Abstract
Dopamine is a retinal neuromodulator secreted from amacrine and interplexiform cells. Activation of dopamine D4 receptors on photoreceptor cells reduces a light-sensitive pool of cAMP. The aim of present study was to evaluate the role of dopamine receptors and cAMP in the regulation of intracellular Ca2+ concentrations ([Ca2+]i) in photoreceptor cells of chick retina. Retinal cells from 6 day-old chicken embryos were isolated and cultured for 5–7 days prior to experiments. Cone photoreceptors were the predominant cell type in these cultures. Dopamine and agonists of dopamine D4 receptors suppressed K+-stimulated uptake of 45Ca2+ and [Ca2+]i, measured with the Ca2+-sensitive fluorescent dye fura-2 AM. The effects of the agonists were blocked by dopamine D2/D4 receptor antagonists or by pertussis toxin. 8Br-cAMP, a cell permeable analog of cAMP, had no effect on inhibition of K+-stimulated 45Ca2+ influx or [Ca2+]i by dopamine D2/D4 receptor agonists. Quinpirole inhibited the increase in cAMP level elicited by K+, which requires Ca2+ influx through voltage-gated Ca2+ channels, but not that induced by the calcium ionophore A23187. Moreover, dopamine had no effect on either forskolin-stimulated or Ca2+/calmodulin-stimulated adenylyl cyclase activity in cell membranes prepared from the cultured cells. These data indicate that the decrease of cAMP elicited by dopamine D4 receptor stimulation may be secondary to decreased [Ca2+]i.
Keywords: photoreceptor, calcium, calcium channel, dopamine receptor, cAMP
1. Introduction
Dopamine is a neuromodulator secreted by amacrine and interplexiform cells of the vertebrate retina. Most retinal cells have dopamine receptors, which mediate the synaptic and paracrine effects of the modulator (reviewed by Witkovsky, 2004). Dopamine receptors are classified into two families, D1 and D2, which are further subdivided based on cloned receptor subtypes (D1 family = D1 and D5 receptors; D2 family = D2, D3, and D4 receptors). Photoreceptor cells possess D2-like receptors, activation of which inhibits melatonin biosynthesis (Iuvone and Besharse, 1986), entrains a circadian oscillator (Cahill and Besharse, 1991), regulates photomechanical movements (Pierce and Besharse, 1985; Hillman et al., 1995), regulates the balance of rod/cone input to second order neurons (Witkovsky et al., 1988; Manglapus et al., 1999), inhibits Na+/K+-ATPase (Shulman and Fox, 1996), and reduces the rod IH current (Akopian and Witkovsky, 1996).
Activation of dopamine D4 receptors reduces a light-sensitive pool of cAMP in photoreceptor cells and this reduction in intracellular cAMP level, in turn, mediates many of dopamine’s effects on photoreceptor physiology (Cohen et al., 1990). However, not all of the effects of dopamine on photoreceptor cell physiology are mediated by cyclic AMP, and other signaling pathways have been implicated (Akopian and Witkovsky, 1996). For example, dopamine may also affect intracellular photoreceptor mechanisms via modulation of intracellular Ca2+ concentration. Ca2+ influx through dihydropyridine sensitive, voltage-gated channels is required for both melatonin biosynthesis (Iuvone and Besharse, 1986; Avendano et al., 1990) and glutamate release (Schmitz and Witkovsky, 1997) by photoreceptor cells. A previous study showed that activation of D2-like receptors on salamander photoreceptor cells decreases intracellular Ca2+ levels ([Ca2+]i) (Thoreson et al., 2002). However, mechanisms whereby dopamine modulates intracellular Ca2+ and cAMP level in photoreceptors are unclear. Therefore, in the present study we investigated the effect of dopamine receptor agonists and antagonists on the intracellular level of cAMP, Ca2+ influx, and [Ca2+]i of chicken cone photoreceptor cells, and the relationship of dopamine receptor-mediated changes of cAMP and [Ca2+]i.
2. RESULTS
2a. Effects of dopamine and quinpirole on 45Ca2+ influx in chicken cone photoreceptor cells cultures
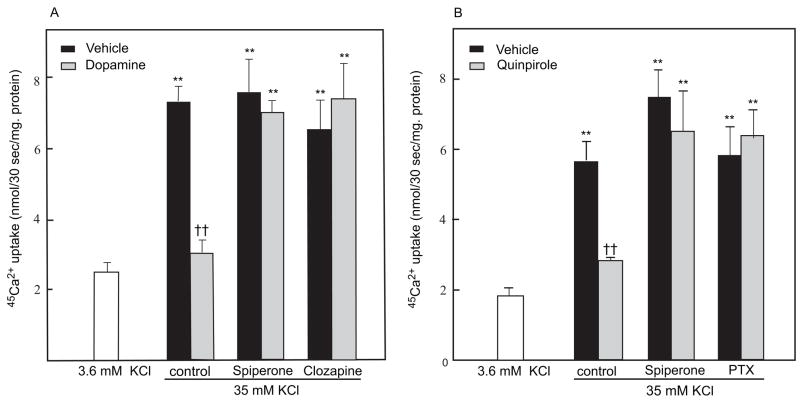
A depolarizing concentration of extracellular K+ (35 mM) elicited a prominent increase in 45Ca2+ influx into cultured retinal cells (p<0.01, compared to basal 3.6 mM K+; Fig. 1A). In cells treated with 1 μM dopamine, influx of 45Ca2+ elicited by 35 mM K+ was significantly reduced (p<0.01; Fig. 1A). This effect of dopamine was blocked by 10 μM clozapine and 10 μM spiperone (p<0.01), dopamine D2-family receptor antagonists. The antagonists had no effect on 45Ca2+ influx in the absence of dopamine (Fig. 1A).
Fig. 1.
Dopamine and quinpirole inhibit calcium influx in chicken photoreceptor cell cultures. Cells were prepared and treated with drugs and 45Ca2+ uptake measured during a 30 sec incubation as described in Experimental Procedures A. Dopamine inhibited calcium influx in cultured chicken photoreceptor cells. Elevated extracellular KCl (35 mM) significantly increased 45Ca2+ influx in cultured chicken photoreceptor cells compared to basal conditions [3.6 mM KCl; **p<0.01]. Dopamine (1 μM) significantly reduced K+-stimulated 45Ca2+ uptake [††p<0.01 vs. vehicle (35 mM KCl)]. The effect of dopamine was blocked by 10 μM spiperone and 10 μM clozapine [**p<0.01 vs. 3.6 mM KCl]; n=6 per group. B. Quinpirole inhibits calcium influx in cultured chicken photoreceptor cells. KCl (35 mM) significantly increased 45Ca2+ influx in cultured chicken photoreceptor cells compared to basal conditions [**p<0.01]. Quinpirole (0.3 μM) inhibited K+-induced 45Ca2+ uptake [††p<0.01 vs. vehicle]. The inhibitory effect of quinpirole was blocked by 10 μM spiperone, [**p<0.01 vs. vehicle] or pretreatment with 50 ng/ml pertussis toxin (PTX) [**p<0.01 vs. vehicle]; n=5–6 per group.
