Abstract
Preclinical studies have implicated the mammalian target of rapamycin (mTOR) pathway in the cell cycle progression and growth of prostate cancer cells. Downstream signaling from PI3′-K/Akt leads to phosphorylation (p) of mTOR at serine 2448 and to activation of its substrate, p70S6Kinase (p70S6K), phosphorylated on threonine 389. This promotes translation and cell cycle progression. Morphoproteomic analysis, that combines both the application of phosphospecific probes directed against putative sites of activation on protein analytes and cellular compartmentalization [1] was carried out on tissue microarray (TMA) slides from 64 cases of primary, previously untreated adenocarcinomas of the prostate. Gleason scores ranged from 6 to 10. High grade prostatic intraepithelial neoplasia (HGPIN), which accompanied the invasive cancer in 20 cases, and 15 non-neoplastic controls from benign prostatic hypertrophy specimens in a separate TMA were also included. Ninety-three percent (93%) of tumors exhibited moderate to strong cytoplasmic/plasmalemmal expression of p-mTOR and eighty-five percent (85%) showed similar staining intensity for p-p70S6K. HGPIN demonstrated comparable and occasionally, stronger expression levels for these protein analytes. Quantitative digital imaging revealed an overall increase in the mean expression levels in HGPIN, reaching statistical significance for p-mTOR (Ser 2448) at p<0.05. Morphoproteomic analysis confirms the constitutive activation of the mTOR pathway in prostate cancer and HGPIN, with relative overexpression of p-mTOR in HGPIN. These findings coincide with preclinical studies in supporting a role for the mTOR pathway in the biology and development of prostate cancer through its putative precursor lesion, HGPIN and in suggesting a potential therapeutic target.
Keywords: mTOR pathway, prostate cancer, high grade PIN, morphoproteomics, tissue microarray
Introduction
Preclinical studies have indicated a role for the mammalian target of rapamycin (mTOR) pathway in the pathogenesis of prostate cancer. Specifically, mTOR signaling has been shown to contribute to cell cycle progression, and inhibition of the mTOR pathway enhances growth reduction in prostate cancer cells [2–3]. Moreover, there is evidence to suggest that androgens induce prostate cancer cell proliferation through both mTOR activation and post-transcriptional increases in cyclin D proteins [4]. In vitro experiments and in vivo xenograft studies using human prostate cancer cells have demonstrated an antitumor effect of CCI-779, an analog of the mTOR inhibitor rapamycin [5]. Finally, overexpression of Akt, an upstream signal transducer involved in the activation of mTOR, has been linked to the formation of a prostatic intraepithelial neoplasia (PIN) lesion in a transgenic mouse model, and this could be reversed by inhibiting mTOR [6].
Against this background, it is noteworthy that the phosphorylative activation of mTOR on serine 2448 can be effected by the downstream signaling of p-Akt [7–9]; the phosphorylation of p70S6K on threonine 389 is occasioned by the signal transduction of p-mTOR [7–8, 10–14], and the phosphorylative activation of p70S6K leads to increased G1 cell cycle progression with tumor cell proliferation [2, 15–17]. Therefore, the purpose of this study was to assess the state of activation of mTOR and p70S6K in primary specimens of prostate cancer and high grade PIN (HGPIN) using a morphoproteomic approach [1] and to compare the expression levels with non-neoplastic prostatic glandular epithelium.
Materials and Methods
Study Groups
After Institutional Review Board (IRB) approval, a total of 64 total prostatectomy specimens with adequate material and/or unequivocal clinical histories of no other malignancies were retrieved from the files of the Department of Laboratory Medicine of Geisinger Medical Center. The prostatectomies had been performed over the span of 11 years and had curative intent. All hematoxylin-eosin (H&E)-stained sections from each case were reviewed and two sections with adequate amount of prostate cancer from each case were selected for inclusion in the tissue microarray. All tissues had been fixed in 10% neutral buffered formalin, routinely processed and embedded in paraffin. Two pathologists (REB and GZ) reviewed the sections without knowing any patient information or clinical outcomes and assigned a Gleason score. Within this group of prostate cancers, a subset of 20 patients with HGPIN was identified. This latter diagnosis was based on established cytologic criteria and the demonstration of a companionate basal cell layer that was immunoreactive for high molecular weight cytokeratin. A separate group comprised of prostate specimens from 15 patients with benign prostate hypertrophy (BPH) was assembled in another tissue microarray to serve as non-neoplastic controls.
Construction of Tissue Microarrays
A manual tissue arrayer device from Beecher Instruments (Sun Prairie, WI) was used to construct the tissue microarrays (TMAs). Areas with adequate amount of prostate cancer or BPH were marked in the paraffin blocks, and one 1 mm diameter core from each case was included in their respective arrays along with 6 cores from control tissues. External controls were provided by an additional slide containing a “sausage” section of different tissues.
Immunohistochemistry using Phosphospecific Probes
Tissue microarray sections, 3 µm thick, were deparaffinized in xylene and rehydrated in a graded series of ethanols. Heat-induced epitope retrieval was performed. Phosphospecific probes to include antibodies directed against mTOR, phosphorylated at serine 2448 and against p70S6K, phosphorylated at threonine 389 were purchased from Cell Signaling Technology, Inc (Beverly, MA). The tissue was then treated with 3% H2O2 and then rinsed with Tween-20 (TBST) buffer. A few drops of diluted normal blocking serum were placed on the tissue and incubated at room temperature. The serum was then blotted off and the slides were incubated with primary antibody overnight at 4°C. The rest of the staining procedure took place on a DAKO Autostainer programmed to treat each slide with diluted biotinylated secondary antibody for 30 minutes. The slides were then rinsed and incubated with DAB (3,3′-diamino-benzidine chromogen solution, DAKO EnVision+ System Kit) for 10 minutes. The slides were rinsed again and counterstained with Gill II hematoxylin, treated with xylene and cover slipped. Positive and negative controls were noted to react appropriately.
