Summary
Ten patients with narcolepsy were given five 20 min opportunities to remain awake throughout the day. Trials were offered at 2 h intervals beginning at 10:00, Polysomnographic variables were monitored during each trial. Sleep latency increased when patients were instructed to maintain wakefulness compared to when instructed to sleep; however, sleep latencies were still lower for narcoleptics than for control subjects. Unexpectedly, we were not always able to document patients’ reports of increased ability to stay awake. The findings suggested that clinical data on symptom control in narcolepsy do not predict ability to stay awake. Objective measures of the ability are potentially more useful in evaluating treatment.
Excessive somnolence is a symptom that is difficult to control in narcolepsy (classification B.6, ASDC 1979), upper airway sleep apnea (classification B.4.a, ASDC 1979), and CNS hypersomnia (classification B.7, ASDC 1979). One problem is the lack of objective and standardized methods to evaluate treatment. The Multiple Sleep Latency test (MSLT) has been useful in documenting the presence of excessive somnolence (Richardson et al. 1978; Carskadon and Dement 1979) and in diagnosing narcolepsy (Mitler et al. 1979; Hartse et al. 1980a). During the MSLT, the patient is required to lie down 5 times during the day and is instructed to attempt to fall asleep at each session. Previous research demonstrates, however, that this multiple nap approach is not particularly useful in evaluating treatment of excessive somnolence. Post-treatment sleep apnea patients fall asleep quite readily (Roth et al. 1980). Furthermore, the treated patient’s ability to sleep on the MSLT is not as relevant as the patient’s ability to stay awake.
We now report on an alternative multiple nap approach designed for measuring ability to stay awake. This polysomnographic procedure, the Maintenance of Wakefulness test (MWT), is an outgrowth of the report by Hartse et al. (1980b) that sleep latency on the MSLT is increased by changing instructions from ‘try to sleep’ to ‘try to stay awake,’ Our protocol involves instructions to stay awake and also requires the patient to sit comfortably throughout the test. Thus the demands more closely approximate common situations of inactivity in which sleepy patients have difficulty staying awake.
Method
Polysomnographic monitoring was done throughout five 20 min trials of ability to maintain wakefulness. Trials were offered at 10:00, 12:00, 14:00, 16:00 and 18:00. The patient was placed in a darkened room, seated in a comfortable chair, and told to stay awake for as long as possible. The only restriction was that the patient must not leave the chair. Between trials, the patient was under surveillance at all times and kept as alert as possible.
Monitored electrographic variables included the electroencephalogram from C3/A2 and O1/A2, the electromyogram from muscles on and beneath the chin, and the electro-oculogram from ROC/A1 and LOC/A2. Scoring criteria were identical to those used for the MSLT (Richardson et al. 1978). Sleep onset was defined by either of the following: (a) 3 consecutive 30 sec epochs of stage 1; or (b) any single, 30 sec epoch of stage 2, 3, 4, or REM sleep. Sleep offset was defined as any two consecutive epochs of wakefulness after sleep onset. Each trial was terminated after any of the following conditions: (a) at minute 20 if no sleep had occurred; (b) after 10 min of continuous sleep as long as the patient had achieved sleep before or during minute 20, or (c) when the patient awakened after minute 20, even if less than 10 min of sleep had occurred. Scoring parameters included sleep latency (elapsed time on each trial before sleep onset) and REM score (number of times that REM sleep occurs with the restriction that only one REM sleep period is scored per trial).
This report comprises data from 10 narcoleptic patients (5 males and 5 females) with a mean age of 43.8 year and 8 volunteer control subjects (4 males and 4 females) with a mean age of 32.9 year. Narcoleptic and control groups did not differ in age (t = 1.76, df =16, P > 0.05). Narcoleptics were tested immediately after a positive diagnosis of narcolepsy by nocturnal polysomnography and MSLT. Patients were without concomitant disorders such as sleep apnea. Narcoleptic patients were polysomnographically monitored during their usual nocturnal sleep prior to the MWT. Control subjects followed identical daytime protocols for the MSLT and MWT.