Quinpirole (0.3 μM), a dopamine D2-family receptor agonist, also significantly reduced depolarization-evoked 45Ca2+ influx (p<0.01) in cultured photoreceptor cells (Fig. 1B). The effect of quinpirole was significantly inhibited by spiperone (10 μM) or by pretreatment with pertussis toxin (50ng/ml) (Fig. 1B).
2b. Effects of dopamine and dopamine receptor agonists on [Ca2+]i in chicken photoreceptor cells labeled with Fura-2AM
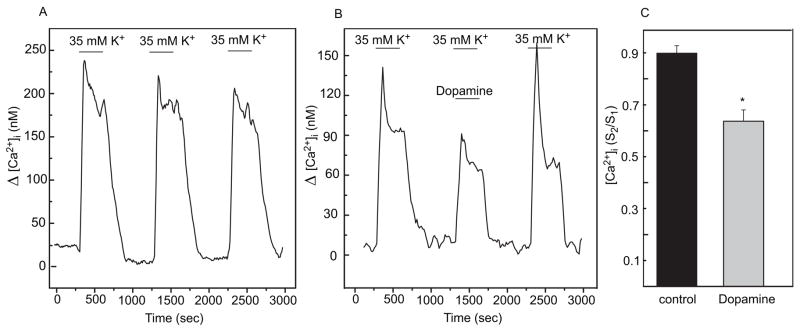
In visually identified photoreceptor cells, three consecutive stimulations with 35 mM K+ produce highly reproducible increases of [Ca2+]i (Fig. 2A), which were shown previously to result from Ca2+ influx through dihydropyridine-sensitive, voltage-gated channels (Uchida and Iuvone, 1999). In control cells, the S2/S1 ratio, where S2 is the response to the 2nd stimulation and S1 the response to the 1st stimulation, was not significantly different from 1.
Fig. 2.
Dopamine inhibits the depolarization-evoked increase in [Ca2+]i in cultured chicken photoreceptor cells. A. Representative trace of [Ca2+]i recording of identified photoreceptor cells treated with three repetitive stimulations with 35 mM KCl. K+-evoked depolarizations produce highly reproducible increases in [Ca2+]i. B. Representative trace of [Ca2+]i recording of identified photoreceptor cells treated with three repetitive stimulations with 35 mM KCl and dopamine (0.1 μM) added during the 2nd stimulation (S2). C. Plots of S2/S1 ratios (S2 = peak area during the 2nd stimulation, and S1 = peak area during the 1st stimulation); n= 12 for controls; n=8 for dopamine; * p<0.05 vs control.
Dopamine (0.1 μM), added during the 2nd stimulation (S2), reduced K+-evoked intracellular Ca2+ influx in photoreceptor cells (Fig. 2B). Dopamine elicited a statistically significant reduction in the S2/S1 ratio (p<0.05, Fig. 2C). The effect of dopamine was reversible upon washout.
Quinpirole (0.3. μM; n=12) significantly suppressed K+ -evoked increase in [Ca2+]i in photoreceptor cells (p<0.05; Fig 3A). The effect of quinpirole was significantly reduced by spiperone (10 μM, p <0.05, n=4) (Fig. 3B). In contrast, SCH 23390 (10 μM, n=16), a selective dopamine D1-like receptor antagonist, did not evoke remarkable changes in [Ca2+]i itself and failed to alter the inhibitory action of quinpirole (Fig. 3C).
Fig. 3.
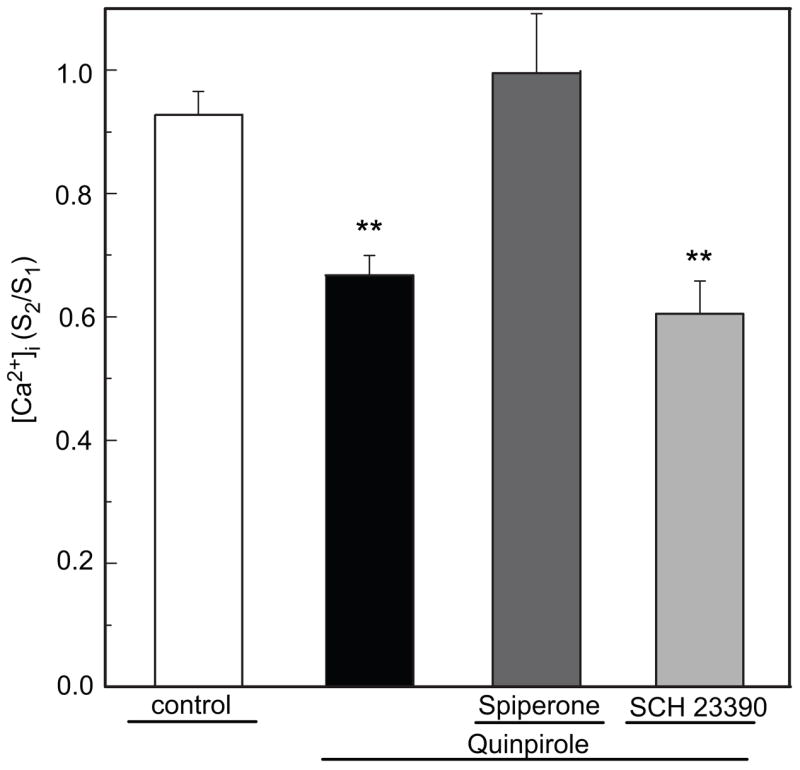
Quinpirole inhibits depolarization-evoked increase in [Ca2+]i in cultured chicken photoreceptor cells. A. Quinpirole (0.3 μM) significantly (**p<0.05 vs control, n=12) reduced the K+-evoked increase in [Ca2+]i in chicken photoreceptor cells. Data expressed as S2/S1 ratio. Inhibitory effect of quinpirole was reduced by the D2/D4 Dopamine receptor antagonist 10 μM spiperone (p>0.05 vs control, n=4). SCH 23390 (10 μM, **p<0.05 vs control, n=16) failed to alter the inhibitory action of quinpirole.
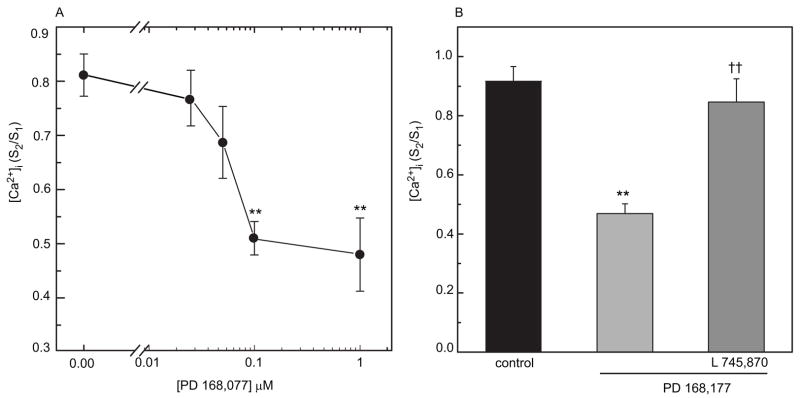
In mouse retina, photoreceptor cells express dopamine D4 receptors, which regulate the light-sensitive pool of cAMP (Cohen and Blazynnski, 1990; Cohen et al., 1992), and the receptors that mediate inhibition of melatonin synthesis in chick retinal photoreceptors in vivo appear to be the D4 subtype (Zawilska et al., 2003). In order to determine if dopamine D4 receptors regulate [Ca2+]i in cultured photoreceptor cells, we tested the effects of a selective D4 receptor agonist, PD 168,077, and a selective D4 antagonist L745,870 (Fig. 4). PD 168,077 (0.1 μM) significantly reduced the K+-evoked increase in [Ca2+]i in photoreceptor cells at concentrations of 0.1 μM and above (p<0.05) (Fig. 4A). This inhibitory effect of PD 168,077 was completely prevented by 1 μM L 745,870 (Fig. 4B). L 745,870 alone did not evoke any significant changes in [Ca2+]i (data not shown).