Scoring of Immunoreactivity of Prostate Cancer, HGPIN and BPH Cells and Cellular Compartmentalization of Chromogenic Signal
Semiquantitative Assessment: Expression levels of the p-mTOR (Ser 2448) and p-p70S6K (Thr 389) protein analytes in target cells were determined using bright-field microscopy and applying a scoring system on a scale of 0–3+ as follows: 0 for no detectable chromogenic signal; 1+ for focal weak staining; 2+ for over 50% of target cells staining; and 3+ for diffuse, strong staining. The cellular compartmentalization of the signal was also indicated as predominantly cytoplasmic/plasmalemmal or nuclear.
Quantitative Assessment: An automated cellular imaging system (ACIS III [DAKO Corporation, Carpinteria, CA]) was used to scan the TMA slides and to create digital images for selection of representative areas and quantitation. Briefly, the ACIS III system consists of two major components: a microscope with electromechanical hardware and a computer with a frame grabber and image processor. It is used to generate two indices: the percentage of immunopositive pixels in a field and the staining intensity score of the cells in immunohistochemistry (IHC) sections from selected representative areas. A region score is generated by the image analyzer. Digital images from scanned TMA sections were examined and representative portions of the images were selected by one of us (BZ). This resulted in multiple representative areas of prostate cancer, HGPIN or non-neoplastic BPH epithelium (luminal + basal cells), respectively for quantitative analysis. The latter involved a TMA application on the ACIS III. This instrument had been preprogrammed for the assessment of cytoplasmic/plasmalemmal or nuclear compartments of p-mTOR (Ser 2448) and p-p70S6K (Thr 389), respectively. A mean total intensity score with standard deviation (SD) was generated for each study group.
Statistical Analyses
Descriptive statistics was used to report the demographic and Gleason scoring data. Chi-square tests were used to correlate the semiquantitative staining expressions with the Gleason score. SAS version 8.1 was used for statistical analysis and p values less than 0.05 were considered significant.
Additionally, the mean intensity scores ± SD and standard error of the mean (SEM) for each study group were analyzed using a Levene's test for equality of variances and a t-test for equality of means.
Results
Sixty-one cases had adequate material on the microarray for analysis of the expression level of p-mTOR (vide infra) and were included in the analysis. The corresponding patients ranged in age from 43 to 81 years with a mean age of 61. Of the 61 patients, 36 (59%) had a Gleason score of 6, 22 (36%) had a Gleason score of 7–8 and 3 patients (5%) had a Gleason score of 9–10.
Semi-quantitative Assessment of p-mTOR and p-p70S6K Expression in Prostate Cancer and Correlation with Gleason Score
Sixty-one (61) out of 64 cases demonstrated good quality, reproducible staining for p-mTOR (Ser 2448), and sixty-two (62) for p-p70S6K (Thr 389). Fifty-seven (57) out of 61 tumors (93%) exhibited moderate to strong (2+ to 3+) cytoplasmic/plasmalemmal expression of p-mTOR (Ser 2448) and fifty-three (53) out of 62 (85%) showed similar moderate to strong intranuclear expression of p-p70S6K (Thr 389). Relatively less cytoplasmic/ plasmalemmal expression of p-mTOR (Ser 2448) appeared to be present overall in the mature (luminal) glandular epithelium of BPH in most cases; however, the companionate basal cell layer was highlighted by strong expression of p-mTOR (Ser 2448). The expression levels of p-p70S6K (Thr 389) in the nuclei of the benign glandular epithelial cells appeared comparable to that seen in the nuclei of prostate cancer. These findings are illustrated in Figures 1 and 2. Of the 36 patients with a Gleason score of 6, 33 (91.6%) had moderate to strong cytoplasmic/ plasmalemmal expression of p-mTOR. Of the 25 patients with Gleason score 7–10, 24 (96%) had moderate to strong cytoplasmic/ plasmalemmal expression of p-mTOR. The difference between the two groups was not statistically significant using a chi-square test (91.6% versus 96%, p>0.05). Notably, variable expression levels were seen in individual patients among these groups and also heterogeneity of expressions could be seen within the same tumor from an individual patient (Figure 2).
Figure 2.