Treatment efficacy of narcoleptics was assessed by subsequent administrations of the MWT at times during therapy when the patient and the attending physician agreed that at least a 25% clinical improvement in symptoms was achieved by medication. Quantification and standardization of clinical evaluation was accomplished by use of a simple clinical status questionnaire that quantified on a 7-point scale the severity of 5 common symptoms of narcolepsy (sleepiness, sleep attacks, cataplexy, sleep paralysis, and hypnagogic hallucinations) during 12 typical daily situations and mood states (working, driving, sitting, reading, watching television, exercising, lying in bed, excitement, boredom, anger, sadness, and tension). Each symptom scale had a value range of 12 (no symptom) to 84 (severe symptom). We report results for the full-scale scores and for the ‘sleepiness’ and ‘sleep attack’ scales individually.
Statistical analyses by parametric tests such as the t-test and the Pearson r were done to assess differences between and within groups and relationships between variates. However, inspection of the sleep latency data disclosed skewness in the control group due to the ceiling effect produced by the 20 min maximum length of each test. Furthermore, the numerical distributions associated with the scales in the clinical status questionnaire are unknown and unlikely to meet all the assumptions of parametric statistical tests. Therefore, we also used non-parametric procedures, the Kolmogorov-Smirnov 2-sample test to assess between group, the Wilcoxon Matched Pairs Signed Ranks test to assess within group differences, and the Spearman r to assess relationships between variates (Siegel 1956). For brevity, we have summarized our data in the parametric terms of group and condition means. However, we have reported results for the more conservative, non-parametric tests. Results for parametrics tests are mentioned only when conclusions differ from their non-parametric counterparts.
Results
Fig. 1 presents mean sleep latencies for control subjects and for drug-free narcoleptic patients. To facilitate graphic presentation the means ± S.E.M. have been plotted. Means ± S.D. for the 10:00 to 18:00 tests respectively were as follows: controls, 19.13 ± 2.47, 18.25 ± 3.74, 16.81 ± 6.08, 16.63 ± 6.27, 18.44 ± 4.42;narcoleptics, 7.85 ± 7.43, 11,75 ± 6.99, 10.60 ± 8.14, 10.70 ± 7.63, 8.65 ± 6.96. Sleep latency averaged over all tests was 17.85 ± 4.38 for controls and 9.94 ± 6.05 for narcoleptics. Kolmogorov-Smirnov results indicated significant group differences on the 10:00 and the 18:00 tests (both χ2 >8.0, df = 2, p<0.02). Moreover, controls differed significantly from narcolepties in terms of average sleep latency over all tests (χ2 = 8.1, df = 2, P< 0.02). The t-tests also disclosed these significant differences and, in addition, indicated a significant difference between controls and narcoleptics on the 12:00 test (t = 2.37, df = 16, P< 0.05). In terms of ‘successful MWT trials’ (20 min without sleep), controls had 80.0% successful trials compared to 26.0% for narcoleptics. Correlations between age and sleep latency on the MWT (narcoleptics: Spearman r = 0.21, n = 10; controls: Spearman r = 0.29, n = 8) were non-significant.
Fig. 1.
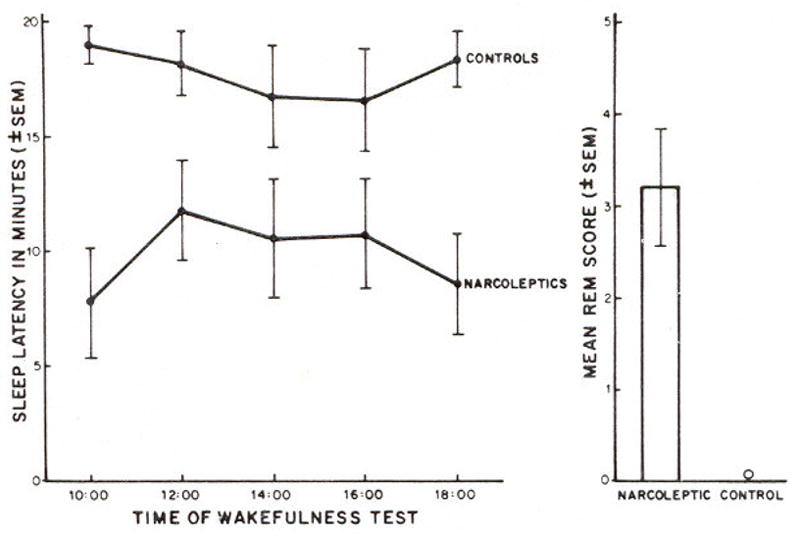
Maintenance of Wakefulness test results for sleep latency as a function of time of test and for number of sleep onset REM sleep periods (REM score) averaged over the 5 tests. Data are from 8 controls and 10 drug-free narcoleptic patients.