Fig. 4.
D4 receptor agonist, PD 168,077, inhibits depolarization-evoked increase in [Ca2+]i in chicken photoreceptor cells. A. The inhibitory effect of PD 168,077 on [Ca2+]i was concentration-dependent (0.025–1.0 μM), with significant inhibition at concentrations of 0.1 μM and above (** p<0.01). B. The inhibitory effect of 0.1 μM PD 168,077 (** p<0.01 vs. control; n=20) was blocked by 1.0 μM L 745,870 (††p<0.01 vs. PD 168,077 n=9).
2c. Relationship of dopamine receptor-mediated changes of intracellular Ca2+ and cAMP
As was shown earlier (Iuvone et al., 1991), the stimulation of cAMP formation by depolarizing concentrations of K+ in this culture preparation requires Ca2+ influx through dihydropyridine-sensitive Ca2+ channels. In the present study, cAMP accumulation was significantly increased by treatment with either 35 mM KCl or the Ca2+ ionophore A23187. Quinpirole significantly suppressed the stimulatory effect of 35 mM KCl on cAMP accumulation (p<0.05), while not significantly affecting the increase of cAMP elicited by A23187 (Table 1). Quinpirole also elicited a small but significant reduction of cAMP accumulation in response to treatment with forskolin (p<0.05; Table 2). Nitrendipine, an antagonist of L-type Ca2+ channels, elicited a comparable inhibition of forskolin-stimulated cAMP accumulation, and the effects of nitrendipine and quinpirole on cAMP accumulation were not additive. These results suggest that activation of dopamine receptors on chick photoreceptor cells reduces cAMP formation, at least in part, by reducing Ca2+ influx through voltage-gated channels.
TABLE 1.
Effect of quinpirole on stimulation of cAMP accumulation elicited by KCl and the calcium ionophore A23187
| Additions | N | cAMP accumulation(% conversion × 103) |
|---|---|---|
| none | 6 | 35.3 ± 3.0 |
| A23187 (1 μM) | 6 | 54.1 ± 4.4a |
| A23187 (1 μM) + quinpirole (0.3 μM) | 5 | 48.1 ± 2.5a |
| KCl (35 mM) | 5 | 61.8 ± 4.7a |
| KCl (35 mM) + quinpirole (0.3 μM) | 6 | 39.7± 2.9b |
Cells were incubated and cAMP accumulation measured as described in Methods.
p< 0.05 vs none ;
p< 0.05 vs KCl
TABLE 2.
Quinpirole and nitrendipine reduce forskolin-stimulated cAMP accumulation
| Additions | N | cAMP accumulation(% conversion x 103) |
|---|---|---|
| none | 5 | 53.7 ± 9.7 |
| quinpirole (0.3 μM) | 5 | 49.9 ± 13.9 |
| nitrendipine (3 μM) | 6 | 50.8 ± 8.2 |
| forskolin (1 μM) | 6 | 847.3 ± 45.0 |
| forskolin (1 μM) + quinpirole (0.3 μM) | 6 | 669.5 ± 38.5a |
| forskolin (1 μM) + nitrendipine (3 μM) | 6 | 628.6 ± 51.8a |
| forskolin (1 μM) + quinpirole (0.3 μM) + nitrendipine (3 μM) | 6 | 605.5 ± 35.8a |
Cells were incubated and cAMP accumulation measured as described in Methods.
p< 0.05 vs forskolin
To determine if the reduction of intracellular Ca2+ elicited by dopamine receptor activation requires a decrease of cAMP and protein kinase A (PKA), cells were pretreated with 8Br-cAMP, a cell-permeable analog of cAMP, in order to maintain activated PKA in the presence of quinpirole. Pretreatment for 5 min with 8Br-cAMP had no effect on K+-evoked 45Ca2+ influx, or on its inhibition by quinpirole (Fig. 5.). A longer pretreatment (1 hr) with 8Br-cAMP produced a small elevation in K+-stimulated [Ca2+]i (Fig. 6A vs 6B; 37 %, p<0.05). However, 8Br-cAMP had no significant effect on the reduction of K+-stimulated [Ca2+]i elicited by PD168,077 (Fig. 6C).
Fig. 5.
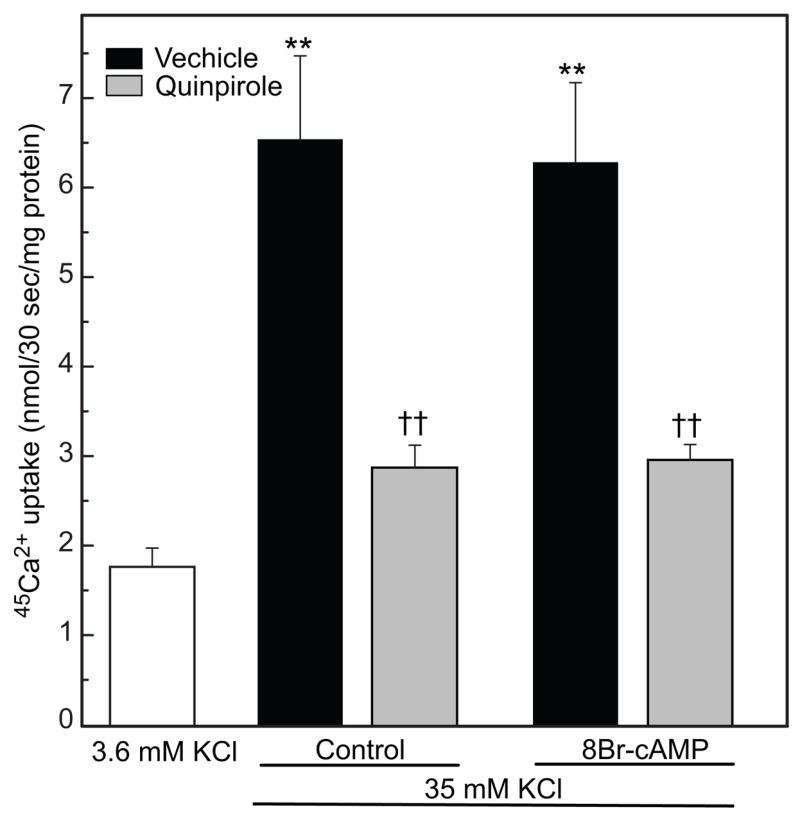
Effect of 8Br-cAMP on depolarization-evoked 45Ca2+ influx. KCl (35 mM) significantly increased 45Ca2+ influx compared to basal conditions [** p<0.01 vs. 3.6 mM K+]; 0.3 μM quinpirole significantly reduced K+-stimulated 45Ca2+ uptake [†† p<0.01 vs. 35 mM KCl]. Pretreating with 2 mM 8Br-cAMP had no effect on K+-stimulated 45Ca2+ influx in the absence (**p<0.01) or presence (††p<0.01) of 0.3 μM quinpirole; n=4–5 per group.
