Abstract
Serrated adenomas (SAs) are part of the distinct serrated polyp pathway of colorectal carcinogenesis characterized by microsatellite instability and deficiency in DNA mismatch repair. Sessile SA is a recently recognized lesion that typically presents as a large sessile polyp, but lacks the conventional dysplasia. It is more frequently found on the right side than on the left side of the colon, and is thought to represent an intermediate form in the hyperplastic polyp to sessile SA, traditional SA, and colon cancer sequence. Many terms have been used and are still in use in the literature to describe this lesion, such as “hyperplastic polyposis”, “giant hyperplastic polyposis,” “large hyperplastic polyps,” “hyperplastic-adenomatous polyposis syndrome,” “giant hyperplastic polyp,” and “mixed epithelial polyp.” The purpose of this paper is to review and clarify the confusing nomenclature, and to provide a framework for understanding the genetic alterations and clinical significance of these precursor lesions in the serrated polyp pathway of colorectal caner.
Keywords: Polyp, hyperplastic, adenomatous, colon, sessile serrated adenoma, carcinoma
Introduction
Epithelial polyps of the colorectum have traditionally been classified into two main groups: neoplastic polyp, or adenoma, and hyperplastic polyp (HPP) [1]. Adenoma has malignant potential and is considered the precursor lesion of colorectal cancer (CRC), whereas HPP is generally regarded as non-neoplastic [2, 3]. Traditional serrated adenoma (TSA) is a rare type of colorectal polyp that has the cytologic features of an adenoma, the architectural features of a HPP, and malignant potential. Serrated adenomas (SAs) have been linked to a distinct “serrated polyp pathway” of colorectal carcinogenesis [4], which is characterized by microsatellite instability (MSI) and deficiency in DNA mismatch repair [5, 6] and is largely independent of the classic “adenoma-to-carcinoma” sequence. A recently recognized lesion in the serrated polyp pathway is the sessile SA. Sessile SA usually presents as a large (usually greater than 1 cm) sessile polyp, but lacks conventional dysplasia. Sessile SA is found more frequently on the right side than on the left side of the colon. It is thought that sessile SAs represent an intermediate form of polyp and thus complete the spectrum from classic HPP to sessile SA, traditional SA, and eventually colon carcinoma. Many terms have been used and are still in use in the literature, such as “hyperplastic polyposis”, “giant hyperplastic polyposis,” “large hyperplastic polyps,” “hyperplastic-adenomatous polyposis syndrome,” “giant hyperplastic polyp,” and “mixed epithelial polyp,” which contribute to the confusion of these lesions [1–7]. Thus, a high interpersonal discrepancy of serrated polyps has been recently reported [8], underscoring the importance of a consistent terminology for such lesions. The purpose of this review is to attempt to clarify the confusing nomenclature, and to review the genetic alterations and clinical significance of these precursor lesions in the serrated polyp pathway of colorectal cancer.
Hyperplastic Polyps
HPPs are typically small (<5 mm in diameter), sessile or slightly raised, mucosal lesions that occur more frequently in the distal colon and rectum than elsewhere in the colon [6]. HPPs occur most frequently in the fifth and sixth decades of life, whereas adenomas usually arise in the sixth and seventh decades [7]. Histologically, HPPs are characterized by a hypermature appearance with an irregular serrated luminal surface contour. Cross sections of hyperplastic crypts have a stellate appearance. The superficial portion of a HPP displays hypermucinous cells, whereas the lower third shows a narrow regenerative crypt. The histologic characteristics of HPPs are shown in (Figure 1). HPPs can be further divided into 3 subtypes based on the character of their mucin – microvesicular serrated polyp (MVSP), goblet cell-rich serrated polyp (GCSP), and mucin-poor serrated polyp (MPSP) [6]. MVSP, also called Type 1 HPP, is the most common type of HPP and correlates with the lesion generally considered to be the typical HPP of the colorectum. It is characterized by vesicular mucin/vesicular cells and numerous goblet cell abnormalities. GCSP, also called Type 2 HPP, reveals increased goblet cells.
Figure 1.
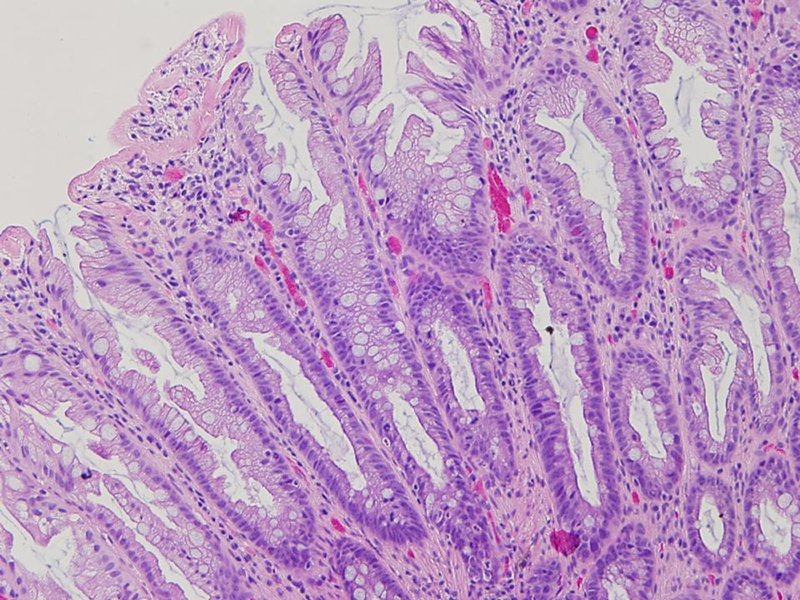
The histologic characteristics of a HPP. Hypermucinous maturation on the surface and serrated architecture in the upper half of the crypt, whereas the lower portion of the crypt reveals a narrow mild hyperchromatic regenerative cells.
