Abstract
Traumatic brain injury (TBI) is a significant clinical problem, yet few effective strategies for treating it have emerged. People that sustain and survive a TBI are left with significant cognitive, behavioral, and communicative disabilities. Apoptotic neuronal death occurs following TBI. Prostate apoptosis response-4 (Par-4) is a death domain-containing protein initially characterized as a critical regulator of apoptosis in prostate cancer cells. We have recently generated and characterized Par-4 transgenic mice in which the expression of the par-4 transgene was limited to cells of neuronal lineage. We now provide evidence that, in cortical neurons from these mice, Par-4 drastically increases apoptotic neuronal death in both in vitro and in vivo models of TBI. In vitro experiments were performed in 7-day-old primary cultures of cortical neurons using a previously published, scratch-induced mechanical trauma model. Neurons that overexpress Par-4 showed not only a significant decrease in overall neuron survival after TBI compared to wild-type cells, but also exhibited a sharper decrease in mitochondrial transmembrane potential, a higher degree of free radical accumulation, and earlier activation of caspase-3 than wild-type cells did. In vivo experiments were performed utilizing a weight drop TBI model. A significantly increased volume of cortical injury and exacerbated activation of caspase-3 were observed in Par-4 transgenic mice when compared to those in wild-type mice. These data suggests that aberrant Par-4 expression exacerbates neuronal cell death following TBI by altering mitochondrial function, enhancing oxidative damage, and execution of apoptosis via caspase activation.
Keywords: Traumatic brain injury, cerebral cortex, apoptosis, prostate apoptosis response-4, cell culture
Introduction
Traumatic brain injury (TBI) is an insult or injury to the brain, not of neurodegenerative nature, but caused by some external force, such as the head suddenly and violently striking or being struck by an object. In the United States, it is estimated that every 15 seconds, someone suffers from a TBI. Over 1 million people are treated in hospital emergency rooms every year, where 50,000 deaths occur, and 80,000 become permanently disabled because of TBI [1, 2]. The combined incidence of Alzheimer's disease, Parkinson's disease, and multiple sclerosis is less than the total number of TBIs experienced. In addition, TBI is one of the leading causes of death among all people below the age of 50 in the United States [2]. The estimated cost for these injuries is more than $60 billion per year [3, 4].
Neurological impairment due to TBI may be temporary or permanent and can cause partial or total functional disability [5]. TBI symptomatology often suggests neuronal loss or damage in key areas of the brain such as the cerebral cortex, hippocampus, thalamus, and the cerebellum [6–8]. Thus, victims of TBI are often left with significant cognitive, behavioral, and communication disabilities [6–8].
Apoptosis may play a role in traumatic neurodegeneration [5, 9–11]. Prostate apoptosis response-4 (Par-4) is a pro-apoptotic leucine zipper protein that plays an important role in neuronal dysfunction and cell death in neurodegenerative disorders [12–15]. Par-4's leucine zipper domain is involved in mediating protein-protein interactions and is also essential for pro-apoptotic functions in neurons [15–18]. A rapid increase in levels of Par-4 expression has been shown to occur in neurons undergoing apoptosis in a variety of paradigms. For example, levels of Par-4 are significantly increased in cortical, hippocampal, and basal forebrain neurons in in vivo and in vitro models of Alzheimer's disease. Significantly higher levels of Par-4 have also been found in lumbar spinal cord samples from ALS patients [15, 16]. Overexpression of Par-4 increases neuron vulnerability to apoptosis and exacerbates neuron cell death, whereas blockade of Par-4 expression attenuates neuronal cell death [16].
Mitochondria play an important role in apoptosis. The organelle releases cytochrome c in response to cell injury [19–23]. Cytochrome c release activates the caspases (a family of proteases that cleave a variety of intracellular proteins) which are the main effectors of apoptosis. Caspase activation leads to morphological changes in the cell which include shrinkage, chromatin condensation, DNA fragmentation, and plasma membrane blebbing. Caspase-3 is the primary executioner caspase. Once activated, caspase-3 cleaves (and deactivates) essential survival proteins such as PARP, XIAP and Lamin B. Overall, these actions amplify the apoptotic signaling cascade, leading to apoptotic cell death [8, 24–28]. Brain trauma also produces a significant amount of oxidative stress, which is known to mediate cell death [24]. Free radicals generated by oxidative stress destroy biological molecules such as proteins, lipids, and nucleic acids [28, 29].
We recently generated and characterized Par-4 transgenic mice in which the expression of the par-4 transgene was limited to cells of neuronal lineage using the neuron-specific enolase (NSE) promoter. Using a combination of in vitro and in vivo cortical impact models of TBI, we found that aberrant Par-4 expression in these mice promotes apoptosis of cortical neurons after TBI by exacerbating mitochondrial dysfunction, increasing accumulation of oxidative free radicals, and activating executioner caspase-3.
Materials and Methods
Generation and Characterization of Neuron-Specific Par-4 Transgenic Mice
We have recently generated and characterized mice transgenic for Par-4 in which expression of the par-4 transgene was limited to cells of neuronal lineage using the NSE promoter. In brief, the Par-4 transgenic mice were generated by microinjection of the pNSE-par4 DNA construct into the pronuclei of fertilized FVB/N ova using the Transgenic Core Facility at the Oklahoma Medical Research Foundation and procedures similar to those described previously [30]. The pNSE-par4 transgenic construct was derived from pNSE-bcl2 by removal of a Hind III and Cla I fragment containing the coding sequence of the human bcl-2 gene, and in frame ligation of a 1.0-kb Hind III/Cla I PCR fragment containing the coding sequence of the rat Par-4 gene. The pNSE-Par4 plasmid was then digested with EcoR I to recover the approximately 4.0 kb NSE-par4 fragment that contains NSE promoter, par-4 cDNA, pA, and SV40 sequence. The purified NSE-Par4 construct was then used for microinjection into zygotes from inbred strain FVB/N. Females were superovulated and mated, zygotes were harvested and fertilized zygotes were injected. Injected zygotes which develop further to the 2-cell stage were reimplanted into the oviduct of pseudo-pregnant Swiss-Webster recipient females. All resulting pups were subject to characterization for transgenic founder animals and further analysis. NSE promoter is active in neurons from as early as E10. We developed a quick PCR-based protocol for genotyping of Par-4 transgenic mice. To confirm the integration of the transgene, genomic DNA from tail biopsies was used to amplify a 236-bp simian virus 40 fragment from pNSE-Par4 vector, which is detectable only in mice transgenic for Par-4, but not in wild-type mice. The primers used for the PCR genotyping protocol were: SV40F: 5′-caggaagctcctctgtgtcc-3′, and SV40R: 5′-tgttgacatttgtgggctgt-3′. Relatively high levels of expression of Par-4 in cortical neurons from the transgenic mice were confirmed by western blot analyses using a monoclonal antibody raised against a recombinant protein corresponding to amino acids 1-334 representing full length Par-4 of rat origin (Santa Cruz Biotechnology, Inc.).