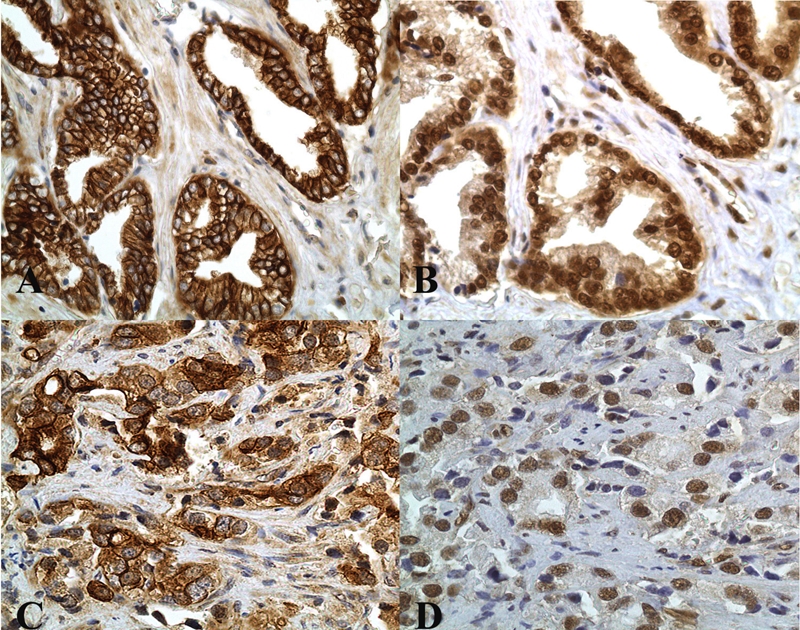
Prostate cancers with Gleason scores of 6 and 9 (top and bottom rows, respectively) and exemplifying constitutive activation of both mTOR (phophorylated at serine 2448) with variable, moderate to strong DAB [brown] chromogenic signal with cytoplasmic/plasmalemmal compartmentalization (A and C) and of p70S6K (phosphorylated at threonine 389 and translocated to the tumoral nuclei (B and D]).Compare and contrast with expression levels in non-neoplastic luminal epithelium in BPH (Figure 1) (original magnification, ×400).
Figure 1.
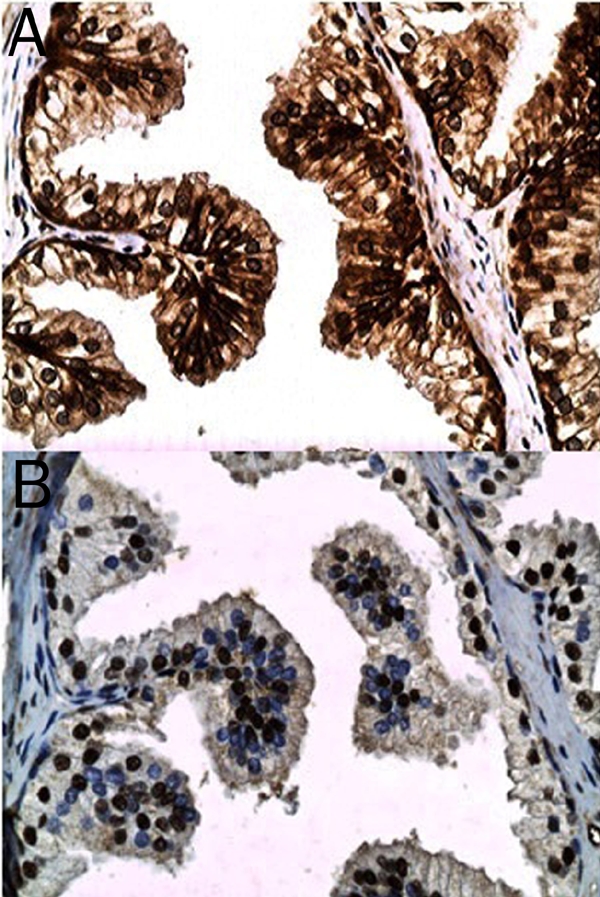
Non-neoplastic glandular epithelium from a case of benign prostatic hypertrophy illustrating the relatively moderate intensity DAB (brown) chromogenic signal for mTOR (phosphorylated at serine 2448) in the plasmalemmal and cytoplasmic compartments of the luminal epithelium and the relatively strong signal in the basal cell layer (A). Variable chromogenic signal for p70S6K (phosphorylated on threonine 389) is noted in the nuclei of the glandular epithelium (B) (original magnification, ×400).
Semi-quantitative Assessment of p-mTOR and p-p70S6K Expression in HGPIN
Twenty out of twenty (100%) cases of HGPIN had moderate to strong (2+ to 3+) cytoplasmic/plasmalemmal expression of p-mTOR (Ser 2448) and nuclear p-p70S6K (Thr 389). Moreover, the staining intensity generally appeared to be greater in HGPIN than in the prostate cancer for p-mTOR and on occasion, for p-p70S6K. This was confirmed in the quantitative assessment (vide infra). These findings are illustrated in Figure 3.
Figure 3.
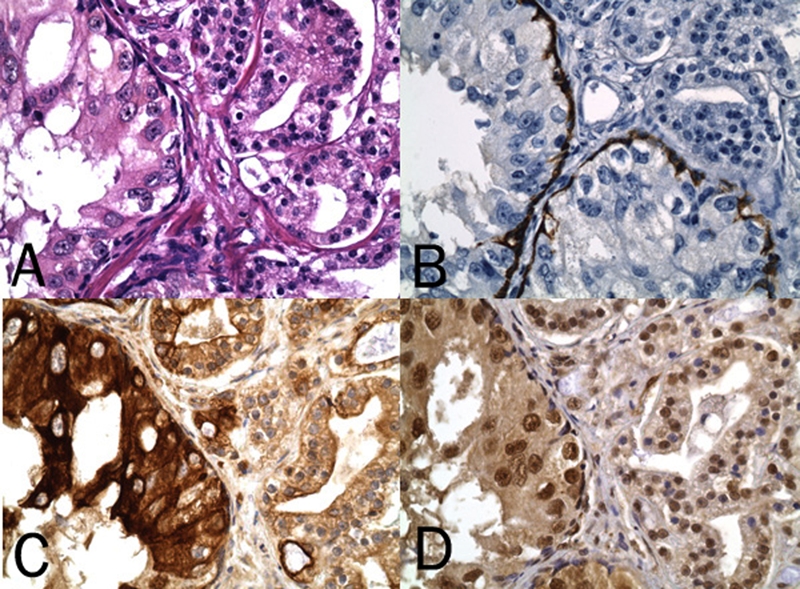
HGPIN and companionate prostate cancer on H&E (A) and with the HGPIN confirmed by IHC staining for high molecular weight cytokeratin of a basal cell layer (B). Note relatively stronger DAB (brown) chromogenic signal for cytoplasmic/ plasmalemmal expression of p-mTOR (Ser 2448) (C) and for nuclear expression of p-p70S6K (Thr 389) (D) in HGPIN (original magnification, ×400).