Fig. 6.
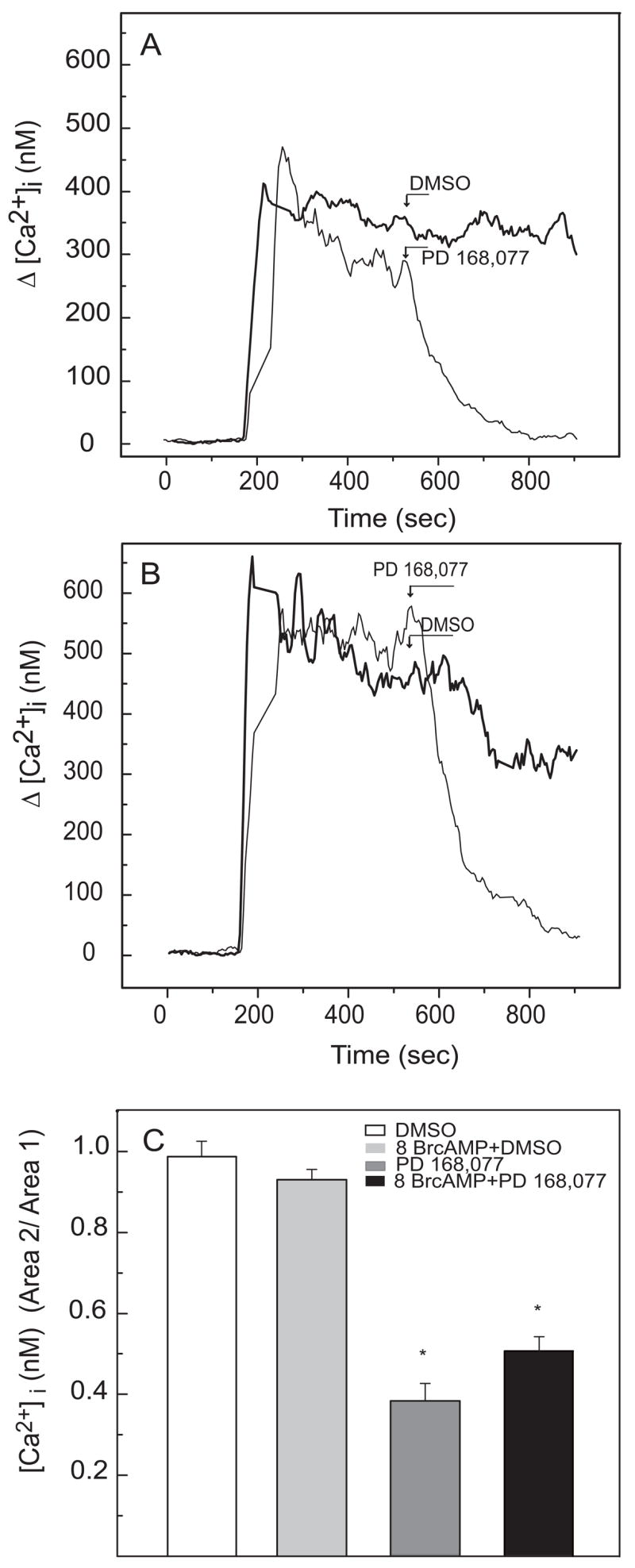
Lack of effect of 8Br-cAMP on the reduction of [Ca2+]i elicited by PD168.077. Cells were pretreated for 1 hour with 3 mM 8Br-cAMP (Fig. 6B) or vehicle (Fig. 6A) prior to stimulation with 35 mM K+ for 13 min; 8Br-cAMP remained in the medium during the recordings. Seven minutes following the introduction of 35 mM K+, cells were treated with PD168,077 (0.5 μM) or vehicle (DMSO) and recordings were continued. To quantify the effect of drug treatment, baseline-subtracted [Ca2+]i values were integrated for the 200 sec prior to addition (Area 1) and the 200 sec following addition (Area 2) of PD168,077 or vehicle. Area 2/Area 1 ratios were determined (Fig. 6C). PD168,077 significantly reduced [Ca2+]i (p<0.01) and 8Br-cAMP had no significant effect on the response to the D4 receptor agonist. Samples sizes were 19 (DMSO), 24 (8Br-cAMP + DMSO), 16 (PD168,077), and 32 (8Br-cAMP + PD168,077). A positive control experiment was conducted to determine if 8Br-cAMP activated protein kinase A (PKA) under these conditions. Cells were analyzed for induction of arylalkylamine N-acetyltransferase (AANAT), a photoreceptor enzyme that is regulated by cAMP-dependent phosphorylation (Alonso-Gomez and Iuvone, 1995; Pozdeyev et al., 2006). Incubation with 8Br-cAMP significantly elevated AANAT activity (Control – 13 ± 0.6; 8Br-cAMP – 40 ± 1.5 pmol N-acetyltryptamine min−1 mg protein−1; p<0.01; N=5 per group).
To determine if dopamine receptors on cultured photoreceptor cells are coupled to adenylyl cyclase, we assessed the effect of quinpirole on adenylyl cyclase activity in membranes prepared from the cell cultures. As shown in Figure 7, adenylyl cyclase activity in membranes was significantly increased by treatment with either forskolin or Ca2+/calmodulin (p<0.01). Quinpirole (10μM) failed to inhibit Ca2+/calmodulin-stimulated or forskolin-stimulated adenylyl cyclase activity.
Fig. 7.
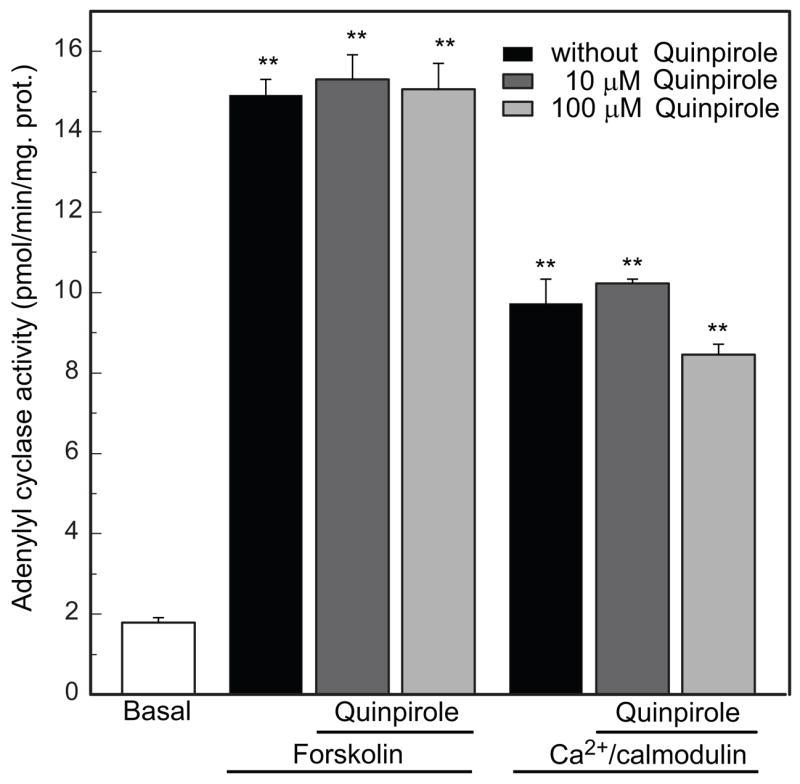
Lack of effect of quinpirole on adenylyl cyclase activity in membranes prepared from cultured chicken photoreceptor cells. Adenylyl cyclase activity (AC) in membranes was significantly increased by treatment with either 10 μM forskolin [n=3, **p<0.01 vs. Basal activity] or 120 nM Ca2+/calmodulin [n=3, **p<0.01 vs. basal activity]. 10 μM and 100 μM quinpirole failed to inhibit either forskolin-stimulated [n=3, **p<0.01 vs. basal activity] or Ca2+/calmodulin-stimulated adenylyl cyclase activity [n=3, **p<0.01 vs. basal activity].