Though serration is less prominent in GCSP, thickening of the mucosa and the muscularis mucosae and irregular thickening of the surface basal membrane are readily seen. MPSP usually has uniform and prominent serration and almost no evidence of mucin.
At the molecular level, the abnormal retention of surface epithelium of HPP is caused by the inhibition of programmed cell death, or apoptosis. Inhibition of apoptosis can also facilitate the development of genetic instability [10]. Recently, researchers have found that HPPs are clonal epithelial growth with underlying genetic alterations implicating components of the mitogen-activated protein (MAP) kinase signaling pathway, notably the oncogenes KRAS [11, 12] and BRAF [13] (Table 1). Mutation of KRAS has been linked to the inhibition of apoptosis through Akt/protein kinase B, which inhibits caspase-9 [14] and BAD [15], and through downregulation of the cell membrane apoptosis receptor, Fas (CD95) [16]. HPPs are also characterized by the epigenetic promoter methylation of genes (CpG island methylator phenotype, CIMP) encoding hMLH1, p16, and MGMT (O-6-methylguanine DNA methyltransferase). Of note, sporadic HPPs were CIMP-negative (not methylated at any locus), but 43% of HPPs from multiple/large HPPs, or hyperplastic polyposis were CIMP-high (two or more methylated loci, P = 0.00001) in a study of 102 HPPs from 17 patients with multiple/large HPPs or polyposis and 14 HPPs from other 12 sporadic patients [17]. However, some traditional SAs of the proximal colon also demonstrate features of CIMP. These findings raise the possibility that HPPs may represent the primordial lesion in the serrated polyp–neoplasia pathway, leading to the formation of SAs and ultimately colorectal carcinomas [18]. This possibility is further supported by the fact that HPPs and SAs have similar age distribution (both occur more often in patients more than 48 years old), and spatial clustering. Also, there appears to be a temporal association between the two lesions. Lazarus et al [19] found that some patients with HPPs later developed traditional SAs and those patients who had traditional SAs more often had HPPs in subsequent screening tests than traditional adenomas. Other studies have shown that MSI and CIMP play significant roles in the pathogenesis of hyperplastic polyposis, an inherited syndrome characterized by multiple, large HPPs and a predilection to familial CRC. An example of hyperplastic polyposis is illustrated in Figure 2.
Table 1.
Molecular abnormalities in serrated polyps of the large intestine
| Normal colon | Small HPP | Large HPP | SSA | TSA | CRC | |
|---|---|---|---|---|---|---|
| CIMP | − | +/− | + | ++ | +++ | ++++ |
| BRAF | − | + | + | + | + | + |
| MSI | − | − | − | + | + | +++ |
Figure 2.
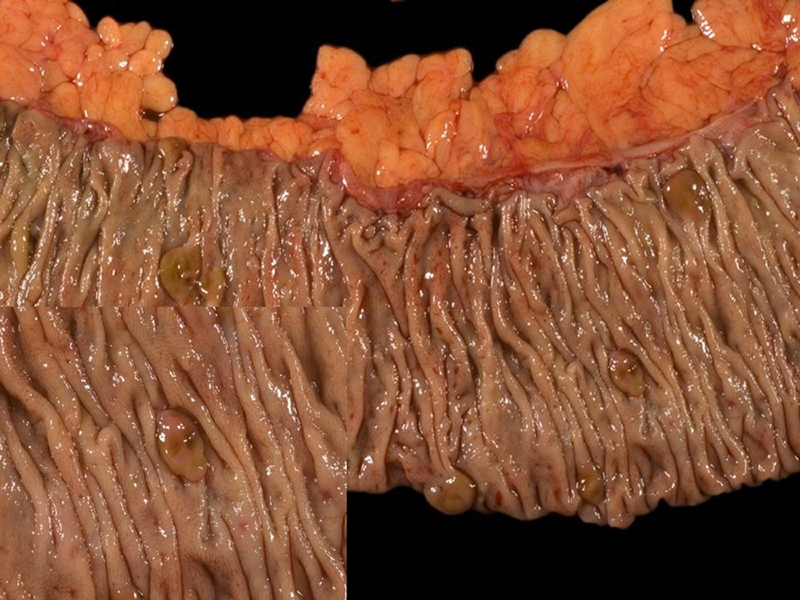
A gross photograph of hyperplastic polyposis. A segment of right colon containing six brownish, sessile serrated polyps. A close-up of a polyp was inserted in the left lower corner.