Culture of Primary Neurons
Dissociated cortical neuronal cultures were prepared from postnatal day 1 mouse pups using methods similar to those described previously [15]. Briefly, cerebral cortical tissues from Par-4 transgenic mice or wild-type control mice were removed and incubated for 15 min in Ca++- and Mg++- free Hank's Balanced Saline Solution (Gibco BRL) containing 0.2% Papain. Cells were dissociated by trituration and plated into polyethyleneimine-coated plastic dishes containing Minimum Essential Medium with Earle's salts supplemented with 10% heat-inactivated fetal bovine serum, 2 mM L-glutamine, 1 mM pyruvate, 20 mM KCl, 10 mM sodium bicarbonate and 1 mM Hepes (pH 7.2). Following cell attachment (3-6 hr post-plating), the culture medium was replaced with Neurobasal Medium with B27 supplements (Gibco BRL).
Delivery of Mechanical Trauma In Vitro
Neurons were grown to 7 days before injury was induced using a published in vitro model of TBI. This model comes from the laboratory of Dennis Choi at the Stanford University Medical Center in Stanford, California, that allows neurons and their intrinsic responses to be studied following mechanical injury without complicating or disrupting normal systemic functions. Neurons in culture were injured by drawing a needle across the bottom of the well, producing a linear tear across the well. A total of four scratches were induced in each well, two running vertically and two horizontally, resulting in a total of 9 quadrants [31]. Cell cultures were placed in an incubator at 37°C until a designated post-trauma time point was reached. Cultures were incubated without change of medium. Experiments were performed immediately after mechanical injury (denoted by 0 hrs) and at 4, 8, 12, and 24 hrs after trauma. Uninjured cultures were used as controls.
Quantification of Cell Survival
Neuron survival was quantified by observing neurons under phase contrast microscopy. Viable neurons in premarked fields were counted before experimental treatment and at specified time points thereafter. Neurons with intact neurites of uniform diameter and a cell body with a smooth round appearance were considered viable, whereas neurons with fragmented neurites and vacuolated soma were considered nonviable [32].
Measurement of Mitochondrial Transmembrane Potential
The dye Rhodamine 123 (Rhd123) was used to measure levels of mitochondrial trans-membrane potential, as previously described [33, 34]. In brief, at designated time points after injury, Neurobasal Medium was withdrawn from each well. Cells were incubated for 15 min in 0.5 mL Locke's Medium (154mM NaCl, 5.6 mM KCl, 2.3 mM CaCl2, 1 mM MgCl2, 3.6 mM NaHCO3, 5 mM glucose, 5 mM HEPES, pH 7.2) containing 5 μM of Rhd123. Cells were washed three times with PBS. Confocal images of cellular fluorescence were acquired using a Nikon microscopic image acquisition system. Levels of fluorescence in neurons were quantified by the average pixel intensity per cell using a Kodak 1D 3.6 imaging analysis software.
Measurement of Cellular Hydrogen Peroxide and Superoxide Levels
Hydrogen peroxide levels were measured by microscopic analysis of cellular dichloro-fluorescein diacetate, which is converted into highly fluorescent dichlorofluorescein (DCF) by hydrogen peroxide [29]. Levels of intracellular superoxide anion radical were measured using hydroethidine (HE), which is oxidized to fluorescent ethidium cation by superoxide [35]. In brief, at designated time points, Neurobasal Medium was withdrawn from each well. Cells were incubated for 15 min in 0.5 mL Locke's Medium containing 5 μM of DCF. Cells were washed afterwards three times with PBS. Microscopic images of cellular fluorescence were acquired and levels of fluorescence in neurons were quantified.
Measurement of Caspase-3 Activation In Vitro
Caspase-3 like protease activity was assessed using a protocol that employs the biotinylated caspase substrate N-acetyl-Asp-Glu-Val-Asp-aldehyde (DEVD) [35]. In brief, Neurobasal Medium was withdrawn from each well at designated time points. Cultured cells were fixed with 4% paraformaldehyde. Cells were then exposed for 10 min to Locke's solution containing 0.01% digitonin and were then incubated for 20 min in the presence of 10 μg/ml DEVD-Caspase-3 Inhibitor I, Biotin Conjugate (Calbiochem). Cells were washed three times with PBS (1mL per wash), incubated for 5 min in PBS containing 0.2% Triton X-100, and then incubated for 20 min in the presence of streptavidin-Oregon Green conjugate (Molecular Probes). Cells were washed twice in PBS, and images of cellular fluorescence were acquired using confocal laser scanning microscope. Levels of fluorescence in neurons were quantified as described for mitochondrial analysis.