Quantitative Assessment of p-mTOR and p-p70S6K Expression in Prostate Cancer, HGPIN and BPH
Quantitation of the chromogenic signals relating to p-mTOR (Ser 2448) and p-p70S6K (Thr 389) in the digital images from the respective microarrays of prostate cancer, HGPIN and BPH (which included luminal with the strongly expressing basal cells for p-mTOR) revealed mean and standard error of the mean (SEM) values for each protein analyte that showed the following pattern: HGPIN>prostate cancer=benign prostatic epithelium. A summary of the numerical data and statistical analyses are found in Table 1 and in the corresponding bar graphs (Figure 4).
Table 1.
Quantitation of p-mTOR (Ser 2448) and p-p70S6K (Thr 389) expression levels in non-neoplastic prostate glands (NL), HGPIN and prostate cancer
| NL | HGPIN | Prostate Cancer | |
|---|---|---|---|
| Mean ± Std Error | Mean ± Std Error | Mean ± Std Error | |
| p-mTOR(Ser 2448) | 169.53 ± 4.09 | 183.78 ± 4.09 | 168.36 ± 2.21 |
| p-p70S6K (Thr 389) | 139.88 ± 1.26 | 148.37 ± 5.78 | 135.12 ± 2.26 |
| p-mTOR (Ser 2448): HGPIN vs Non-neoplastic Prostate Glands (p= 0.019) | |||
| HGPIN vs Prostate Cancer (p=0.002) | |||
| p-p70S6K (Thr 389): HGPIN vs Prostate Cancer (p= 0.012) | |||
Figure 4.

Graphic representation of mean intensity expression levels as generated on the ACIS III image analyzer for p-mTOR (Ser 2448) and p-p70S6K (Thr 389). Patient categories include those with benign glandular epithelium from BPH cases (NL) vs High HGPIN vs prostate cancer (CA). Note higher mean expression levels for HGPIN cases (also see Table 1).
Discussion
Morphoproteomic analysis of primary clinical specimens, as applied in this study, reveals that components of the mTOR pathway are constitutively activated, as well as moderately to strongly expressed in the majority (up to 93%) of cases of prostate cancer (independent of the Gleason score), and also unregulated in all foci of HGPIN vis-à-vis non-neoplastic prostatic luminal epithelium in the case of p-mTOR. Such constitutive activation is evidenced by: 1. the moderate to strong expression of p-mTOR and p-p70S6K, phosphorylated on putative sites of activation, namely serine 2448 and threonine 389, respectively [13, 15, 18]; and 2. nuclear translocation and compartmentalization of p-p70S6K (Thr 389) [19–23]. Moreover, morphoproteomic analysis incorporates the correlative expression of other protein analytes to reinforce the interpretation that a given signal transduction pathway is indeed present and operative in tumors [1]. Such appears to be the case with both prostate cancer and HGPIN.
To expand on this, we have identified, using computer-assisted mining of the US National Library of Medicine's data base, correlative expressions of protein analytes, as detected by immunohistochemical expression, which can be associated with an activated mTOR signal transduction pathway in prostate cancer and HGPIN. These include: 1. detection of upstream proteins acting as signaling ligands or tyrosine kinases, and which then activate the PI3’-K/Akt pathway, specifically: a) epidermal growth factor receptor (EGFR) and its ligand, transforming growth factor alpha (TGF-α [24]; b) the insulin-like growth factor axis comprising type I insulin-like growth factor receptor (IGF-IR) and its signaling ligands, IGF-I and IGF-II [25] and c) platelet-derived growth factor receptor-alpha (PDGFR-α) and its signaling ligand PDGF-AA [26–27]; 2. a decreased expression level of the phosphatase and tensin homologue deleted on chromosome 10 (PTEN) protein, that normally inhibits/moderates signaling through the PI3’-K/Akt pathway, occurring in association with genomic deletions in 68% of prostate cancer and 23% of HGPIN [28]; 3. a correlative downstream association between reduced PTEN protein expression and an increase in mTOR signaling pathway markers [29]; 4. constitutive activation of p-Akt (Ser 473) [30–31] which coincides with our finding of phosphorylative activation of mTOR on serine 2448 [13, 15, 18]; 5. constitutive activation of the parallel ras/Raf kinase/ extracellular signal-regulated (ERK) pathway that includes p-ERK 1/2 (Thr 202/Tyr 204) [30, 32]; this latter signal transducer collaborates with the mTOR pathway in the activation of p70S6K and the phosphorylative inactivation of 4E-BP1 [33–37]; 6. the expression of downstream effectors of mTOR pathway signaling, namely higher expression levels of hypoxia-inducible factor 1 alpha (HIF-1α) and vascular endothelial growth factor (VEGF) [38, 39] and of the downstream effector of p-p70S6K in the form of p-S6 expression [26, 40]; and 7. the impact of convergent signaling on cell cycle progression as evidenced by a progressive increase in the Ki-67 proliferation indices along with continuum from benign to HGPIN to prostate cancer [41–42]. These correlative expressions and their interactions in the protein circuitry are schematically depicted in Figure 5.