Although it is likely that HPPs from the proximal colon are linked histogenetically with the subset of CRCs with high levels of MSI (MSI-H), DNA hypermethylation, or both, it is clear that most HPPs on the right side of the colon will not become malignant. In a postmortem study conducted in New Zealand, Jass et al [20] found only 43 right-side HPPs among the 333 cases of colorectal adenomas studied. It was estimated that only about 1% of right-side HPPs develop into MSI-H cancers [20]. This finding suggests that, except for lesions of hyperplastic polyposis, there is a limited benefit to be gained by removing a relatively small, sporadic lesion from the thin-walled right colon of an elderly person.
Traditional Serrated Adenomas
The term “serrated adenoma” was first introduced into the pathology literature in 1990 to describe a rare type of colorectal polyp that had the cytologic features of an adenoma and the architectural features of a HPP [1]. Some authors had described SAs as “atypical HPPs” [21] or as “villous adenomas” [22]. To clarify the confusion of terminology, it has been proposed that serrated adenoma be called “traditional serrated adenoma” (TSA) to distinguish it from sessile serrated adenoma (to be discussed further).
The main histological features of TSA are the uniform population of abnormal epithelial cells which are columnar cells with eosinophilic cytoplasm and centrally placed, elongated nuclei that are hyperchromatic and display somewhat pseudostratification (Figure 3). Staining of these cells with the proliferation marker Ki-67 (Mib-1) reveals minimal proliferation index in contrast to the traditional tubular adenoma and tubulovillous adenoma. In addition, cell proliferation in TSAs mainly occurs within the crypt base, and occasional mitoses may be observed in the upper crypt zone. Overall, there is a definite transition from a basal proliferative zone to a more superficial zone of maturing cells [23–25]. By contrast, proliferating cells in traditional adenomas are located throughout the crypts and even within the surface epithelium [23–25]. Thus, it can be difficult to differentiate traditional adenomas from TSAs with high-grade dysplasia, which also have proliferating cells in the upper crypt and surface epithelium [23]. In some cases, immunohistochemical markers such as Ki-67, Bcl-2 and p21 are useful to help distinguish TSAs from both HPPs and adenomas. Generally, the levels of these markers in TSAs are intermediate between levels in HPPs and adenomas [26–28].
Figure 3.
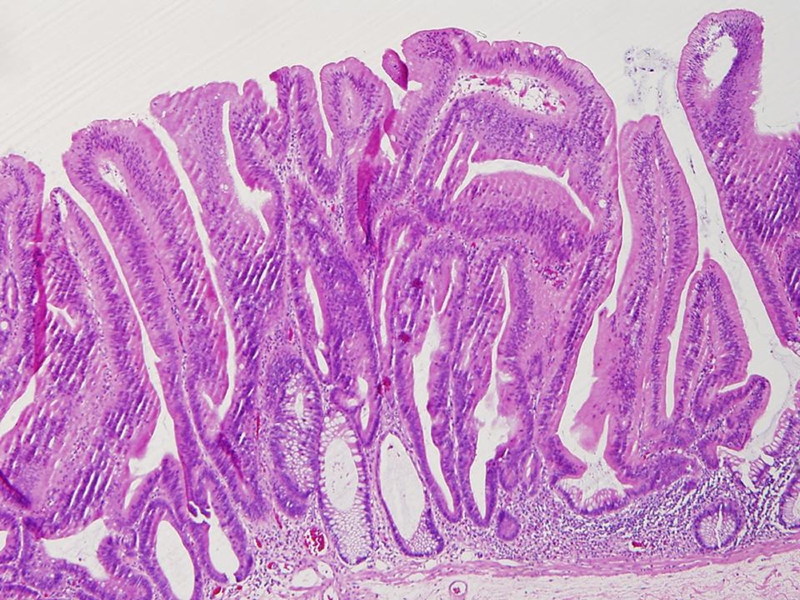
The histologic characteristics of a TSA. A noticeable serrated architecture, eosinophilic epithelium with hyperchromatic “pencillate” nuclei.
TSAs are characterized by a heterogeneous group of changes at the molecular level, with features intermediate between those of HPPs and adenomas. A high proportion of TSAs has BRAF mutations and DNA methylation. In mixed polyps (those with both HPP and adenoma components), both components have identical microsatellite mutations, but the dysplastic component is more likely to be TSA than adenoma, despite the fact that adenoma is much more common than TSA [5]. At the molecular level, TSAs and HPPs share three common features: mutation of genes in the MAP kinase signaling pathway (BRAF or KRAS) [12, 13], extensive DNA methylation [28–30], and gastric metaplasia [31, 32]. In addition, the transition of HPPs and mixed polyps to invasive carcinoma has been documented [33–35]. These studies found that TSAs may arise from either HPPs or sessile SAs, or they may develop de novo.