Animals and Surgical Procedures In Vivo
A total of 80 animals were used in the present study for in vivo experiments and were all housed in group cages on a 12 hr light/dark cycle with food and water available ad libitum. All animal procedures were approved by the institutional animal care and use committee (IACUC). Mice were anesthetized with 2.5% Avertin (0.583 g/kg body wt) and placed in a stereotaxic frame before TBI. The head was positioned in the horizontal plane with the nose bar set at negative 1 [33]. A 3 mm craniotomy was made 2 mm left laterally of the saggital suture and centered between bregma and lambda [35]. A small piece of skull bone was carefully removed without disruption of the dura [33]. Once exposed, the cortex was injured using a published weight drop procedure. A 10-gram, 2.5 mm in diameter metal rod was positioned through a guide shaft mounted to the stereotaxic frame. The guidance tube was positioned directly over the craniotomy site and the rod weight was released from a height of 1 cm creating a 10g/cm impact on the exposed cortex [34]. After injury, Close Liquid Suture (BVC Switzerland) was laid on the dura and allowed to dry. The skin was then sutured together and the animals were placed back in cages on a heating pad to recover from the procedure. Sham animals underwent the same procedures without cortical impact [36–39]. All animals survived the surgical procedures up to 48 hrs.
Perfusion and Quantification of Brain Damage In Vivo
At designated time points of 8, 24, and 48 hrs, animals were anesthetized with 2.5% Avertin (0.583 g/kg body wt) and exsanguinated by cardiac perfusion with saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.3. Brains were removed and post-fixed for 12-24 hrs [40]. Frozen brains were then sectioned at 16 μm in the coronal plane with a freezing microtome, where every third section was mounted onto a glass slide. Glass slides were previously coated overnight with PEI then dried before mounting of brain sections onto slides. Nissl staining was used to quantify the extent of damage sustained to the brain after TBI, according to standard protocols [33]. Every third brain section was stained with cresyl violet. Nissl stained images of each section at the site of the injury and the contralateral cortex were acquired using a Nikon microscope. The extent of cortical injury was quantified at 8, 24, and 48 hrs post-injury by color images of Nissl-stained brain sections at 10 × magnifications using a digital Nikon camera mounted on a light microscope. Sham operated animals were used as controls. The area of healthy, uninjured tissue in each hemisphere was measured using image analysis software (NIH Image version J). The area of the entire cortical region was determined using the Cavalieri method [41]. The damaged cortex was characterized by determining the volume of the cortical lesion or the lack of staining in remaining tissue and was clearly delineated from healthy tissue. The amount of cortical damage was expressed as a percentage of the total cortical volume [36].
Assessment of Caspase-3 Activation In Vivo by Immunofluorescent Microscopy
Activation of caspase-3 following TBI in mouse brain in vivo was assessed using a method similar to that described previously [42]. A polyclonal antibody specific for activated form of caspase-3 (Cell Signaling Technology, Danvers, MA, U.S.A.) was used that detects endogenous levels of the large fragment (17/19 kDa) of activated caspase-3 resulting from cleavage adjacent to Asp175. This antibody does not recognize full length caspase-3 or other cleaved caspases. In brief, brain sections were incubated at 4°C overnight in blocking solution (10% goat serum, 1% BSA, 0.1% Triton X-100 in PBS, pH 7.4). The sections were then incubated with the primary anti-activated caspase-3 antibody in PBS at the 1:500 dilutions for 90 minutes. After three washes in PBS, biotinylated anti-rabbit IgG secondary antibody was applied to sections and incubated at room temperature in the dark for 1 hr. Sections were then washed three times and incubated in the dark with Fluorescein Avidin D for 10 min at room temperature. Brain sections were washed for 5 min in PBS buffer. The sections were mounted, analyzed, and digitally photographed. Images of immunofluorescence of activated caspase-3 were acquired using a digital Nikon camera, mounted on a light microscope with 10 × objective. The average pixel intensity per cell was determined using the Kodak 1D 3.6 imaging analysis software.
Results
Par-4 Exacerbates Death of Cortical Neurons Following Mechanical Injury In Vitro
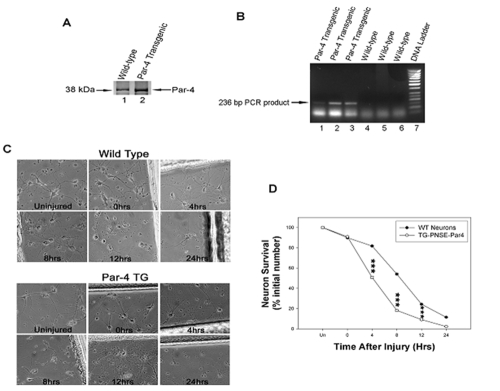
We recently generated Par-4 transgenic mice in which expression of the par-4 transgene was limited to cells of neuronal lineage using the NSE promoter. Transgenic mice were characterized by both Western blot analysis of Par-4 protein expression and PCR genotyping of integration of the transgene. As shown in Figure 1A, levels of Par-4 were clearly higher in Par-4 transgenic mice than those in wild-type animals. Genotype of the transgenic animals were also confirmed using genomic DNA from tail biopsies to PCR amplify a 236-bp simian virus 40 fragment from pNSE-Par4 vector, which is detectable only in mice transgenic for Par-4, but not in wild-type mice (Figure 1B). Primary cultures of mouse cortical neurons from wild-type and Par-4 transgenic mice were established and cultured for 7 days. Photographs of cells under the phase contrast microscope were collected at time points immediately after the scratch injury, denoted by (i.e., ∼0 hrs) and at 4, 8, 12, and 24 hrs and in uninjured cultures as a control for both wild-type and Par-4 transgenic cultures (Figure 1C). Neuronal assessment was determined by counting the number of healthy neurons within 2 mm of the injury (scratch mark). We observed a progressive decline in the percentage of surviving cells after the injury induction in both wild-type and Par-4 transgenic neurons (Figures 1C and 1D). However, at 4 hrs, we observed a significant difference in the percentage of cell survival after mechanical injury between wild-type and Par-4 transgenic cultures. By 4 hrs, approximately 50% of neurons that overexpressed Par-4 were seen to be nonviable. In contrast, it took nearly twice as long (i.e., ∼8 hrs) to see similar results in neurons from wild-type mice. By 24 hrs, a small percentage of neurons from the wild-type remained viable, while neurons from Par-4 transgenic mice were almost entirely eliminated. These results indicate that overexpression of Par-4 exacerbates cell death after mechanical injury in cortical neurons.