Figure 5.
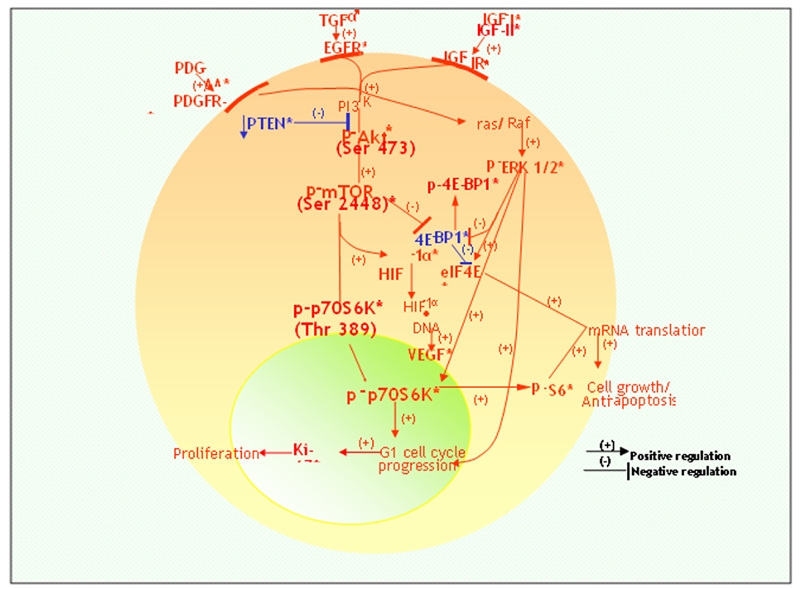
A schematic composite of the complex protein circuitries identified by the authors and others [24–44] utilizing immunohistochemistry in cases of prostate cancer and HGPIN and depicting their interrelationships with the mTOR pathway. Specifically, downstream signaling by the tyrosine kinases, PDGFR-alpha, EGFR and IGF-IR and their signaling ligands, proceeds through the PI3′-K/Akt and ras /Raf kinase/ERK1/2 pathways. The former is facilitated in some cases by a reduction in PTEN, an inhibitor of PI3′-K and leads to phosphorylative activation of mTOR and in turn its effector, p70S6K; and the latter (p-ERK 1/2) converges on p70S6K promoting cell cycle progression. Their impact is evidenced by progressively increased proliferation indices (Ki-67 expression/ percentages) and cell proliferation. Such bipathway stimulation and signal transduction also leads to the phosphorylative inactivation of 4E-BP1 (p-4E-BP1), thereby inhibiting ((−)) 4E-BP-1 and resulting in unbound eIF4E to effect mRNA translation and the synthesis of proteins that promote cell growth and anti-apoptosis. Relatedly, p-p70S6K promotes phosphorylation of S6 ribosomal protein (p-S6) that also participates in mRNA translations. Notably, HIF-1alpha induced by mTOR signaling is relevant to VEGF production and coincides with the increased expression levels of these two protein analytes in prostate cancer and HGPIN. * indicates established immunohistochemical findings with respect to protein analytes; PDGFR: platelet-derived growth factor receptor; TGF: transforming growth factor; EGFR: epidermal growth factor receptor; IGF: insulin-like growth factor; PI3′-K: phosphatidylinositol 3-kinase; PTEN: phosphatase and tensin homologue deleted on chromosome 10; mTOR: mammalian target of rapamycin; ERK: extracellular signal-regulated kinase; HIF: hypoxia-inducible factor; VEGF: vascular endothelial growth factor; 4E-BP1: 4E-binding protein-1; eIF4E: eukaryotic initiation factor 4E.
The potential pathogenetic and therapeutic implications of the concomitantly higher expression levels of constitutively activated components of the mTOR pathway in HGPIN vis-à-vis the luminal (glandular) epithelium of BPH and some prostate cancers are noteworthy. This was readily evident to visual inspection on bright-field microscopy and was supported by the higher mean expression levels by quantitative digital imaging. In a parallel and complementary study by Kremer and colleagues [29], similar observations were noted for inactivated p-4E-BP1 and activated p-S6, namely higher mean long scores in PIN. Because HGPIN is generally regarded as the direct precursor of invasive prostate cancer [43–44], the constitutive and apparently overexpressed mTOR pathway in the majority of HGPIN cases relative to benign glandular epithelium has pathogenetic implications in the developmental phase of invasive cancer. Moreover, it could present a therapeutic target to keep HGPIN from progressing and invasive cancer from developing. This would require identifying HGPIN in biopsy specimens from individual patients and confirming the constitutive activation of the mTOR pathway, so that applying appropriate therapy with rapamycin or a rapamycin analog might be considered and the rationale defended. As such, this represents a new and specific dimension under the concept of personalized and preventive medicine. Parenthetically such an approach accords with certain stated objectives of the U.S. National Cancer Institute to apply treatments or interventions at the individual level that keep cancers from starting or progressing.