Methylation and inactivation of the DNA repair genes MLH1 and MGMT have been proposed as critical steps in the development of genetic instability. Subsets of CRCs with an unusually high number of methylated CpG islands have been described as having the CIMP. It is possible that as many as 20% of CRCs with this phenotype evolve through the serrated polyp pathway. Indeed, many molecular changes identified in MSI-H cancers are present in serrated lesions, including CpG island methylation of hMLH1 in proximal HPPs, increased CpG island methylation with loss of expression of hMLH1 and MSI in TSAs, and the aforementioned BRAF mutation as a potentially late event in the progression from HPP to TSA [35]. Researchers have suggested that the CIMP is the engine that drives the serrated polyp–carcinoma pathway. In their study, Beach et al [36] found that sporadic MSI-H cancers commonly lack some of the genetic alterations identified in traditional adenomas, such as in the APC gene. This group also found the rates of cell proliferation and apoptosis of dysplastic cells in both the upper and lower crypts were intermediate between those of HPPs and tubulovillous adenomas [26].
Since the first detailed description of TSAs appeared in 1990 [1], the proportion of colorectal polyps diagnosed as TSAs has increased. In a study of over 18,000 lesions originally diagnosed as polyps, Longacre et al characterized approximately 0.6% as TSAs and found that they were more likely to occur in the proximal colon (35.4%) than either adenomas (23.3%) or HPPs (7.5%) [37]. In a recent review of pathologic specimens from Finland, however, Lazarus et al [17] found that 15.9% of colorectal polyps were diagnosed as TSAs and suggested that other pathologists might have misdiagnosed TSAs with high-grade dysplasia as adenomas.
The risk of malignant transformation of TSAs appears to be higher than that of traditional adenomas. In a study of 40 Norwegian subjects with adenomas, only 1 developed CRC over the 10-year period of the study [38]. In a consecutive series of patients with polyps diagnosed between 1978 and 1982 and followed until the end of 2000, the incidence of CRC in patients with SAs (5%) was greater than but not statistically different from the incidence of CRC among patients with adenomas (2.2%) [17]. Another study reported a patient who refused to have a TSA resected and developed advanced cancer at the site of the lesion within 2 years [39].
Sessile Serrated Adenomas
Torlakovic and Snover first described a distinct morphological subtype of colorectal polyps in the context of hyperplastic polyposis [40]. These polyps, termed sessile SAs (SSAs), are characterized by their large size, exaggerated serration, and dilated horizontal crypts. The diagnosis of SSA is based mainly on architectural features that seem to emanate from the abnormal proliferation and decreased apoptosis that is the basis for the abnormal growth in these serrated polyps. The architectural features of SSA include branching of crypts, dilatation of the base of the crypts, and a peculiar growth pattern of crypts which grow parallel to the muscularis mucosae, creating an L-shaped crypt. These distinct growth features, readily recognizable at the low-power magnification, are accompanied by the presence of mature cells with goblet cells at the serrated base of the crypt (Figure 4). In contrast, the lower third of the crypts in HPPs remains narrow and is lined with proliferative cells. SSAs can be further distinguished from HPPs on the basis of the exaggerated serration in their lower crypts, increased crypt branching or “boot-shaped” crypts, crypt dilatation, an epithelial-to-stromal ratio of > 50%, mitoses in the mid to upper levels of crypts, and minimal cytologic atypia (such as vesicular nuclei with conspicuous nucleoli) in the upper crypts [41]. However, distinguishing between the two is sometimes difficult, because both can have focal serrations and sessile serrated adenoma may, like HPP, lack dysplastic features. Further-more, because of the sessile nature of the polyp, a poorly oriented specimen may not reveal the characteristic features. In our daily practice, when initial biopsy results of clinically visible polyps are nondiagnostic, we routinely reorientate the specimens and section deeper tissue levels, which often reveal diagnostic features for HPP or SSA [42].
Figure 4.