Figure 1.
Neurons that overexpress Par-4 show an exacerbated rate of cell death following the mechanical insult compared to wild-type neurons. A. Representative Western blotting analysis showing high level expression of Par-4 protein in cortical neurons from transgenic mice. Primary cultures of cortical neurons were established and western blot analyses were performed in 7-day old cultures using a monoclonal antibody raised against a recombinant protein corresponding to amino acids 1-334 representing full length PAR4 of rat origin (Santa Cruz Biotechnology, Inc.). Relatively high levels of Par-4 protein were detected in neurons from Par-4 transgenic mice (lane 2), while only minimal levels of endogenous Par-4 were observed in normal neurons from wild-type mice (lane 1). B. Genotyping of Par-4 transgenic mice using a PCR-based protocol. We have developed a quick PCR-based assay of the DNA from tail biopsies to amplify a 236-bp simian virus 40 fragment from pNSE-Par4 vector, which is detectable only in mice transgenic for Par-4 (lanes 1-3), but not in wild-type mice (lanes 4-6). C. Phase contrast images of cortical neurons from wild-type and Par-4 transgenic mice before and after injury. Overall neuron survival decreases at each time point. D. Graph shows percentage of surviving cells at each time point as indicated: uninjured (Un), immediately after injury (0), 4, 8, 12 and 24 hrs after injury. A higher percentage of cell death was observed in Par-4 transgenic cortical neurons compared to wild-type neurons. Values are the means and standard derivations of determinations from eight separate cultures (20-30 neurons analyzed per culture). ***p<0.001 compared with corresponding values in cells from wild-type mice. ANOVA with Scheffe's post-hoc tests.
Par-4 Alters Mitochondrial Transmembrane Potential in Cortical Neurons after Mechanical Injury
We next examined the effect of Par-4 on mitochondrial function in cortical neurons after mechanical injury. Experiments were performed at several time points immediately after the scratch injury (i.e., ∼0 hrs) and at 4, 8, 12, and 24 hrs and in uninjured cultures as a control for both wild-type and Par-4 transgenic cultures. As seen in Figure 2A, Rhd123 fluorescence begins to diminish immediately after the mechanical injury in Par-4 transgenic neurons. Similar findings are not present in wild-type cells until around 12 hrs after injury. Figure 2B shows a progressive decrease in the mitochondrial membrane potential, as reflected by the attenuation of Rhd123 fluorescence in wild-type neurons, and an even sharper decrease in fluorescence from neurons that overexpress Par-4. Consistent with phase contrast imaging, we observed a leftward shift in the curve for Par-4 transgenic mice, indicating that the mitochondrial membrane becomes compromised much quicker in Par-4 transgenic neurons than in wild-type neurons.
Figure 2.
Neurons that overexpress Par-4 exhibit enhanced mitochondrial dysfunction after mechanical injury compared to wild-type neurons. A. Rhd123 fluorescence (a measure of mitochondrial transmembrane potential) was measured before injury and at specific time intervals in both wild-type (top left) and Par-4 transgenic neurons (bottom left). Fluorescence intensity diminishes after injury, indicating the mitochondrial membrane potential has been compromised. B. Graph shows the mean average pixel intensity at each time point before and after injury. Par-4 transgenic neurons have significantly lower fluorescence intensity, beginning immediately after mechanical injury, when compared to wild-type neurons. Values are the means and standard derivations of determinations from eight separate cultures (20-30 neurons analyzed per culture). ***p<0.001 compared with corresponding values in cells from wild-type mice. ANOVA with Scheffe's post-hoc tests.
Par-4 Increases Accumulation of Superoxide and Hydrogen Peroxide in Cortical Neurons Following Mechanical Injury
Oxidative stress plays a major role in TBI-induced neuronal injury. We measured levels of intracellular superoxide anion radical with hydroethidine, which is oxidized to fluorescent ethidium cation by superoxide. The fluorescent dye DCF was used to quantify relative levels of intracellular hydrogen peroxide. Experiments were performed in uninjured cell cultures and 12 hrs post injury in both wild-type and Par-4 transgenic neurons. As shown in Figure 3, uninjured cells exhibited relatively low amounts of superoxide and hydrogen peroxide in both wild-type and Par-4 transgenic neurons. However, at 12 hrs, there is a significantly greater amount of superoxide and hydrogen peroxide produced in Par-4 transgenic neurons compared to that found in wild-type neurons.
Figure 3.
Cortical neurons overexpressing Par-4 show an increased accumulation of superoxide and hydrogen peroxide following mechanical injury compared to wild-type neurons. A. HE fluorescence (a measure of superoxide levels) and C. DCF fluorescence (a measure of hydrogen peroxide levels) were measured before injury and 12 hrs post injury in both wild-type (top) and Par-4 transgenic neurons (bottom). Fluorescence intensity of HE and DCF increases 12 hrs post injury in wild-type and Par-4 transgenic cortical neurons. B. Bar graph shows a higher accumulation of superoxide in neurons that overexpress Par-4 compared to wild-type neurons. D. Similar results are observed for the production of hydrogen peroxide in neurons overexpressing Par-4 when compared to wild-type neurons. Values are the means and standard derivations of determinations from eight separate cultures (20-30 neurons analyzed per culture). ***p<0.001 compared with corresponding values in cells from wild-type mice. ANOVA with Scheffe's post-hoc tests.