In summary, morphoproteomic analysis in this study has confirmed the constitutive activation of components of the mTOR pathway, p-mTOR (Ser 2448) and p-p70S6K (Thr 389), along with moderate to strong expression in the majority of prostate cancers and in all cases of HGPIN. Moreover, our demonstration of p-p70S6K (Thr 389) appears to be the first report of such findings in primary prostate cancer and HGPIN specimens, from a review of the literature. Correlative expression of upstream and downstream protein circuitry and effectors demonstrated by other investigators coincides with and helps to complete the morpho-proteomic profile around the mTOR pathway in prostate cancer and HGPIN. Finally, the relative overexpression of activated mTOR pathway components in HGPIN and specifically of p-mTOR has both pathogenetic and therapeutic implications that can be applied to individual patients in an effort to help prevent the progression to invasive cancer.
Acknowledgments
The authors would like to thank Ms. Laurie Kneller-Waller, Ms. Tina Brosius and Mr. Glen Kauwell for their technical assistance at Geisinger Medical Center. We are also grateful for the technical contributions of Mr. Richard A. Breckenridge and Ms. Pamela K. Johnston and the secretarial and graphic design assistance provided by Ms. Bheravi Patel at the University of Texas Health Science Center-Houston Medical School. Finally, we thank Mr. G. Craig Wood and Dr. Steven Shen for help with the statistical analyses.
References
- 1.Brown RE. Morphoproteomics: exposing protein circuitries in tumors to identify potential therapeutic targets in cancer patients. Exp Rev Proteomics. 2005;2:337–348. doi: 10.1586/14789450.2.3.337. [DOI] [PubMed] [Google Scholar]
- 2.Gao N, Zhang Z, Jiang BH, Shi X. Role of PI3K/AKT/mTOR signaling in the cell cycle progression of human prostate Cancer. Biochem Biophys Res Comm. 2003;310:1124–1132. doi: 10.1016/j.bbrc.2003.09.132. [DOI] [PubMed] [Google Scholar]
- 3.Van der Poel HG. Mammalian target of Rapamycin and 3-phosphatidylinositol 3-kinase pathway inhibition enhances growth inhibition of transforming growth factor-β1 in prostate cancer cells. J Urol. 2004;172:1333–1337. doi: 10.1097/01.ju.0000138829.97838.19. [DOI] [PubMed] [Google Scholar]
- 4.Xu Y, Chen SY, Ross KN, Balk SP. Androgens induce prostate cancer cell proliferation through mammalian target of rapamycin activation and post-transcriptional increases in cyclin D proteins. Cancer Res. 2006;66:7783–7792. doi: 10.1158/0008-5472.CAN-05-4472. [DOI] [PubMed] [Google Scholar]
- 5.Wu L, Birle DC, Tannock IF. Effects of the mammalian target of rapamycin inhibitor CCI-779 used alone or with chemotherapy on human prostate cancer cells and xenografts. Cancer Res. 2005;65:2825–2831. doi: 10.1158/0008-5472.CAN-04-3137. [DOI] [PubMed] [Google Scholar]
- 6.Majumder PK, Febbo PG, Bikoff R, Berger R, Xue Q, McMahon LM, Manola J, Brugarolas J, McDonnell TJ, Golub TR, Loda M, Lane HA, Sellers WR. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med. 2004;10:594–601. doi: 10.1038/nm1052. [DOI] [PubMed] [Google Scholar]
- 7.Lin F, Zhang PL, Yang XJ, Prichard JW, Lun M, Brown RE. Morphoproteomic and molecular concomitants of an overexpressed and activated mTOR pathway in renal cell carcinomas. Ann Clin Lab Sci. 2006;36:283–293. [PubMed] [Google Scholar]
- 8.Cao X, Kambe F, Moeller LC, Refetoff S, Seo H. Thyroid hormone induces rapid activation of Akt/protein kinase B-mammalian target of rapamycin-p70S6K cascade through phosphatidylinositol 3-kinase in human fibroblasts. Mol Endocrinol. 2005;19:102–112. doi: 10.1210/me.2004-0093. [DOI] [PubMed] [Google Scholar]
- 9.Navé BT, Ouwens M, Withers DJ, Alessi DR, Shepherd PR. Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem J. 1999;344:427–431. [PMC free article] [PubMed] [Google Scholar]
- 10.Xu G, Zhang W, Bertram P, Zheng XF, McLeod H. Pharmacogenomic profiling of the PI3K/PTEN-AKT-mTOR pathway in common human tumors. Int J Oncol. 2004;24:893–900. [PubMed] [Google Scholar]
- 11.Pullen N, Dennis PB, Andjelkovic M, Dufner A, Kozma SC, Hemmings BA, Thomas G. Phosphorylation and activation of p70s6k by PDK1. Science. 1998;279:707–710. doi: 10.1126/science.279.5351.707. [DOI] [PubMed] [Google Scholar]