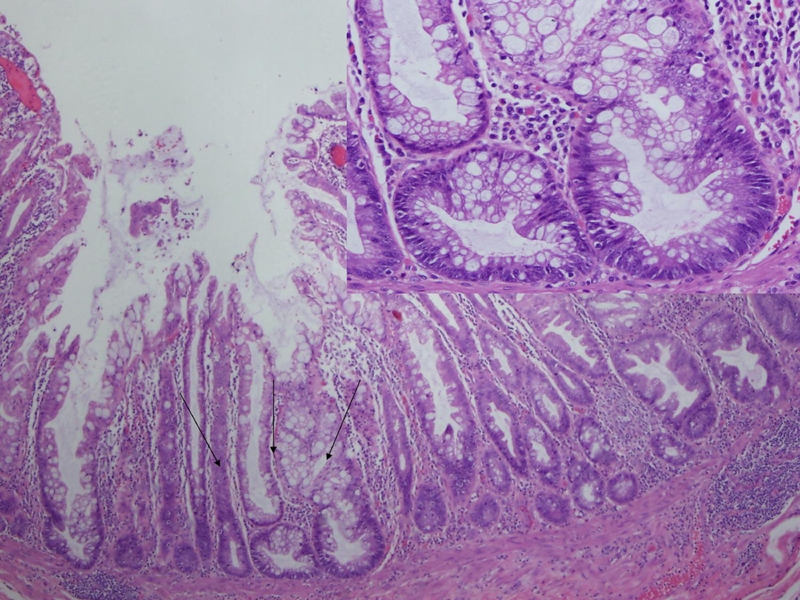
The histologic characteristics of a SSA. The SSA cytologically resembles hyperplastic polyps and has some of the architectural features of serrated adenomas. SSA is distinguished from hyperplastic polyps on the basis of exaggerated serration in the lower crypts, increased crypt branching or ‘boot-shaped’ or “L-shaped” crypts, crypt dilation, an epithelial to stromal ratio of > 50%, and mitoses in the mid to upper levels of crypts. A close-up of the arrowed area was shown on the right upper corner.
Although SSAs have cytologic features resembling those of HPPs, they have some of the architectural features of TSAs. SSAs can be considered large HPP variants, or possibly intermediates between HPPs and TSAs. At the molecular level, SSAs are characterized by frequent mutation of BRAF and DNA methylation, whereas traditional HPPs are more likely to have KRAS mutation and less extensive DNA methylation. A study of HPPs and TSAs revealed an inverse relationship between BRAF mutations and KRAS mutations [43], which supports grouping serrated polyps as a spectrum of lesions associated with activation of the MAP kinase pathway. In addition to CIMP, the acquisition of BRAF mutations with methylation of hMLH1 is an important genetic alteration found in many cases of SSA. Loss of expression of MSI, particularly hMLH1, in SSA is readily demonstrated by immunohistochemistry (Figure 5). However, SSAs are morphologically and molecularly distinct from TSAs and lack the conventional dysplasia seen in other adenomas. Thus, SSA, like TSA, probably represents a fairly distinctive lesion along the serrated polyp–neoplasia pathway of colorectal carcinogenesis. SSAs are thought to represent approximately 2% of all polyps removed colonoscopically, over 8% of all polyps that would have been previously diagnosed as HPPs [44], and around 18% of all serrated polyps [45].
Figure 5.
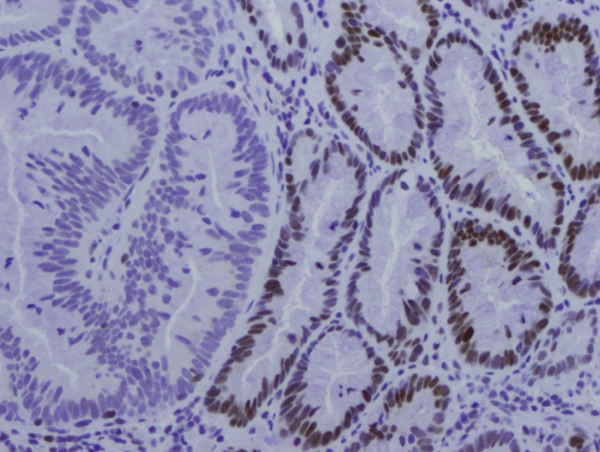
Immunohistochemistry of hMLH1 showing significant decrease/absence of hMLH1 in the portion of SSA (left side) and normal expression of hMLH1 in the adjacent colonic epithelium.
The question then arises: how do we report serrated polyp of the colorectum and how do we manage those polyps? Though nomenclature of colorectal serrated polyps is not generalized, a panel of terminology for reporting serrated polyps of the large intestine has been recently proposed [6]. A modified classification of colorectal serrated polyps is summarized in Table 2. It should be pointed out not every lesion is classic, and a lesion with combination of any components of large HPP, TSA, SSA, and typical adenoma (tubular adenoma/tubulovillous adenoma) is common (Figure 6). The key of pathology reporting is a good communication and understanding between pathologists and clinicians, who should work closely as a team to manage these lesions. Specially, most small rectosigmoid HPPs are innocuous. However, HPPs ≥ 1 cm that arise proximal to the rectosigmoid may not be entirely innocuous lesions, which should be excised and followed up after surgery to ensure that the excision is complete. Similarly, any lesion reported as a mixed polyp, TSA, or SSA should be completely excised, if possible, and the patient should be monitored for recurrence and development of other similar lesions [6]. The follow-up interval is unclear. However, the development of any degree of dysplasia would imply that the lesion has developed into an adenoma, and so further follow-up should be done according to the guidelines outlined for adenomas [45].
Table 2.