3. Discussion
Photoreceptor cells are influenced by neuromodulators, including dopamine (Cohen et al., 1992; Akopian and Witkovsky, 1996), adenosine (Blazynski et al., 1991, Stella et al., 2003), melatonin (Wiechman et al., 2003), nitric oxide (Kurenny et al., 1994), and somatostatin (Akopian et al., 2000). However, little is know about the signaling mechanisms that mediate effects of these neuromodulators on photoreceptor functions. Previous studies have shown that activation of dopamine D2-like receptors affects Ca2+ currents and intracellular Ca2+ levels in salamander photoreceptors (Stella et al., 2000; Thoreson et al., 2002) and decreases cAMP levels in mouse photoreceptors (Cohen and Blazynski, 1990; Cohen et al., 1992). The current study extends these findings to chicken cone photoreceptors and investigates the relationship between dopamine receptor-mediated changes of cAMP and intracellular Ca2+.
Dopamine plays an important role in the regulation of visual processes related to mechanisms of light adaptation (Witkovsky et al., 2004). In chicken retina, dopamine release is stimulated by light and has a diurnal rhythm with the highest rate of release during the daytime (Zawilska et al., 2003; Megaw et al., 2006). Synaptic and paracrine effects of dopamine in retinal cells are mediated by multiple types of dopaminergic receptors expressed by retinal neurons and glia (Witkovsky et al., 2004).
In photoreceptor cells, light inhibits adenylyl cyclase activity and decreases the level of cAMP (Cohen et al., 1982). In darkness, membranes of photoreceptor cells are partially depolarized (Hagins et al., 1970) and Ca2+ enters the cells through dihydropyridine sensitive, voltage-gated calcium channels (Uchida and Iuvone 1999; Schmitz and Witkovsky, 1997). A sustained increase of intracellular Ca2+ (Uchida and Iuvone, 1999) evokes the activation of cAMP synthesis (Iuvone et al., 1991). Light, which hyperpolarizes photoreceptor membranes, inhibits cAMP formation, an effect that can be mimicked by Ca2+ channel blockers in depolarized photoreceptor cells (Iuvone et al., 1991). It was suggested that this effect of light is partially mediated by activation of dopamine D4 receptors (Cohen et al., 1992, Nir et al., 2002).
Stella and Thoreson (2000) reported that protein kinase A (PKA) inhibitors mimic the effect of D2 receptor stimulation on the activation of Ca2+ currents in salamander rod and cone photoreceptor cells and that an activation of PKA occluded the effect of receptor stimulation. These results suggest that effects of dopamine on Ca2+ currents are secondary to its effect on cAMP formation. However, our data support an alternative hypothesis, that the inhibition of Ca2+ influx by dopamine is at least partially independent of changes in cAMP and that inhibition of cAMP formation may be a secondary consequence of decreased intracellular calcium.
The present study demonstrates that dopamine D2/D4 agonists inhibit depolarization-evoked stimulation of 45Ca2+ influx and [Ca2+]i in chicken cone photoreceptor cells. Effects on [Ca2+]i of the PD 168,077 and L 745,870, selective for the dopamine D4 receptor, strongly support involvement of that receptor subtype in the regulation of Ca2+ dynamics in chicken cones.
Several observations suggest that regulation of voltage-gated Ca2+ channels in chick photoreceptor cells by dopamine D4 receptors may occur by a cAMP-independent mechanism and that the cAMP response to D4 receptor activation may be secondary to decreased Ca2+ influx. The cell-permeable analog of cAMP, 8Br-cAMP, had no effect on K+-stimulated 45Ca2+ influx or on its inhibition by quinpirole in cultured chicken photoreceptor cells. Similarly, 8Br-cAMP had no significant on D4 receptor-mediated reductions in K+-stimulated [Ca2+]i. These results are consistent with observations that dopamine D4 receptor-mediated inhibition of L-type Ca2+ currents in cerebellar granule cells is not affected by cAMP and occurs by a mechanism that is independent of inhibition of adenylyl cyclase (Mei et al., 1995). Depolarization of cultured chick photoreceptor cells stimulates cAMP formation by a mechanism that involves Ca2+ influx through dihydropyridine-sensitive channels and, presumably, activation of a Ca2+/calmodulin-stimulated adenylyl cyclase (Iuvone et al., 1991). Dopamine and quinpirole inhibit the stimulation of cAMP formation in response to K+-evoked depolarization, which opens voltage-gated Ca2+ channels, but have no effect on the stimulation of cAMP elicited by the Ca2+ ionophore A23187. Moreover, quinpirole had no effect on forskolin- or calmodulin-stimulated adenylyl cyclase activity in membranes prepared from the cultured cells, consistent with previous studies that were unable to show an effect of quinpirole on adenylyl cyclase activity in chick retina homogenate (Zawilska et al., 1995). These findings suggest that dopamine D4 receptors may not be directly coupled to adenylyl cyclase and that activation of dopamine receptors on chicken photoreceptor cells reduces cAMP formation, at least in part, by reducing Ca2+ influx through voltage-gated channels.
4. Experimental Procedures
Cell cultures
Photoreceptor cell cultures were prepared from embryonic day 6 chicken retina as described by Adler et al. (1984). Cells were seeded into 60 mm Falcon culture dishes (Becton-Dickson, Franklin Lakes, NJ) or 35 mm glass bottom microwell culture dishes (MatTec Co, Ashland, MA, USA), precoated with polyornithine, at an initial density of 3.9 × 106 or 1.5 × 106 cells per dish. Cells were cultured for 5–7 days in medium 199 supplemented with 10% fetal bovine serum, linoleic acid-BSA (110 μg/ml), 2 mM glutamine and penicillin G (100 U/ml) at 37°C under a humidified atmosphere of 5–6% CO2 in air.
Cultured embryonic cone photoreceptor cells were identified by morphological criteria established previously (Adler et al., 1984), including: a highly polarized cell body; a single, short, usually unbranched neurite; and a refractile lipid droplet characteristic of chick cones. Photoreceptor cells are the predominant cell type in these cultures (Iuvone et al., 1990).