- 12.Dennis PB, Pullen N, Kozma SC, Thomas G. The principal rapamycin-sensitive p70 (s6k) phosphorylation sites, T-229 and T-389, are differentially regulated by rapamycin-insensitive kinase kinases. Mol Cell Biol. 1996;16:6242–6251. doi: 10.1128/mcb.16.11.6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valentinis B, Navarro M, Zanocco-Marani T, Edmonds P, McCormick J, Morrione A, Sacchi A, Romano G, Reiss K, Baserga R. Insulin receptor substrate-1, p70S6K, and cell size in transformation and differentiation of hemopoietic cells. J Biol Chem. 2000;275:25451–25459. doi: 10.1074/jbc.M002271200. [DOI] [PubMed] [Google Scholar]
- 14.Ali SM, Sabatini DM. Structure of S6 kinase 1 determines whether raptor-mTOR or rictor-mTOR phosphorylates its hydrophobic motif site. J Biol Chem. 2005;280:19445–19448. doi: 10.1074/jbc.C500125200. [DOI] [PubMed] [Google Scholar]
- 15.Brown RE, Zhang PL, Lun M, Zhu S, Pellitteri PK, Riefkohl W, Law A, Wood GC, Kennedy TL. Morphoproteomic and pharmacoproteomic rationale for mTOR effectors as therapeutic targets in head and neck squamous cell carcinoma. Ann Clin Lab Sci. 2006;36:273–282. [PubMed] [Google Scholar]
- 16.Gao N, Flynn DC, Zhang Z, Zhong XS, Walker V, Liu KJ, Shi X, Jiang BH. G1 cell cycle progression and the expression of G1 cyclins are regulated by PI3K/AKT/mTOR/p70S6K1 signaling in human ovarian cancer cells. Am J Physiol-Cell Physiol. 2004;287:C281–291. doi: 10.1152/ajpcell.00422.2003. [DOI] [PubMed] [Google Scholar]
- 17.Giles FJ, Albitar M. Mammalian target of rapamycin as a therapeutic target in leukemia. Curr Mol Med. 2005;5:653–661. doi: 10.2174/156652405774641034. [DOI] [PubMed] [Google Scholar]
- 18.Weng QP, Kozlowski M, Belham C, Zhang A, Comb MJ, Avruch J. Regulation of the p70 S6 kinase by phosphorylation in vivo. Analysis using site-specific anti-phosphopeptide anti-bodies. J Biol Chem. 1998;273:16621–16629. doi: 10.1074/jbc.273.26.16621. [DOI] [PubMed] [Google Scholar]
- 19.Ferrari S, Thomas G. S6 phosphorylation and the p70s6k/p85s6k. Crit Rev Biochem Mol Biol. 1994;29:385–413. doi: 10.3109/10409239409083485. [DOI] [PubMed] [Google Scholar]
- 20.Kozma SC, Thomas G. p70s6k/p85s6k: mechanism of activation and role in mito-genesis. Semin Cancer Biol. 1994;5:255–260. [PubMed] [Google Scholar]
- 21.Reinhard C, Fernandez A, Lamb NJ, Thomas G. Nuclear localization of p85s6k: functional requirement for entry into S phase. EMBO J. 1994;13:1557–1565. doi: 10.1002/j.1460-2075.1994.tb06418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edelmann HM, Kühne C, Petritsch C, Ballou LM. Cell cycle regulation of p70 S6 kinase and p42/p44 mitogen-activated protein kinases in Swiss mouse 3T3 fibroblasts. J Biol Chem. 1996;271:963–971. doi: 10.1074/jbc.271.2.963. [DOI] [PubMed] [Google Scholar]
- 23.Kim SJ, Kahn CR. Insulin stimulates p70 S6 kinase in the nucleus of cells. Biochem Biophys Res Comm. 1997;234:681–685. doi: 10.1006/bbrc.1997.6699. [DOI] [PubMed] [Google Scholar]
- 24.Leav I, McNeal JE, Ziar J, Alroy J. The localization of transforming growth factor alpha and epidermal growth factor receptor in stromal and epithelial compartments of developing human prostate and hyperplastic, dysplastic, and carcinomatous lesions. Hum Pathol. 1998;29:668–675. doi: 10.1016/s0046-8177(98)90274-x. [DOI] [PubMed] [Google Scholar]
- 25.Liao Y, Abel U, Grobholz R, Hermani A, Trojan L, Angel P, Mayer D. Up-regulation of insulin-like growth factor axis components in human primary prostate cancer correlates with tumor grade. Hum Pathol. 2005;36:1186–1196. doi: 10.1016/j.humpath.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 26.Fudge K, Bostwick DG, Stearns ME. Platelet-derived growth factor α and β chains and the alpha and beta receptors in prostatic intra-epithelial neoplasia. Prostate. 1996;29:282–286. doi: 10.1002/(SICI)1097-0045(199611)29:5<282::AID-PROS2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 27.Fudge K, Wang CY, Stearns ME. Immunohistochemistry analysis of platelet-derived growth factor α and β chains and platelet-derived growth factor alpha and beta receptor expression in benign prostatic hyperplasias and Gleason-graded human prostate adeno-carcinomas. Mod Pathol. 1994;7:549–554. [PubMed] [Google Scholar]
- 28.Yoshimoto M, Cutz JC, Nuin PA, Joshua AM, Bayani J, Evans AJ, Zielenska M, Squire JA. Interphase FISH analysis of PTEN in histologic sections shows genomic deletions in 68% of primary prostate cancer and 23% of high-grade prostatic intra-epithelial neoplasias. Cancer Genet Cytogenet. 2006;169:128–137. doi: 10.1016/j.cancergencyto.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Kremer CL, Klein RR, Mendelson J, Browne W, Samadzedeh LK, Vanpatten K, Highstrom L, Pestano GA, Nagle RB. Expression of mTOR signaling pathway markers in prostate cancer progression. Prostate. 2006;66:1203–1212. doi: 10.1002/pros.20410. [DOI] [PubMed] [Google Scholar]