Terminology for reporting the colorectal serrated polyps
| Hyperplastic polyp |
| Microvesicular (optional) |
| Goblet cell-rich (optional |
| Mucin-poor (optional) |
| Sessile serrated adenoma (SSA) |
| Traditional serrated adenoma (TSA) |
| Mixed serrated polyp (list the components of the polyp in parentheses, e.g. mixed sessile serrated adenoma-tubular adenoma) |
| Sessile serrated polyp (for an equivocal diagnosis of small SSA and HPP, one should make a comment, indicating favor one or the other based on the location and size, e.g. a large right-sided polyp “favor” SSA, a small left-sided polyp “favor” HPP) |
Figure 6.
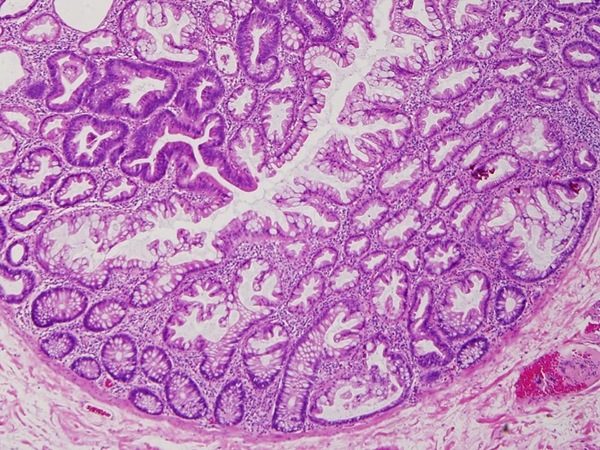
Mixed serrated polyp with tubular adenoma (left upper corner) and sessile serrated adenoma (SSA, right lower corner).
Conclusions
Studies have indicated that serrated polyps are a group of heterogeneous precursor lesions in the serrated polyp pathway of colorectal carcinogenesis, which includes large, and multiple HPPs, SSAs, and TSAs. These serrated lesions have potential for malignant transformation to colorectal carcinoma. Because of their increased malignant potential, any lesion reported as a mixed polyp, TSA, or SSA should be completely excised, if possible. A given serrated polyp can contain mixed components of any combination of large HPP, TSA, and/or SSA. Appropriate terminology, pathology report and communications between a pathologist and clinicians are important in the management of the colorectal serrated polyps. Reliable biomarker(s) predicting the aggressiveness of serrated polyps have not yet been developed, but demographic, pathologic, and molecular markers (e.g. mutation of BRAF, MSI and CIMP) are possible candidates.
Acknowledgments
The authors would like to thank Mrs. A Hines for secretarial assistance and Mrs. Aton for scientific editing of the manuscript.
References
- 1.Jass JR. Serrated adenoma of the colorectum and the DNA-methylator phenotype. Nat Clin Pract Oncol. 2005;2:398–405. doi: 10.1038/ncponc0248. [DOI] [PubMed] [Google Scholar]
- 2.Kaye GI, Fenoglio CM, Pascal RR, Lane N. Comparative electron microscopic features of normal, hyperplastic, and adenomatous human colonic epithelium. Variations in cellular structure relative to the process of epithelial differentiation. Gastroenterology. 1973;64:926–945. [PubMed] [Google Scholar]
- 3.Muto T, Bussey HJ, Morson BC. The evolution of cancer of the colon and rectum. Cancer. 1975;36:2251–2270. doi: 10.1002/cncr.2820360944. [DOI] [PubMed] [Google Scholar]
- 4.Jass JR. Serrated route to colorectal cancer: back street or super highway? J Pathol. 2001;193:283–285. doi: 10.1002/1096-9896(200103)193:3<283::AID-PATH799>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 5.Iino H, Jass JR, Simms LA, Young J, Leggett B, Ajioka Y, Watanabe H. DNA microsatellite instability in hyperplastic polyps, serrated adenomas, and mixed polyps: a mild mutator pathway for colorectal cancer? J Clin Pathol. 1999;52:5–9. doi: 10.1136/jcp.52.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snover DC, Jass JR, Fenoglio-Preiser C, Batts KP. Serrated polyps of the large intestine. Am J Clin Pathol. 2005;124:380–391. doi: 10.1309/V2EP-TPLJ-RB3F-GHJL. [DOI] [PubMed] [Google Scholar]
- 7.Morimoto LM, Newcomb PA, Ulrich CM, Bostick RM, Lais CJ, Potter JD. Risk factors for hyperplastic and adenomatous polyps: evidence for malignant potential? Cancer Epidemiol Biomarkers Prev. 2002;11:1012–1018. [PubMed] [Google Scholar]
- 8.Odze RD, Batts K, Goldstein N, Hamilton S, Jass J, O'Brien M, Riddell R, Snover, Wang H. Interobserver variability in the diagnosis of hyperplastic and serrated colonic polyps. Modern Pathol. 2007;1(Suppl):247. [Google Scholar]
- 9.Hayashi T, Yatani R, Apostol J, Stemmermann GN. Pathogenesis of hyperplastic polyps of the colon: a hypothesis based on ultrastructure and in vitro cell kinetics. Gastroenterology. 1974;66:347–356. [PubMed] [Google Scholar]
- 10.Jass JR, Whitehall VL, Young J, Leggett BA. Emerging concepts in colorectal neoplasia. Gastroenterology. 2002;123:862–876. doi: 10.1053/gast.2002.35392. [DOI] [PubMed] [Google Scholar]