Intracellular calcium measurement
Estimates of intracellular free Ca2+ concentration were obtained by image analysis of individual cells preloaded with Fura-2AM (Molecular Probes, Eugene, OR, USA; 0.67 mM) at a concentration of 2 μM, as described previously (Uchida and Iuvone, 1999). Fura-2AM was prepared in DMSO containing pluronic F-127 (Molecular Probes, Eugene, OR, USA; 20% in DMSO). Cells were incubated at 37°C for 60 min in atmosphere of 5% CO2 in air with Fura-2AM and then superfused at a rate of 0.5 ml/min with basal salt solution (BSS; in mM: 125 NaCl; 3.6 KCl; 1.12 CaCl2; 1.2 MgCl2; 10 d-Glucose; 25 mM Tris/HCl at pH 7.2). All experiments were performed at room temperature (20 ± 2 °C).
Three consecutive depolarizations with a salt solution containing 35 mM K+, generally of 6 min duration, were applied with 10 min periods of superfusion with 3.6 mM K+ between them. During the first, no drugs were applied to cells. Dopamine receptor agonists or vehicles were added to the salt solution during the second depolarization.
Effects of agonists and antagonists on concentration of free intracellular calcium ([Ca2+]i) were quantified by determining the ratio of the peak area corresponding to the second stimulation (S2) to that of the first stimulation (S1), and comparing it to the S2/S1 ratio of vehicle treated cells. The baseline-subtracted peak areas were analyzed using Origin 6.0 (Microcal Software, Inc., Northhampton, MA). Values are presented as Δ [Ca2+]i to illustrate changes in response to K+ treatment.
In one series of experiments, cells were pretreated for 1 hour with 8Br-cAMP prior to stimulation with 35 mM K+ for 13 min. Approximately seven minutes following the introduction of 35 mM K+, cells were treated with PD168,077 or vehicle (DMSO) and recordings were continued. To quantify the effect of drug treatment, baseline-subtracted [Ca2+]i values were integrated for the 200 sec prior to addition (Area 1) and the 200 sec following addition (Area 2) of PD168,077 or vehicle. Area 2/Area 1 ratios were determined and statistically analyzed.
Excitation light was provided by a 300 W xenon lamp (ORC, Azusa, CA, USA). Excitation wavelengths of 340 nm and 380 nm were applied for 0.3–0.5 sec, and fluorescence emitted by Fura-2AM was collected by a Nikon Fluor 40 Ph 3DL objective and detected with a Cohu 4915 CCD camera (San Diego, CA, USA). Four frames at each wavelength were averaged to calculate an emission ratio. Unless otherwise noted, emission ratios were sampled once every 10 sec. For each experiment, a standard curve (0 – 1,350 nM) was constructed in vitro using Calcium Calibration Buffer Kit #2 and Fura-2 pentasodium (Molecular Probes, Eugene, OR, USA), which was used for estimation of calcium concentrations. Cells were selected for recording based on an apparent 340/380 nm ratio close to 1, which represents a basal intracellular calcium concentration of 50–100 nM. Digitized signals of the area of cells outlined with an image analysis program (InCyt Im2; Intracellular Imaging Inc, Cinncinati, OH, USA) were averaged to calculate calcium concentrations.
Measurement of 45Ca2+ influx in photoreceptor cells
K+ depolarization-evoked 45Ca2+ influx was measured by the method of Wei et al (1989), with incubations at room temperature (20 ± 2°C). Culture medium of 60 mm dishes was replaced with BSS containing 3.6 mM KCl and preincubated for 5 min. Cells were then treated with dopamine receptor antagonists (10 μM clozapine and 10 μM spiperone) or 8Br-cAMP for 5 min, with 0.1μM dopamine and 0.3 μM quinpirole added during the last minute. Pertussis toxin (50 ng/ml) was added 18 hrs prior to the experiment. 45Ca2+ (1 μCi) was added and uptake was subsequently measured for 30 sec in the presence of 1.12 mM CaCl2 and 3.6 or 35 mM KCl. 45Ca2+ uptake was stopped by three rapid washes (15 sec each) with ice-cold BSS (3.6 mM KCl). Cells were extracted in 0.5M NaOH, and radioactivity determined by liquid scintillation counting. Protein content of cells was determined by the method of Lowry et al. (1951), using bovine serum albumin (BSA) as standard.
Assay of cAMP formation
The synthesis of [3H]cyclic AMP in cells prelabeled with [3H]adenine was determined by a modification of the method of Shimizu et al. (1969), as described previously (Gan et al., 1995). Culture medium was removed by aspiration and replaced by 3 ml of balanced salt solution (BSS: in mM: NaCl, 125.4; KCl, 3.6; MgCl2, 1.2; CaCl2, 1.15; NaHCO3, 22.6; Na2HPO4, 0.4; NaH2PO4, 0.1; Na2SO4, 1.2; D-glucose, 10) containing 5 μCi of [2,8-3H] adenine (20.7 Ci/mmol). Cells were returned to the incubator for 2 h, after which the [3H]adenine solution was replaced by 2.5 ml of BSS. After a 10 min preincubation with quinpirole, nitrendipine, or vehicle, 1 ml of BSS containing test compounds (35 mM KCl, A23187, forskolin, or vehicles) was added and the samples were incubated for an additional 20 min. The incubation was terminated by addition of 0.25 ml of 77% trichloroacetic acid. Culture dishes were scraped with a spatula, and cells and medium were transferred to tubes. The dishes were washed with 0.5 ml of BSS, and the wash was added to the tubes. An aliquot (50 μ1) of 10 mM cAMP was added as carrier. Samples were homogenized and centrifuged at 30,000 g for 10 min. Aliquots (50 μl) of supernatant fraction were taken for determination of total radioactivity. [3H]cAMP was isolated by sequential chromatography on Dowex 50W-X4 and alumina as described by Minneman et al. (1979), except that the bed dimension of the Dowex 50W-X4 columns was 0.8 × 3 cm. The data are expressed as percent conversion ([3H]cAMP × 100/total 3H).
Adenylyl cyclase assay
Calcium-stripped photoreceptor membranes were prepared following the method of Gnegy and Treisman (1981). Photoreceptor cells from 60 mm dishes were scraped in 75 μl TEMG buffer (10 mM Tris-maleate, pH 7.5, 1.2 mM EGTA, 1 mM MgSO4, 10 μM GTP), resultant suspensions pooled, and cells disrupted with a teflon on glass homogenizer. Membranes were precipitated by centrifugation (20,000 g, 5°C, 20 min). The pellet was resuspended and washed twice more in TEMG buffer. Finally, the pellets were resuspended in TEM buffer (10 mM Tris-Maleate, pH 7.5, 1.2 mM EGTA, 1 mM MgSO4) and stored at −70°C (final protein concentration: 1.3–2 mg prot/ml).
Adenylyl cyclase activity assay was conducted essentially according to the method described by Gnegy and Treisman (1981). Briefly, 186.1 μl of a reaction mix (final concentrations: 80 mM Tris-Maleate (pH 7.5), 10 μM GTP, 1 mM DTT, 5 mM MgSO4, 0.5 mM cAMP, 5 mM phosphocreatine, 250 μg/tube creatine phosphokinase, 1 unit/tube adenosine deaminase, 50 μM ATP and 0.5 μCi/tube [α-32P]ATP) was added to each reaction tube. Next, either 10 μM forskolin, 100 μM CaCl2 plus 120 nM calmodulin, 100 μM quinpirole and H20 were added to complete a volume of 235 μl. This mix was preincubated for 10 min at 37°C. Finally, 15 μl of membrane preparation (approx. 20–30 μg prot) was added to initiate the reaction. The calculated free-Ca2+ concentration under these conditions was 28 μM. After 10 min, the reaction was terminated by the addition of 750 μl of a solution that contained 9.33% TCA and [3H]cAMP (4,000 dpm) as internal standard. Reaction tubes were centrifuged (20,000 g, 10 min, 4°C) and 1 ml of supernatant was used for [32P]cAMP isolation following the chromatographic procedure used for cAMP accumulation experiments. Recovery, as assessed by [3H]cAMP, was 68–80 %. Assays were performed in triplicate and data were corrected for recovery.