Compared with MSLT data, MWT data showed increased sleep latencies for narcoleptics, but not for controls. Mean sleep latency for narcoleptics was 2.44 ± 1.78 min on the MSLT and 9.94 ± 6.05 min on the MWT (Wilcoxon Matched Pairs T = 1, n = 10, P < 0.01). For controls, mean sleep latency was 15.43 ± 3.27 min on the MSLT and 17.85 ± 4.38 min on MWT (Wilcoxon Matched Pairs T = 4, n = 7 with one tie, P = NS). Mean sleep latency on the MWT was independent of mean sleep latency on the MSLT for narcoleptics (Spearman r = −0.12, n = 10, P = NS) and for controls (Spearman r = 0.34, n = 8, P = NS).
It was also clear that narcoleptics showed abnormal transitions into REM sleep in this protocol in spite of the fact that they were sitting. The number of sleep onset REM sleep periods over the 5 sessions of the MWT (REM score) averaged 0.0 ± 0.0 for controls and 3.2 ± 2.0 for narcoleptics.
Eight narcoleptics were tested while drug-free and while on medication. Average full-scale clinical status questionnaire scores improved from a pretreatment level of 193.4 to a posttreatment level of 90.9. Data for these patients are presented in Table I. Of particular interest was the absence of objective change with medication for patients 2, 3 and 6 in spite of considerable subjective improvement. These findings suggest that subjective clinical criteria for evaluating treatment cannot reliably predict ability to stay awake.
TABLE I.
Pre- and posttreatment subjective and MWT variables for 8 patients with narcolepsy.
| Patient | Clinical status questionnaire
|
Medication | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Full-scale
|
Sleepiness
|
Sleep attacks
|
MWT sleep latency
|
||||||
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | ||
| 1 | 109 | 90 | 39 | 38 | 34 | 16 | 19.2 | 20.0 | pemoline, 18.75 mg |
| 2 | 119 | 83 | 39 | 30 | 38 | 16 | 12.4 | 11.8 | pemoline, 18.75 mg |
| 3 | 135 | 92 | 48 | 27 | 30 | 12 | 18.8 | 18.2 | pemoline, 18.75 mg |
| 4 | 246 | 99 | 76 | 41 | 76 | 22 | 3.7 | 9.2 | pemoline, 18.75 mg and protriptyline, 10 mg |
| 5 | 365 | 68 | 76 | 15 | 79 | 17 | 2.0 | 6.7 | protriptyline, 30 mg |
| 6 | 283 | 148 | 70 | 54 | 70 | 34 | 6.5 | 6.5 | pemoline, 56.25 mg and Protriptyline, 60 mg |
| 7 | 127 | 84 | 12 | 36 | 52 | 12 | 4.8 | 6.1 | pemoline, 37.5 mg and protriptyline, 40 mg |
| 8 | 163 | 63 | 66 | 15 | 61 | 12 | 7.7 | 17.4 | pemoline, 37.5 mg |
| Mean | 193.3 | 90.9 | 53.3 | 32.0 | 55.0 | 17.6 | 9.4 | 12.0 | |
| Wilcoxon | |||||||||
| Matched Pairs T value | 0 | 5 | 0 | 3 | |||||
| N | 8 | 8 | 8 | 7(one tie) | |||||
| P | 0.01 | NS | 0.01 | NS | |||||
Discussion
For a conceptual and historical perspective, it is important to note that the MWT is a simple modification of the MSLT (Richardson et al. 1978). In turn, the MSLT itself is a modification of protocols first used in nap studies by Tepas (1967), Weitzman et al. (1974), Carskadon and Dement (1975), Moses et al. (1975) and Webb and Agnew (1975). Thus, a multiple nap approach has been used for some time to quantify changes in ability to sleep. The MWT is used in this study to quantify changes in ability to stay awake. The test has the same rationale and face validity as the MSLT because it is a direct measure of the ability in question.