Cortical Neurons Overexpressing Par-4 Show an Increased Activation of Caspase-3
Activation of caspase-3 represents a point-of-no-return step of execution of cell death in the apoptotic pathways. We measured the amount of activated caspase-3 following mechanical injury to cultured cortical neurons. Experiments were performed at time intervals immediately after mechanical injury (0 hrs) and at 4, 8, 12, and 24 hrs post-injury and in uninjured cultures as a control, in both wild-type and Par-4 transgenic neurons. Consistent with data from cell survival and mitochondrial membrane potential studies, we found a significant increase in caspase-3 fluorescence intensity as time progresses after injury in both wild-type and Par-4 transgenic neurons (Figure 4A). However, we noticed an earlier activation of caspase-3 in Par-4 transgenic neurons. There was a great amount of caspase-3 activation by 4 hrs in Par-4 transgenic neurons, whereas it took 8 hrs to see similar results from wild-type cultures. This trend continues to be evident at 8, 12, and 24 hrs after mechanical injury (Figure 4B). By 24 hrs, essentially all neurons from Par-4 transgenic mice have succumbed to cell death and this may explain the decline in the overall amount of DEVD fluorescence, whereas a small percentage of cells from wild-type remain alive and viable.
Figure 4.
Par-4 overexpression in cortical neurons facilitates an earlier activation of caspase-3, contributing to a quicker progression through cell death. A. Levels of DEVD fluorescence (a measure of caspase-3 activation) were measured before injury and at specific time points after injury. Caspase-3 becomes activated after mechanical injury in both wild-type (top left) and Par4 transgenic neurons (bottom left). B. Quantification of DEVD fluorescence shows a higher degree of caspase-3 activation at each time point in Par-4 transgenic neurons compared to that in wild-type neurons, except at 24 hrs, when essentially all neurons overexpressing Par-4 have perished. Values are the means and standard derivations of determinations from eight separate cultures (20-30 neurons analyzed per culture). ***p<0.001. ANOVA with Scheffe's post-hoc tests.
Cortical Damage Increases Following Weight Drop Injury in Par-4 Transgenic Mice In Vivo
We then performed in vivo experiments to corroborate the data obtained in cell cultures. We utilized a published weight drop model of cortical impact. Relative area of cortical damage was quantified by Nissl staining in coronal brain sections. As shown in Figure 5A, each animal demonstrates trauma to the cerebral cortex directly below the impact site, while the contralateral cortex remains unaffected. The amount of cortical damage progresses at each time point, with a larger volume of cortical damage in mice that overexpress Par-4. Figure 5B shows a significant difference in volume of the cortical lesion between Par-4 transgenic and wild-type mice.
Figure 5.
Traumatic brain injury is exacerbated in Par-4 transgenic mice compared to wild-type mice. A. Coronal sections through the damaged cerebral cortex of adult mice were stained with cresyl violet. Nissl staining was measured in sham-operated mice and at 8, 24, and 48 hr intervals after cortical impact. Cortical damage is observed in the ipsilateral cortex (Ips), while the contralateral cortex (Ctl) is unaffected in wild-type (top left) and Par-4 transgenic mice (bottom left). B. Graph shows the percentage of cortical lesion volume in ipsilateral (Ips) and contralateral (Ctl) sides of the cortical impact in both wild-type and Par-4 transgenic mice. A significantly larger cavity was produced in the ipsilateral cortex (Ips) by 8 hrs and more prominently by 24 and 48 hrs after cortical impact in mice that overexpress Par-4 when compared to wild-type mouse brains. The damaged portion of the cortex was characterized by faint or lack of staining and was clearly delineated from healthy brain tissue. The amount of cortical damage is expressed as a percentage of total cortical volume, calculated using the Cavalieri principle (see Materials and Methods). Values are the means and standard derivation of determinations made from coronal brain sections through the area of impact and the contralateral side of impact. *p<.0.05, ***p<0.001 compared with corresponding values in sections from wild-type mice. ANOVA with Scheffe's post-hoc tests.
Activation of Caspase-3 Increases in Cerebral Cortex of Par4 Transgenic Mice Following Cortical Impact
Using immunofluorescent histochemistry and an antibody that recognizes activated caspase-3, we examined if there was an increased activation of caspase-3 in cortical neurons from Par-4 transgenic mice after TBI in vivo. As seen in Figure 6A, the amount of caspase-3 activation in the cortical neurons was seen to gradually increase after TBI in both wild-type and Par-4 transgenic animals. However, there was a significant difference in the amount of caspase-3 activation between wild-type and Par-4 transgenic animals. Animals that overexpress Par-4 showed a much greater degree of activation of caspase-3 in the cortical neurons after TBI. We also measured caspase-3 activation in the contralateral cortex where no differences between wild-type and transgenic animals were observed (Figure 6B). These in vivo data is consistent with data from cell cultures, and provides physiologically meaningful evidence for the involvement of Par-4 in neuronal cell death following cortical impact.
Figure 6.
Caspase-3 activation is significantly greater in transgenic mice that overexpress Par-4 compared to wild-type mice. A. Coronal sections were immunostained with the mouse caspase-3 primary antibody, which only recognizes and labels the cleaved, activated form of caspase-3. Caspase-3 activation was measured in sham-operated mice and at 8, 12, and 24 hr intervals after weight drop. Caspase-3 activation increases at each time point in the ipsilateral cortex (Ips), while activation in the contralateral cortex (Ctl) remains unchanged in both wild-type (top left) and Par-4 transgenic mice (bottom left). B. Graph shows a higher degree of caspase-3 activation in Par-4 transgenic mice following cortical impact in the ipsilateral cortex (Ips), but not in the contralateral cortex (Ctl). Values are the means and standard derivations of determinations made from coronal brain sections through the area of impact and adjacent to the area of impact. ***p<0.001 compared with corresponding values in cells from wild-type mice. ANOVA with Scheffe's post-hoc tests.
Discussion
Apoptosis represents one of the major mechanisms in neuronal injury following TBI [6–8]. The generation and characterization of neuron-specific Par-4 transgenic mice provided a unique model to investigate the roles of aberrant Par-4 expression in neuronal apoptosis after TBI. Although mice that overexpress Par-4 did not express an overt abnormal phenotype, we found that they are significantly more vulnerable to TBI-induced neuronal death. We used a combination of in vitro and in vivo models of cortical impact induced TBI, and found that Par-4 plays a critical role in the progressive death of cortical neurons. This conclusion is supported by several pieces of experimental evidence obtained from this study: (1) in both in vitro and in vivo models, death of cortical neurons following mechanical impact was significantly exacerbated by Par-4 in transgenic animals; (2) mitochondrial dysfunction and accumulation of free radicals were significantly increased after TBI in cortical neurons from Par-4 transgenic mice; and (3) in both in vitro and in vivo models, activation of caspase-3, a critical executioner caspase in apoptosis, was significantly enhanced by Par-4 after TBI. These results identify Par- 4 as a critical contributor to neuronal apoptosis in models of TBI.
