;126:517–524. doi: 10.1111/j.1469-8137.1994.tb04250.x. [DOI] [PubMed] [Google Scholar]
- Trappe JM. Selection of fungi for ectomycorrhizal inoculation in nurseries. Annu Rev Phytopathol. 1977;15:203–222. doi: 10.1146/annurev.py.15.090177.001223. [DOI] [Google Scholar]
- Turnau K, Kottke I, Oberwinkler F. Element localization in mycorrhizal roots of Pteridium aquilinum L. Kuhn collected from experimental plots treated with cadmium dust. New Phytol. 1993;123:313–324. doi: 10.1111/j.1469-8137.1993.tb03741.x. [DOI] [Google Scholar]
- Van Tichelen KK, Colpaert JV, Van Assche JA. Development of arbuscular mycorrhizas in a heavy metal-contaminated soil amended with a metal immobilizing substance. In: Azcon-Aguilar C, Barea JM, editors. Mycorrhizas in integrated systems: from genes to plant development. EUR 16728, Luxembourg: European Commission; 1996. pp. 479–482. [Google Scholar]
- Zarb J, Walters DR. Polyamine biosynthesis in the ectomycorrhizal fungus Paxillus involutus exposed to zinc. Lett Appl Microbiol. 1995;21:93–95. doi: 10.1111/j.1472-765X.1995.tb01014.x. [DOI] [Google Scholar]
- Zarb J, Walters DR. Polyamine biosynthesis in the ectomycorrhizal fungus Paxillus involutus exposed to lead. Mycol Res. 1996;100:486–488. [Google Scholar]