The MWT appears to measure a different ability than the MSLT for our patient group. Sleep latency increases 300% when instructions are changed from ‘try to sleep’ on the MSLT to ‘try to remain awake’ on the MWT. The pretreatment MWT scores are not subject to the ‘floor effect’ that characterizes the MSLT scores for untreated patients with excessive somnolence. Sleep onset REM periods are obtained for most narcoleptic patients and sleep latency on the MWT is relatively independent of age. Moreover, our findings show that the MWT can document change or the absence of change in ability to maintain wakefulness even when clinical evidence indicates that sleepiness is under control. We plan to routinely use this test to evaluate status in patients under treatment for disorders of excessive somnolence. Such evaluation is needed to monitor and improve treatment. The medico-legal issues surrounding the determination of disability demand objective data such as those provided by the MWT.
Footnotes
This research was supported in part by The Office of Mental Health of The State of New York, and by Grant No. NS 17304 from NINCDS to Dr. Mitler.
References
- Association of Sleep Disorders Centers (H. Roffwarg) Diagnostic classification of sleep and arousal disorders. Sleep Disorders Classification Committee. Sleep. 1979;2:1–137. [PubMed] [Google Scholar]
- Carskadon M, Dement W. Sleep studies on a 90-minute day. Electroenceph clin Neurophysiol. 1975;39:145–155. doi: 10.1016/0013-4694(75)90004-8. [DOI] [PubMed] [Google Scholar]
- Carskadon M, Dement W. Effects of total sleep loss on sleep tendency. Percept Mot Skills. 1979;48:495–506. doi: 10.2466/pms.1979.48.2.495. [DOI] [PubMed] [Google Scholar]
- Hartse K, Roth T, Zorick F, Moyles T. REM sleep episodes during multiple daytime naps of narcoleptic subjects. Sleep Res. 1980a;9:203. [Google Scholar]
- Hartse K, Roth T, Zorick F, Zammit G. The effect of instruction upon sleep latency during multiple daytime naps of normal subjects. Sleep Res. 1980b;9:123. [Google Scholar]
- Mitler M, Van den Hoed J, Carskadon M, Richardson G, Park R, Guillerminault C, Dement W. REM sleep episodes during the multiple sleep latency test in narcoleptic patients. Electroenceph clin Neurophysiol. 1979;46:479–481. doi: 10.1016/0013-4694(79)90149-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses J, Hord D, Lubin A, Johnson L, Naitoh P. Dynamics of nap sleep during a 40-hour period. Electroenceph clin Neurophysiol. 1975;39:627–633. doi: 10.1016/0013-4694(75)90075-9. [DOI] [PubMed] [Google Scholar]
- Richardson G, Carskadon M, Flagg W, Van den Hoed J, Dement W, Mitler M. Excessive daytime sleepiness in man: multiple sleep latency measurements in narcoleptic vs. control subjects. Electroenceph clin Neurophysiol. 1978;45:621–627. doi: 10.1016/0013-4694(78)90162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth T, Hartse K, Zorick F, Conway W. Multiple naps and the evaluation of daytime sleepiness in patients with upper airway sleep apnea. Sleep. 1980;3:425–439. [PubMed] [Google Scholar]
- Siegel S. Nonparametric Statistics for the Behavorial Sciences. McGraw-Hill; New York: 1956. [Google Scholar]
- Tepas D. Evolved brain response as a measure of human sleep and wakefulness. Aerospace Med. 1967;38:148–153. [PubMed] [Google Scholar]
- Webb W, Agnew H. Sleep efficiency for sleep-wake cycles of varied length. Psychophysiology. 1975;12:637–641. doi: 10.1111/j.1469-8986.1975.tb00063.x. [DOI] [PubMed] [Google Scholar]
- Weitzman E, Nogeire C, Perlow M, Fukushima D, Sassin J, McGregor P, Gallagher T, Hellman L. Effects of a prolonged three-hour sleep-wake cycle on sleep stages, plasma cortisol, growth hormone and body temperature in man. J clin Endocr Metab. 1974;38:1018–1030. doi: 10.1210/jcem-38-6-1018. [DOI] [PubMed] [Google Scholar]