Several potential mechanisms may be considered for the observed exacerbation of cell death after TBI in cortical neurons that overexpress Par-4. A shift in the balance between pro- and anti-apoptotic factors towards the expression of proteins that promote cell death may be one underlying mechanism. Of importance, Par-4-dependent cell death seems to be mediated by decreased transcription of the anti-apoptotic bcl-2 [43]. Ectopic expression of Bcl-2 abrogates Par-4 activity [43, 44]. At least in certain types of cells, the cell death-enhancing actions of Par-4 seem to require Par-4 translocation from the cytoplasm to the nucleus where it directly regulates bcl-2 promoter activity [44, 45]. Thus, it is possible that Par-4 functions as a suppressor of bcl-2 transcription to enhance neuronal death following TBI. Another potential mechanism by which Par-4 enhance neuronal death following TBI is through binding to the atypical protein kinase C (ζPKC). ζPKC is involved in activation of NF-κB, a transcription factor that often confers anti-apoptotic activity by enhancing the expression of inhibitors of apoptosis (IAPs). Par-4 interacts with ζPKC and inhibits its activity. These potential mechanisms need to be further investigated.
An essential role for Par-4 in TBI-induced neuronal injury can be demonstrated by examining if blocking Par-4 activity confers significant protection against neuronal cell death after TBI. Par-4 activity can be significantly attenuated using several different approaches. In previous studies, we have found that Par-4 contains a leucine zipper domain that mediates protein-protein interactions essential for sensitization of cells to apoptosis [15, 46–49]. Overexpression of a deletion mutant of Par-4 that encodes only the leucine zipper domain of Par-4 (Leu.zip) has been shown to block Par-4 activity in a dominant negative fashion by inhibiting binding of other protein(s) with the full-length Par-4 [15, 46–49]. We also found that apoptosis antagonizing transcription factor (AATF) is an endogenous interaction partner and potent antagonist of Par-4 activity [49]. In addition, we have demonstrated that Par-4 expression can be effectively silenced by RNA interference (RNAi) in vitro and in vivo [13, 40, 49]. Experiments are now underway in our lab to determine if blocking Par-4 expression would significantly reduce neuronal injury following TBI.
TBI is a major clinical problem with exceptionally high morbidity and mortality. There has been no effective treatment for TBI. Although the precise mechanisms by which Par-4 alters mitochondrial function and various elements of neuronal cell death pathways after TBI still need to be further investigated, this study identifies Par-4 as a potential target for developing therapeutic strategies for TBI.
Acknowledgments
We thank Paul Tompkins in the Department of Neurosurgery at the University of Oklahoma Health Sciences Center for his excellent technical assistance in establishing the mouse cortical impact models. This work was supported by a research startup fund to Q.G. from The University of Oklahoma Health Sciences Center (OUHSC).
References
- 1.DeFord SM, Wilson MS, Rice AC, Clausen T, Rice LK, Barabnova A, Bullock R, Hamm RJ. Repeated mild brain injuries result in cognitive impairment in B6C3F1 mice. J Neurotrauma. 2002;19:427–438. doi: 10.1089/08977150252932389. [DOI] [PubMed] [Google Scholar]
- 2.Tweedie D, Milman A, Holloway HW, Li Y, Harvey BK, Shen H, Pistell PJ, Lahiri DK, Hoffer BJ, Wang Y, Pick CG, Greig NH. Apoptotic and behavioral sequelae of mild brain trauma in mice. J Neurosci Res. 2007;85:805–815. doi: 10.1002/jnr.21160. [DOI] [PubMed] [Google Scholar]
- 3.Vink R, Nimmo AJ, Cernak I. An overview of new and novel pharmacotherapies for use in traumatic brain injury. Clin Exp Pharmacol Physiol. 2001;28:919–921. doi: 10.1046/j.1440-1681.2001.03548.x. [DOI] [PubMed] [Google Scholar]
- 4.Hatton J. Pharmacological treatment of traumatic brain injury: a review of agents in development. CNS Drugs. 2001;15:553–581. doi: 10.2165/00023210-200115070-00005. [DOI] [PubMed] [Google Scholar]
- 5.Wong J, Hoe NW, Zhiwei F, Ng I. Apoptosis and traumatic brain injury. Neurocrit Care. 2005;3:177–182. doi: 10.1385/NCC:3:2:177. [DOI] [PubMed] [Google Scholar]
- 6.Raghupathi R, Graham DI, McIntosh TK. Apoptosis after traumatic brain injury. J Neurotrauma. 2000;17:927–938. doi: 10.1089/neu.2000.17.927. [DOI] [PubMed] [Google Scholar]
- 7.Raghupathi R. Cell death mechanisms following traumatic brain injury. Brain Pathol. 2004;14:215–22. doi: 10.1111/j.1750-3639.2004.tb00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keane RW, Kraydieh S, Lotocki G, Alonso OF, Aldana P, Dietrich WD. Apoptotic and antiapoptotic mechanisms after traumatic brain injury. J Cereb Blood Flow Metab. 2001;21:1189–1198. doi: 10.1097/00004647-200110000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Dressler J, Hanisch U, Kuhlisch E, Geiger KD. Neuronal and glial apoptosis in human traumatic brain injury. Int J Legal Med. 2006 doi: 10.1007/s00414-006-0126-6. [DOI] [PubMed] [Google Scholar]