Protein content of cell membranes was determined by the method of Lowry et al. (1951), using bovine serum albumin (BSA) as standard.
Arylalkylamine-N-acetyltransferase (AANAT)
AANAT activity was assayed in cell homogenates as described previously (Pozdeyev et al., 2006), by measuring the catalytic conversion of tryptamine to N-acetyltryptamine.
Statistical analysis
Data are expressed mean ± standard error of the mean (S.E.M.), and were analyzed for statistical significance by one-way analysis of variance (ANOVA) with Student-Newman-Keuls multiple comparison test.
Materials
Materials were obtained from the following sources: [α-32P]ATP (spec. act. 3000 mCi/mg), [2,8-3H]cAMP (31.3 Ci/mmol), [2,8-3H]adenine (20.7 Ci/mmol), 45Ca2+ (30 mCi/mg) from DuPont/New England Nuclear (Boston, MA); ATP, GTP, cyclic AMP, EGTA, mannitol, creatine phosphokinase (E.C. 2.7.3.2), adenosine deaminase (E.C. 3.5.4.4), phosphocreatine, DL-dithiothreitol, calmodulin, Trizma base, poly-L-ornithine (MW 30,000–70,000), linoleic acid/bovine serum albumin, dopamine, SCH 23390, L 745,870 and PD 168,077 from Sigma Aldrich Co. (St. Louis, MO). Forskolin, and A23187, Calbiochem (La Jolla, CA); L-glutamine, trypsin 0.25% from GIBCO (Grand Islang, NY, USA); fetal bovine serum from Hyclone (Logan, UT, USA) or Atlanta Biologicals (Atlanta, GA, USA); Fura–2AM, Fura-2 pentasodium, Pluronic F 127, and Calcium Calibration Kit 2 from Molecular Probes Inc. (Eugene, OR, USA).
Acknowledgments
This work was supported by NIH grants EY014764 and EY004864. The authors gratefully acknowledge the assistance of Dr. Nikita Pozdeyev, Dr. Rashidul Haque, and Ms. Jane Abbey.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature references
- 1.Adler R, Lindsey JD, Elsner CL. Expression of cone-like properties by chick embrio retina cells in glial-free monolayer cultures. J Cell Biol. 1984;99:1173–1178. doi: 10.1083/jcb.99.3.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akopian A, Johnson J, Gabriel R, Brecha N, Witkovsky P. Somatostatin modulates voltage-gated K+ and Ca2+ currents in rod and cone photoreceptors of the salamander retina. J Neurosci. 2000;20:929–936. doi: 10.1523/JNEUROSCI.20-03-00929.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akopian A, Witkovsky P. D2 dopamine receptor-mediated inhibition of a hyperpolarization-activated current in rod photoreceptors. J Neurophysiol. 1996;76:1828–1835. doi: 10.1152/jn.1996.76.3.1828. [DOI] [PubMed] [Google Scholar]
- 4.Alonso-Gómez AL, Iuvone PM. Melatonin biosynthesis in cultured chick retinal photoreceptor cells: calcium and cyclic AMP protect serotonin N-acetyltransferase from inactivation in cycloheximide-treated cells. J Neurochem. 1995;65:1054–1060. doi: 10.1046/j.1471-4159.1995.65031054.x. [DOI] [PubMed] [Google Scholar]
- 5.Avendano G, Butler BJ, Iuvone PM. K+- Evoked depolarization incceases serotonin N-acetyltransferase activity in photoreceptor-enriched retinal cell cultures. Involvement of calcium influx through L-type calcium channels. Neurochem Int. 1990;17:117–126. doi: 10.1016/0197-0186(90)90075-5. [DOI] [PubMed] [Google Scholar]
- 6.Blazynski C, Perez MT. Adenosine in vertebrate retina: localization, receptor characterization, and function. Cell Mol Neurobiol. 1991;11:463–484. doi: 10.1007/BF00734810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cahill GM, Besharse JC. Resetting the circadian clock in cultured Xenopus eyecups: regulation of retinal melatonin rhythms by light and D2 dopamine receptors. J Neurosci. 1991;11:2959–2971. doi: 10.1523/JNEUROSCI.11-10-02959.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen AI. Increased levels of 3′,5′-cyclic adenosine monophosphate induced by cobaltous ion or 3-isobutylmethylxantine in the incubated mouse retina: evidence concerning location and response to ions and light. J Neurochem. 1982;38:781–796. doi: 10.1111/j.1471-4159.1982.tb08699.x. [DOI] [PubMed] [Google Scholar]
- 9.Cohen AI, Blazynski C. Dopamine and its agonists reduce a light-sensitive pool of cyclic AMP in mouse photoreceptors. Vis Neurosci. 1990;4:43–52. doi: 10.1017/s0952523800002753. [DOI] [PubMed] [Google Scholar]
- 10.Cohen AI, Todd RD, Harmon S, O’Malley KL. Photoreceptors of mouse retinas possess D4 receptors coupled to adenylate cyclase. Proc Natl Acad Sci USA. 1992;89:12093–12097. doi: 10.1073/pnas.89.24.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gnegy M, Treisman G. Effect of calmodulin on dopamine-sensitive adenylate cyclase activity in rat striatal membranes. Mol Pharmacol. 1981;19:256–263. [PubMed] [Google Scholar]
- 12.Gan J, Alonso-Gomez AL, Avendano G, Johnson B, Iuvone PM. Melatonin biosynthesis in photoreceptor-enriched chick retinal cell cultures: role of cyclic AMP in the K+-evoked, Ca2+-dependent induction of serotonin N-acetyltransferase activity. Neurochem Int. 1995;27:147–155. doi: 10.1016/0197-0186(95)00035-7. [DOI] [PubMed] [Google Scholar]
- 13.Hagins WA, Penn RD, Yoshikami S. Dark current and photocurrent in retinal rods. Biophys J. 1970;10:380–412. doi: 10.1016/S0006-3495(70)86308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hillman DW, Lin D, Burnside B. Evidence for D4 receptor regulation of retinomotor movement in isolated teleost cone inner-outer segments. J Neurochem. 1995;64:1326–1335. doi: 10.1046/j.1471-4159.1995.64031326.x. [DOI] [PubMed] [Google Scholar]
- 15.Iuvone PM, Avendano G, Butler BJ, Adler R. Cyclic AMP-dependent induction of serotonin N-acetyltransferase activity in photoreceptor-enriched chick retinal cell cultures: characterization and inhibition by dopamine. J Neurochem. 1990;2:673–682. doi: 10.1111/j.1471-4159.1990.tb04186.x. [DOI] [PubMed] [Google Scholar]
- 16.Iuvone PM, Besharse JC. Dopamine receptor-mediated inhibition of serotonin N-acetyltransferase activity in retina. Brain Res. 1986;26:168–176. doi: 10.1016/0006-8993(86)90525-1. [DOI] [PubMed] [Google Scholar]