- 11.Otori K, Oda Y, Sugiyama K, Hasebe T, Mukai K, Fukii T, Tajiri H, Yoshida S, Fukushima S, Esumi H. High frequency of K-ras mutations in human colorectal hyperplastic polyps. Gut. 1997;40:660–663. doi: 10.1136/gut.40.5.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ajioka Y, Watanabe H, Jass JR, Yokota Y, Kobayashi M, Nishikura K. Infrequent K-ras codon 12 mutation in serrated adenomas of human colorectum. Gut. 1998;42:680–684. doi: 10.1136/gut.42.5.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan TL, Zhao W, Leung SY, Yuen ST. BRAF and KRAS mutations in colorectal hyperplastic polyps and serrated adenomas. Cancer Res. 2003;63:4878–4881. [PubMed] [Google Scholar]
- 14.Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 15.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 16.Fenton RG, Hixon JA, Wright PW, Brooks AD, Sayers TJ. Inhibition of Fas (CD95) expression and Fas-mediated apoptosis by oncogenic Ras. Cancer Res. 1998;58:3391–3400. [PubMed] [Google Scholar]
- 17.Chan AO, Issa JP, Morris JS, Hamilton SR, Rashid A. Concordant CpG island methylation in hyperplastic polyposis. Am J Pathol. 2002;160:529–536. doi: 10.1016/S0002-9440(10)64872-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Brien MJ, Yang S, Clebanoff JL, Mulcahy E, Farraye FA, Amorosino M, Swan N. Hyperplastic (serrated) polyps of the colorectum: relationship of CpG island methylator phenotype and K-ras mutation to location and histologic subtype. Am J Surg Pathol. 2004;28:423–434. doi: 10.1097/00000478-200404000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Lazarus R, Junttila OE, Karttunen TJ, Makinen MJ. The risk of metachronous neoplasia in patients with serrated adenoma. Am J Clin Pathol. 2005;123:349–359. doi: 10.1309/VBAG-V3BR-96N2-EQTR. [DOI] [PubMed] [Google Scholar]
- 20.Jass JR, Young PJ, Robinson EM. Predictors of presence, multiplicity, size and dysplasia of colorectal adenomas. A necropsy study in New Zealand. Gut. 1992;33:1508–1514. doi: 10.1136/gut.33.11.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jass JR, Filipe MI, Abbas S, Falcon CA, Wilson Y, Lovell D. A morphologic and histochemical study of metaplastic polyps of the colorectum. Cancer. 1984;53:510–515. doi: 10.1002/1097-0142(19840201)53:3<510::aid-cncr2820530324>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 22.Goldman H, Ming S, Hickock DF. Nature and significance of hyperplastic polyps of the human colon. Arch Pathol. 1970;89:349–345. [PubMed] [Google Scholar]
- 23.Rubio CA, Kato Y, Hirota T, Muto T. Flat serrated adenomas of the colorectal mucosa in Japanese patients. In Vivo. 1996;10:339–343. [PubMed] [Google Scholar]
- 24.Komori K, Ajioka Y, Watanabe H, Oda K, Nimura Y. Proliferation kinetics and apoptosis of serrated adenoma of the colorectum. Pathol Int. 2003;53:277–283. doi: 10.1046/j.1440-1827.2003.01476.x. [DOI] [PubMed] [Google Scholar]
- 25.Kang M, Mitomi H, Sada M, Tokumitsu Y, Takahashi Y, Igarashi M, Katsumata T, Okayasu I. Ki-67, p53, and Bcl-2 expression of serrated adenomas of the colon. Am J Surg Pathol. 1997;21:417–423. doi: 10.1097/00000478-199704000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Ban S. Small hyperplastic polyps of the colorectum showing deranged cell organization: a lesion considered to be a serrated adenoma? Am J Surg Pathol. 1999;23:1158–1160. doi: 10.1097/00000478-199909000-00026. [DOI] [PubMed] [Google Scholar]
- 27.Mitomi H, Sada M, Kobayashi K, Igarashi M, Mori A, Kanazawa H, Nishiyama Y, Ihara A, Otani Y. Different apoptotic activity and p21(WAF1/CIP1), but not p27(Kip1), expression in serrated adenomas as compared with traditional adenomas and hyperplastic polyps of the colorectum. J Cancer Res Clin Oncol. 2003;129:449–455. doi: 10.1007/s00432-003-0478-y. [DOI] [PubMed] [Google Scholar]
- 28.Ladas SD, Kitsanta P, Triantafyllou K, Tzathas C, Spiliadi C, Raptis SA. Cell turnover of serrated adenomas. J Pathol. 2005;206:62–67. doi: 10.1002/path.1739. [DOI] [PubMed] [Google Scholar]