- 10.Lau A, Arundine M, Sun HS, Jones M, Tymianski M. Inhibition of caspase-mediated apoptosis by peroxynitrite in traumatic brain injury. J Neurosci. 2006;26:11540–11553. doi: 10.1523/JNEUROSCI.3507-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yildirim E, Ozisik K, Ozisik P, Emir M, Yildirim E, Misirlioglu M, Tuncer S, Kilinc K. Apoptosis-related gene bcl-2 in lung tissue after experimental traumatic brain injury in rats. Heart Lung Circ. 2006;15:124–129. doi: 10.1016/j.hlc.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Chung H, Seo S, Moon M, Park S. IGF-I inhibition of apoptosis is associated with decreased expression of prostate apoptosis response-4. J Endocrinol. 2007;194:77–85. doi: 10.1677/JOE-07-0073. [DOI] [PubMed] [Google Scholar]
- 13.Xie J, Awad KS, Guo Q. RNAi knockdown of Par-4 inhibits neurosynaptic degeneration in ALS-linked mice. J Neurochem. 2005;92:59–71. doi: 10.1111/j.1471-4159.2004.02834.x. [DOI] [PubMed] [Google Scholar]
- 14.Xie J, Chang X, Zhang X, Guo Q. Aberrant induction of Par-4 is involved in apoptosis of hippocampal neurons in presenilin-1 M146V mutant knock-in mice. Brain Res. 2001;915:1–10. doi: 10.1016/s0006-8993(01)02803-7. [DOI] [PubMed] [Google Scholar]
- 15.Guo Q, Fu W, Xie J, Luo H, Sells SF, Geddes JW, Bondada V, Rangnekar VM, Mattson MP. Par-4 is a mediator of neuronal degeneration associated with the pathogenesis of Alzheimer disease. Nat Med. 1998;4:957–962. doi: 10.1038/nm0898-957. [DOI] [PubMed] [Google Scholar]
- 16.Xie J, Guo Q. Par-4 inhibits choline uptake by interacting with CHT1 and reducing its incorporation on the plasma membrane. J Biol Chem. 2004;279:28266–28275. doi: 10.1074/jbc.M401495200. [DOI] [PubMed] [Google Scholar]
- 17.El-Guendy N, Rangnekar VM. Apoptosis by Par-4 in cancer and neurodegenerative diseases. Exp Cell Res. 2003;283:51–66. doi: 10.1016/s0014-4827(02)00016-2. [DOI] [PubMed] [Google Scholar]
- 18.Dhillon HS, Dong GX, Yurek DM, Estus S, Rangnekar VM, Dendle P, Prasad RM. Regional expression of Par-4 mRNA and protein after fluid percussion brain injury in the rat. Exp Neurol. 2001;170:140–148. doi: 10.1006/exnr.2001.7685. [DOI] [PubMed] [Google Scholar]
- 19.Hara MR, Snyder SH. Cell signaling and neuronal death. Annu Rev Pharmacol Toxicol. 2007;47:117–141. doi: 10.1146/annurev.pharmtox.47.120505.105311. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan PG, Rabchevsky AG, Waldmeier PC, Springer JE. Mitochondrial permeability transition in CNS trauma: cause or effect of neuronal cell death? J Neurosci Res. 2005;79:231–239. doi: 10.1002/jnr.20292. [DOI] [PubMed] [Google Scholar]
- 21.Khodorov BI, Storozhevykh TP, Surin AM, Yuryavichyus AI, Sorokina EG, Borodin AV, Vinskaya NP, Khaspekov LG, Pinelis VG. The leading role of mitochondrial depolarization in the mechanism of glutamate-induced disruptions in Ca2+ homeostasis. Neurosci Behav Physiol. 2002;32:541–547. doi: 10.1023/a:1019819925257. [DOI] [PubMed] [Google Scholar]
- 22.Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 23.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341:233–249. [PMC free article] [PubMed] [Google Scholar]
- 24.Cole K, Perez-Polo JR. Neuronal trauma model: in search of Thanatos. Int J Dev Neurosci. 2004;22:485–496. doi: 10.1016/j.ijdevneu.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 25.Slee EA, Adrain C, Martin SJ. Executioner caspase-3, -6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J Biol Chem. 2001;276:7320–7326. doi: 10.1074/jbc.M008363200. [DOI] [PubMed] [Google Scholar]
- 26.Nijhawan D, Honarpour N, Wang X. Apoptosis in neural development and disease. Annu Rev Neurosci. 2000;23:73–87. doi: 10.1146/annurev.neuro.23.1.73. [DOI] [PubMed] [Google Scholar]
- 27.Slee EA, Adrain C, Martin SJ. Serial killers: ordering caspase activation events in apoptosis. Cell Death Differ. 1999;6:1067–1074. doi: 10.1038/sj.cdd.4400601. [DOI] [PubMed] [Google Scholar]
- 28.Hutchins JB, Barger SW. Why neurons die: cell death in the nervous system. Anat Rec. 1998;253:79–90. doi: 10.1002/(SICI)1097-0185(199806)253:3<79::AID-AR4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 29.Guo Q, Sebastian L, Sopher BL, Miller MW, Glazner GW, Ware CB, Martin GM, Mattson MP. Neurotrophic factors [activity-dependent neurotrophic factor (ADNF) and basic fibroblast growth factor (bFGF)] interrupt excitotoxic neurodegenerative cascades promoted by a PS1 mutation. Proc Natl Acad Sci USA. 1999;96:4125–4130. doi: 10.1073/pnas.96.7.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farlie PG, Dringen R, Rees SM, Kannourakis G, Bernard O. bcl-2 transgene expression can protect neurons against developmental and induced cell death. Proc Natl Acad Sci USA. 1995;92:4397–4401. doi: 10.1073/pnas.92.10.4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tecoma ES, Monyer H, Goldberg MP, Choi DW. Traumatic neuronal injury in vitro is attenuated by NMDA antagonists. Neuron. 1989;2:1541–1545. doi: 10.1016/0896-6273(89)90042-1. [DOI] [PubMed] [Google Scholar]