- 17.Iuvone PM, Gan J, Avendano G. K(+)-evoked depolarization stimulates cyclic AMP accumulation in photoreceptor-enriched retinal cell cultures: role of calcium influx through dihydropyridine-sensitive calcium channels. J Neurochem. 1991;57:615–621. doi: 10.1111/j.1471-4159.1991.tb03792.x. [DOI] [PubMed] [Google Scholar]
- 18.Kurenny DE, Moroz L, Turner RW, Sharkey KA, Barnes S. Modulation of ion channels in rod photoreceptors by nitric oxide. Neuron. 1994;13:315–324. doi: 10.1016/0896-6273(94)90349-2. [DOI] [PubMed] [Google Scholar]
- 19.Lowry H, Rosebrough NJ, Farr AL, Randall RJ. Protein measurements with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 20.Manglapus MK, Iuvone PM, Underwood H, Pierce ME, Barlow RB. Dopamine mediates circadian rhythms of rod-cone dominance in the Japanese quail retina. J Neurosci. 1999;19:4132–4134. doi: 10.1523/JNEUROSCI.19-10-04132.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Megaw PL, Boelen MG, Morgan IG, Boelen MK. Diurnal patterns of dopamine release in chicken retina. Neurochem Int. 2006;48:17–23. doi: 10.1016/j.neuint.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Mei YA, Griffon N, Buquet C, Martres MP, Vaudry H, Schwartz JC, Sokoloff P, Cazin L. Activation of dopamine D4 receptor inhibits an L-type calcium current in cerebellar granule cells. Neurosci. 1995;68:107–116. doi: 10.1016/0306-4522(95)00116-z. [DOI] [PubMed] [Google Scholar]
- 23.Nir I, Harrison JM, Haque R, Low MJ, Grandy DK, Rubinstein M, Iuvone PM. Dysfunctional light-evoked regulation of cAMP in photoreceptors and abnormal retinal adaptation in mice lacking dopamine D4 receptors. J Neurosci. 2002;22:2063–2073. doi: 10.1523/JNEUROSCI.22-06-02063.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pierce ME, Besharse JC. Circadian regulation of retinomotor movements. I Interaction of melatonin and dopamine in the control of cone length. J Gen Physiol. 1985;86:671–689. doi: 10.1085/jgp.86.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pozdeyev N, Taylor C, Haque R, Chaurasia SS, Visser A, Thazyeen A, Du Y, Fu H, Weller J, Klein DC, Iuvone PM. Photic Regulation of arylalkylamine N-acetyltransferase binding to 14-3-3 proteins in retinal photoreceptor cells. J Neurosci. 2006;26:9153–9161. doi: 10.1523/JNEUROSCI.1384-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimizu H, Daly JW, Creveling CR. A radioisotopic method for measuring the formation of adenosine 3′,5′-cyclic monophosphate in incubated slices of brain. J Neurochem. 1969;16:1609–1619. doi: 10.1111/j.1471-4159.1969.tb10360.x. [DOI] [PubMed] [Google Scholar]
- 27.Schmitz Y, Witkovsky P. Dependence of photoreceptor glutamate release on a dihydropyridine-sensitive calcium channel. Neurosci. 1997;78:1209–1216. doi: 10.1016/s0306-4522(96)00678-1. [DOI] [PubMed] [Google Scholar]
- 28.Shimizu H, Daly JW, Creveling CR. A radioisotopic method for measuring the formation of adenosine 3′,5′-cyclic monophosphate in incubated slices of brain. J Neurochem. 1969;16:1609–1619. doi: 10.1111/j.1471-4159.1969.tb10360.x. [DOI] [PubMed] [Google Scholar]
- 29.Shulman LM, Fox DA. Dopamine inhibits mammalian photoreceptor Na+,K+-ATPase activity via a selective effect on the alpha3 isozyme. Proc Natl Acad Sci USA. 1996;93:8034–8039. doi: 10.1073/pnas.93.15.8034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stella SL, Jr, Thoreson WB. Differential modulation of rod and cone calcium currents in tiger salamander retina by D2 dopamine receptors and cAMP. Eur J Neurosci. 2000;12:3537–3548. doi: 10.1046/j.1460-9568.2000.00235.x. [DOI] [PubMed] [Google Scholar]
- 31.Stella SL, Jr, Bryson EJ, Cadetti L, Thoreson WB. Endogenous adenosine reduces glutamatergic output from rods through activation of A2-like adenosine receptors. J Neurophysiol. 2003;90:165–174. doi: 10.1152/jn.00671.2002. [DOI] [PubMed] [Google Scholar]
- 32.Thoreson WB, Stella SL, Jr, Bryson EI, Clements J, Witkovsky P. D2-like dopamine receptors promote interactions between calcium and chloride channels that diminish rod synaptic transfer in the salamander retina. Vis Neurosci. 2002;19:235–247. doi: 10.1017/s0952523802192017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uchida K, Iuvone PM. Intracellular Ca2+ concentrations in cultured chicken photoreceptor cells: sustained elevation in depolarized cells and the role of dihydropyridine-sensitive Ca2+ channels. Mol Vis. 1999;5:1. [PubMed] [Google Scholar]
- 34.Wei XY, Rutledge A, Zhong Q, Ferrante J, Triggle DJ. Ca2+ channels in chick neural retina cells characterized by 1,4-dihydropyridine antagonists and activators. Can J Physiol Pharmacol. 1989;67:506–514. doi: 10.1139/y89-080. [DOI] [PubMed] [Google Scholar]
- 35.Wiechmann AF, Vrieze MJ, Dighe R, Hu Y. Direct modulation of rod photoreceptor responsiveness through a Mel(1c) melatonin receptor in transgenic Xenopus laevis retina. Invest Ophthalmol Vis Sci. 2003;44:4522–4531. doi: 10.1167/iovs.03-0329. [DOI] [PubMed] [Google Scholar]
- 36.Witkovsky P. Dopamine and retinal function. Doc Ophthalmol. 2004;108:17–40. doi: 10.1023/b:doop.0000019487.88486.0a. [DOI] [PubMed] [Google Scholar]
- 37.Witkovsky P, Stone S, Besharse JC. Dopamine modifies the balance of rod and cone inputs to horizontal cells of the Xenopus retina. Brain Res. 1988;449:332–336. doi: 10.1016/0006-8993(88)91048-7. [DOI] [PubMed] [Google Scholar]
- 38.Zawilska JB, Rosiak J, Berezinska M, Nowak JZ. L-745,870 suppresses the nighttime serotonin N-acetyltransferase activity in chick retina: in vivo evidence for agonist activity at D4-dopamine receptors. J Neural Transm. 2003;110:219–227. doi: 10.1007/s00702-002-0787-3. [DOI] [PubMed] [Google Scholar]
- 39.Zawilska JB, Derbiszewska T, Sek B, Nowak JZ. Dopamine-dependent cyclic AMP generating system in chick retina and its relation to melatonin biosynthesis. Neurochem Int. 1995;27:535–543. doi: 10.1016/0197-0186(95)00033-5. [DOI] [PubMed] [Google Scholar]