- 30.Malik SN, Brattain M, Ghosh PM, Troyer DA, Prihoda T, Bedolla R, Kreisberg JI. Immunohistochemical demonstration of phospho-Akt in high Gleason grade prostate cancer. Clin Cancer Res. 2002;8:1168–1171. [PubMed] [Google Scholar]
- 31.Ayala G, Thompson T, Yang G, Frolov A, Li R, Scardino P, Ohori M, Wheeler T, Harper W. High levels of phosphorylated form of Akt-1 in prostate cancer and non-neoplastic prostate tissues are strong predictors of biochemical recurrence. Clin Cancer Res. 2004;10:6572–6578. doi: 10.1158/1078-0432.CCR-04-0477. [DOI] [PubMed] [Google Scholar]
- 32.Price DT, Della Rocca G, Guo C, Ballo MS, Schwinn DA, Luttrell LM. Activation of extracellular signal-regulated kinase in human prostate cancer. J Urol. 1999;162:1537–1542. [PubMed] [Google Scholar]
- 33.Tsutsumi N, Yonemitsu Y, Shikada Y, Onimaru M, Tanii M, Okano S, Kaneko K, Hasegawa M, Hashizume M, Maehara Y, Sueishi K. Essential role of PDGFRalpha-p70S6K signaling in mesenchymal cells during therapeutic and tumor angiogenesis in vivo: role of PDGFRalpha during angiogenesis. Circ Res. 2004;94:1186–1194. doi: 10.1161/01.RES.0000126925.66005.39. [DOI] [PubMed] [Google Scholar]
- 34.Iwasaki H, Eguchi S, Ueno H, Marumo F, Hirata Y. Endothelin-mediated vascular growth requires p42/p44 mitogen-activated protein kinase and p70 S6 kinase cascades via transactivation of epidermal growth factor receptor. Endocrinology. 1999;140:4659–4668. doi: 10.1210/endo.140.10.7023. [DOI] [PubMed] [Google Scholar]
- 35.Morley SJ. Signalling through either the p38 or ERK mitogen-activated protein (MAP) kinase pathway is obligatory for phorbol ester and T cell receptor complex (TCR-CD3)-stimulated phosphorylation of initiation factor (eIF) 4E in Jurkat T cells. FEBS Lett. 1997;418:327–332. doi: 10.1016/s0014-5793(97)01405-1. [DOI] [PubMed] [Google Scholar]
- 36.Herbert TP, Tee AR, Proud CG. The extracellular signal-regulated kinase pathway regulates the phosphorylation of 4E-BP1 at multiple sites. J Biol Chem. 2002;277:11591–11596. doi: 10.1074/jbc.M110367200. [DOI] [PubMed] [Google Scholar]
- 37.Davies CC, Mason J, Wakelam MJ, Young LS, Eliopoulos AG. Inhibition of phosphatidyl-inositol 3-kinase- and ERK MAPK-regulated protein synthesis reveals the pro-apoptotic properties of CD40 ligation in carcinoma cells. J Biol Chem. 2004;279:1010–1019. doi: 10.1074/jbc.M303820200. [DOI] [PubMed] [Google Scholar]
- 38.Lekas A, Lazaris AC, Deliveliotis C, Chrisofos M, Zoubouli C, Lapas D, Papathomas T, Fokitis I, Nakopoulou L. The expression of hypoxia-inducible factor-1alpha (HIF-1alpha) and angiogenesis markers in hyperplastic and malignant prostate tissue. Anticancer Res. 2006;26:2989–2993. [PubMed] [Google Scholar]
- 39.Wang L, Chen ZJ, Wang QT, Cao WF, Jian Y, Wang SX, Zhang BH. [Expression of hypoxia-inducible factor 1 alpha and vascular endothelial growth factor in prostate cancer and its significance] Zhonghua Nan Ke Xue. 2006;12:57–59. [PubMed] [Google Scholar]
- 40.Amornphimoltham P, Patel V, Sodhi A, Nikitakis NG, Sauk JJ, Sausville EA, Molinolo AA, Gutkind JS. Mammalian target of rapamycin, a molecular target in squamous cell carcinomas of the head and neck. Cancer Res. 2005;65:9953–9961. doi: 10.1158/0008-5472.CAN-05-0921. [DOI] [PubMed] [Google Scholar]
- 41.Tamboli P, Amin MB, Schultz DS, Linden MD, Kubus J. Comparative analysis of the nuclear proliferative index (Ki-67) in benign prostate, prostatic intraepithelial neoplasia, and prostatic carcinoma. Mod Pathol. 1996;9:1015–1019. [PubMed] [Google Scholar]
- 42.Veliiković LJ, Dordević B, Rancić G, Marjanović G. Expression of nuclear Ki-67 antigen in prostatic high grade intraepithelial neoplasia and prostatic carcinoma. Vojnosanit Pregl. 2007;64:325–330. doi: 10.2298/vsp0705325j. [DOI] [PubMed] [Google Scholar]
- 43.Montironi R, Mazzucchelli R, Lopez-Beltran A, Cheng L, Scarpelli M. Mechanisms of disease: high-grade prostatic intraepithelial neoplasia and other proposed preneoplastic lesions in the prostate. Nat Clin Pract Urol. 2007;4:321–332. doi: 10.1038/ncpuro0815. [DOI] [PubMed] [Google Scholar]
- 44.Man YG, Gardner WA. Focal degeneration of basal cells and the resultant auto-immunoreactions: A novel mechanism for prostate tumor progression and invasion. Med Hypotheses. 2007 doi: 10.1016/j.mehy.2007.05.015. (in press) [DOI] [PubMed] [Google Scholar]