- 32.Zhu H, Fu W, Mattson MP. The catalytic subunit of telomerase protects neurons against amyloid beta-peptide-induced apoptosis. J Neurochem. 2000;75:117–124. doi: 10.1046/j.1471-4159.2000.0750117.x. [DOI] [PubMed] [Google Scholar]
- 33.Xie J, Guo Q. Apoptosis antagonizing transcription factor protects renal tubule cells against oxidative damage and apoptosis induced by ischemia-reperfusion. J Am Soc Nephrol. 2006;17:3336–3346. doi: 10.1681/ASN.2006040311. [DOI] [PubMed] [Google Scholar]
- 34.Xie J, Guo Q. AATF protects neural cells against oxidative damage induced by amyloid beta-peptide. Neurobiol Dis. 2004;16:150–157. doi: 10.1016/j.nbd.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 35.Guo Q, Sebastian L, Sopher BL, Miller MW, Ware CB, Martin GM, Mattson MP. Increased vulnerability of hippocampal neurons from presenilin-1 mutant knock-in mice to amyloid beta-peptide toxicity: central roles of superoxide production and caspase activation. J Neurochem. 1999;72:1019–1029. doi: 10.1046/j.1471-4159.1999.0721019.x. [DOI] [PubMed] [Google Scholar]
- 36.Saatman KE, Feeko KJ, Pape RL, Raghupathi R. Differential behavioral and histopathological responses to graded cortical impact injury in mice. J Neurotrauma. 2006;23:1241–1253. doi: 10.1089/neu.2006.23.1241. [DOI] [PubMed] [Google Scholar]
- 37.Sullivan PG, Bruce-Keller AJ, Rabchevsky AG, Christakos S, Clair DK, Mattson MP, Scheff SW. Exacerbation of damage and altered NF-kappa B activation in mice lacking tumor necrosis factor receptors after traumatic brain injury. J Neurosci. 1999;19:6248–6256. doi: 10.1523/JNEUROSCI.19-15-06248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeKosky ST, Goss JR, Miller PD, Styren SD, Kochanek PM, Marion D. Upregulation of nerve growth factor following cortical trauma. Exp Neurol. 1994;130:173–177. doi: 10.1006/exnr.1994.1196. [DOI] [PubMed] [Google Scholar]
- 39.Nakagawa Y, Nakamura M, McIntosh TK, Rodriguez A, Berlin JA, Smith DH, Saatman KE, Raghupathi R, Clemens J, Saido TC, Schmidt ML, Lee VM, Trojanowski JQ. Traumatic brain injury in young, amyloid-beta peptide overexpressing transgenic mice induces marked ipsilateral hippocampal atrophy and diminished Abeta deposition during aging. J Comp Neurol. 1999;411:390–398. [PubMed] [Google Scholar]
- 40.Xie J, Guo Q. Par-4 is a novel mediator of renal tubule cell death in models of ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2007;292:F107–F115. doi: 10.1152/ajprenal.00083.2006. [DOI] [PubMed] [Google Scholar]
- 41.Michel RP, Cruz-Orive LM. Application of the Cavalieri principle and vertical sections method to lung: estimation of volume and pleural surface area. J Microsc. 1988;150:117–136. doi: 10.1111/j.1365-2818.1988.tb04603.x. [DOI] [PubMed] [Google Scholar]
- 42.Srinivasan A, Roth KA, Sayers RO, Shindler KS, Wong AM, Fritz LC, Tomaselli KJ. In situ immunodetection of activated caspase-3 in apoptotic neurons in the developing nervous system. Cell Death Differ. 1998;5:1004–1016. doi: 10.1038/sj.cdd.4400449. [DOI] [PubMed] [Google Scholar]
- 43.Qiu G, Ahmed M, Sells SF, Mohiuddin M, Weinstein MH, Rangnekar VM. Mutually exclusive expression patterns of Bcl-2 and Par-4 in human prostate tumors consistent with down-regulation of Bcl-2 by Par-4. Oncogene. 1999;18:623–631. doi: 10.1038/sj.onc.1202344. [DOI] [PubMed] [Google Scholar]
- 44.Cheema SK, Mishra SK, Rangnekar VM, Tari AM, Kumar R, Lopez-Berestein G. Par-4 transcriptionally regulates Bcl-2 through a WT1-binding site on the bcl-2 promoter. J Biol Chem. 2003;278:19995–20005. doi: 10.1074/jbc.M205865200. [DOI] [PubMed] [Google Scholar]
- 45.El-Guendy N, Zhao Y, Gurumurthy S, Burikhanov R, Rangnekar VM. Identification of a unique core domain of par-4 sufficient for selective apoptosis induction in cancer cells. Mol Cell Biol. 2003;23:5516–5525. doi: 10.1128/MCB.23.16.5516-5525.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sells SF, Han SS, Muthukkumar S, Maddiwar N, Johnstone R, Boghaert E, Gillis D, Liu G, Nair P, Monnig S, Collini P, Mattson MP, Sukhatme VP, Zimmer SG, Wood DP, Jr, McRoberts JW, Shi Y, Rangnekar VM. Expression and function of the leucine zipper protein Par-4 in apoptosis. Mol Cell Biol. 1997;17:3823–3832. doi: 10.1128/mcb.17.7.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rangnekar VM. Apoptosis mediated by a novel leucine zipper protein Par-4. Apoptosis. 1998;3:61–66. doi: 10.1023/a:1009666705875. [DOI] [PubMed] [Google Scholar]
- 48.Mattson MP, Duan W, Chan SL, Camandola S. Par-4: an emerging pivotal player in neuronal apoptosis and neurodegenerative disorders. J Mol Neurosci. 1999;13:17–30. doi: 10.1385/JMN:13:1-2:17. [DOI] [PubMed] [Google Scholar]
- 49.Guo Q, Xie J. AATF inhibits aberrant production of amyloid beta peptide 1–42 by interacting directly with Par-4. J Biol Chem. 2004;279:4596–4603. doi: 10.1074/jbc.M309811200. [DOI] [PubMed] [Google Scholar]