- 29.Park SJ, Rashid A, Lee JH, Kim SG, Hamilton SR, Wu TT. Frequent CpG island methylation in serrated adenomas of the colorectum. Am J Pathol. 2003;162:815–822. doi: 10.1016/S0002-9440(10)63878-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wynter CV, Walsh MD, Higuchi T, Leggett BA, Young J, Jass JR. Methylation patterns define two types of hyperplastic polyp associated with colorectal cancer. Gut. 2004;53:573–580. doi: 10.1136/gut.2003.030841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biemer-Huttmann AE, Walsh MD, McGuckin MA, Simms LA, Young J, Leggett BA, Jass JR. Mucin core protein expression in colorectal cancers with high levels of microsatellite instability indicates a novel pathway of morphogenesis. Clin Cancer Res. 2000;6:1909–1916. [PubMed] [Google Scholar]
- 32.Biemer-Huttmann AE, Walsh MD, McGuckin MA, Ajoka Y, Watanabe H, Leggett BA, Jass JA. Immunohistochemical staining patterns of MUC1, MUC2, MUC4, and MUC5AC mucins in hyperplastic polyps, serrated adenomas, and traditional adenomas of the colorectum. J Histochem Cytochem. 1999;47:1039–1048. doi: 10.1177/002215549904700808. [DOI] [PubMed] [Google Scholar]
- 33.Urbanski SJ, Kossakowska AE, Marcon N, Bruce WR. Mixed hyperplastic adenomatous polyps–an underdiagnosed entity. Report of a case of adenocarcinoma arising within a mixed hyperplastic adenomatous polyp. Am J Surg Pathol. 1984;8:551–556. [PubMed] [Google Scholar]
- 34.Kudo T, Matsumoto T, Esaki M, Yada S, Hirashashi M, Kayashima K, Yao T, Iida M. Small invasive colonic cancer occurring in a hyperplastic polyp. Endoscopy. 2004;36:825–828. doi: 10.1055/s-2004-825831. [DOI] [PubMed] [Google Scholar]
- 35.Oh K, Redston M, Odze RD. Support for hMLH1 and MGMT silencing as a mechanism of tumorigenesis in the hyperplastic-adenoma-carcinoma (serrated) carcinogenic pathway in the colon. Hum Pathol. 2005;36:101–111. doi: 10.1016/j.humpath.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 36.Beach R, Chan AO, Wu TT, White JA, Morris JS, Lunagomez S, Broaddus RR, Issa JP, Hamilton SR, Rashid A. BRAF mutations in aberrant crypt foci and hyperplastic polyposis. Am J Pathol. 2005;166:1069–1075. doi: 10.1016/S0002-9440(10)62327-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Longacre TA, Fenoglio-Preiser CM. Mixed hyperplastic adenomatous polyps/ serrated adenomas. A distinct form of colorectal neoplasia. Am J Surg Pathol. 1990;14:524–537. doi: 10.1097/00000478-199006000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Eide TJ. Risk of colorectal cancer in adenoma-bearing individuals within a defined population. Int J Cancer. 1986;38:173–176. doi: 10.1002/ijc.2910380205. [DOI] [PubMed] [Google Scholar]
- 39.Yamauchi T, Watanabe M, Hasegawa H, Yamamoto S, Endo T, Kabeshima Y, Yorozuya K, Yamamoto K, Kitajima M. Serrated adenoma developing into advanced colon cancer in 2 years. J Gastroenterol. 2002;37:467–470. doi: 10.1007/s005350200068. [DOI] [PubMed] [Google Scholar]
- 40.Torlakovic E, Snover DC. Serrated adenomatous polyposis in humans. Gastroenterology. 1996;110:748–755. doi: 10.1053/gast.1996.v110.pm8608884. [DOI] [PubMed] [Google Scholar]
- 41.Higuchi T, Sugihara K, Jass JR. Demographic and pathological characteristics of serrated polyps of colorectum. Histopathology. 2005;47:32–40. doi: 10.1111/j.1365-2559.2005.02180.x. [DOI] [PubMed] [Google Scholar]
- 42.Tan D, Ross W. Colorectal polyps: Clinical significance of endoscopical and pathological correlation. Am J Clin Pathol. (in press) [PubMed] [Google Scholar]
- 43.Yang S, Farraye FA, Mack C, Posnik O, O'Brien MJ. BRAF and KRAS Mutations in hyperplastic polyps and serrated adenomas of the colorectum: relationship to histology and CpG island methylation status. Am J Surg Pathol. 2004;28:1452–1459. doi: 10.1097/01.pas.0000141404.56839.6a. [DOI] [PubMed] [Google Scholar]
- 44.Torlakovic E, Skovlund E, Snover DC, Torlakovic G, Nesland JM. Morphologic reappraisal of serrated colorectal polyps. Am J Surg Pathol. 2003;27:65–81. doi: 10.1097/00000478-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 45.Cunningham KS, Riddell RH. Serrated mucosal lesions of the colorectum. Curr Opin Gastroenterol. 2006;22:48–53. doi: 10.1097/01.mog.0000198074.52287.16. [DOI] [PubMed] [Google Scholar]


