Abstract
A recent report indicates that patients with squamous cell carcinoma of the anal canal (SCCAC) and intraepithelial lymphocytes have a poor prognosis. Against that background, histological sections from 277 consecutive SCCACs were reviewed searching for cases with massive tumor-infiltrating lymphocytes (TILs; ≥ 50 lymphocytes /100 tumor cells). Of the 277 SCCACs, 8 (3%) had massive TILs. These 8 patients (all females) had both more advanced clinical stage than the remaining 269 control SCCAC patients. Follow-up studies revealed that the 8 patients with SCCACs having massive TILs had a much better 15 years survival rate than control SCCAC patients. It is concluded that despite SCCAC patients with massive TILs had a more advanced clinical stage than SCCAC controls, SCCAC with massive TILs patients had a longer survival rate (with no deaths after 5 years) than control cases. The search via proteomic methodology for the lymphocyte-attracting tumor protein might bring forward a novel co-adjuvant therapy, capable to increase prolonged survival time in patients having SCCAC without massive TILs.
Keywords: Squamous cell carcinoma, anal canal, intratumoral lymphocytes
Introduction
Data from most population-based cancer registries worldwide indicates that the incidence of squamous cell carcinoma of the anal canal (SCCAC) ranges from 0.5 to 1.0 per 100,000 in women and from 0.3 to 0.8 per 100,000 in men [1]. In the United States, SCCAC accounts for about 1.5% of all gastrointestinal cancers [2].
In recent years, a remarkable increase in the incidence of SCCAC has been recorded in many countries [3–5], including those in Scandinavia [6, 7]. In Denmark, the incidence of SCCAC raised 2.5 fold in men and 5 fold in women between 1943 and 1994 [8]. In Sweden, approximately 100 new cases are being diagnosed each year [7]. In the Stockholm-Gotland Health Care Region, with a population of 1.9 million, a steadily increasing frequency has been documented, from 14 cases in 1985 to 27 cases in 2003. SCCAC is frequently related to chronic infection with human papilloma virus (HPV) [8–11].
Several follow-up studies have shown that patients with malignant tumors displaying intra-epithelial lymphocytes have a favourable prognosis [12–16]. This phenomenon was demonstrated in patients with colorectal [12] or esophageal [13] carcinomas. Surprisingly, the significance of intra-epithelial lymphocytes in SCCAC was not investigated until very recently. Grabenbauer et al [17] found in 38 patients with SCCAC a mean of 3.8 intra-epithelial lymphocytes/100 tumor cells (range 0–17.3). Patients with tumor-infiltrating lymphocytes (TILs) had a poor prognosis.
In previous work, we investigated 308 consecutive cases of SCCAC [7, 18]. More recently, we diagnosed a case of SCCAC displaying an unusually high number of TILs. The finding of a case of SCCAC with a high number of TILs prompted us to investigate whether similar cases had been “missed” in earlier studies [18].
Material and Methods
The histological sections from 277 consecutive SCCAC cases were reviewed. Following the recommendations of Mangano et al for another organ [19], the number of lymphocytes in SCCACs was assessed in an area (25 ×) having a priori the highest number of TILs. Cases having ≥50 TILs/100 tumour cells were regarded as with massive TILs. SCCAC cases with and without massive TILs were subsequently compared with their clinical stage and with survival at ≥5 years follow-up.
A team consisting of a surgeon and a radiation/medical oncologist performed the clinical assessment of the patients. Endo-anal ultrasound, CT scans, and MRI was used in the pre-treatment evaluation [7]. Staging was performed, by applying the TNM classification of the International Union Against Cancer (UICC) [20]. Stage IIIA and IIIB tumors of the anal canal have been grouped together as stage III.
Statistical analysis was performed using the Spearman´s rank correlation test to compare the significance of the difference between the groups. Statistical significance was set at p<0.05. The cumulative proportion of surviving patients was assessed by the Kaplan-Meier survival curve-method.
Results
The histological evaluation of the 277 SCCACs revealed massive TILs in 8 cases (3%). The number of TILs varied from 53 to 89 TILs/100 tumour cells with a mean of 61 TILs/100 tumour cells. A representative image is shown in Figure 1. All 8 cases (100%) with massive TILs were females. Among the remaining 269 control cases, only 79% (210 cases) were females (Table 1).
Figure 1.
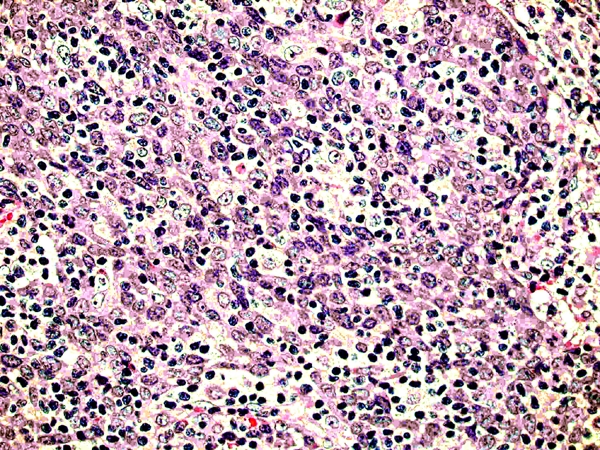
A case of SCCAC with massive TILs (H&E, × 400).
Table 1.
Clinicopathological data of the 277 patients with SCCAC
| SCCAC with massive TILs (n=8) | SCCAC without massive TILs (n= 269) | |
|---|---|---|
| Age | ||
| median | 72 | 69 |
| range | (51–82) | (28–92) |
| Gender | ||
| male | 0 | 59 |
| female | 8 | 210 |
| Clinical stage | ||
| I | 0 (0%) | 44 (16%) |
| II | 3 (37.5%) | 152 (56%) |
| III | 5 (62.5) | 73 (27%) |
| IV | 0 (0%) | 0 (0%) |
When tumors were classified according to clinical stage, all 8 (100%) SCCACs with massive TILs were clinical stage II and III, the rate for SCCAC control cases being only 83% (224/269). Table 1 also shows that as many as 63% (5/8) of the cases were clinical stage III. On the other hand, only 27% (73/269) of the SCCAC control cases were clinical stage III (p<0.05).
When the cumulative proportion of surviving patients was tested by the Kaplan-Meier method in SCCACs massive TILs and in control SCCAC cases, no difference in survival was found between the two groups at 60 months (5-years followup). On the other hand, between 5 years and 15-years follow-up, all SCCAC cases having massive TILs survived whereas SCCACs without TILs continued to die of the tumor (Figure 2).
Figure 2.
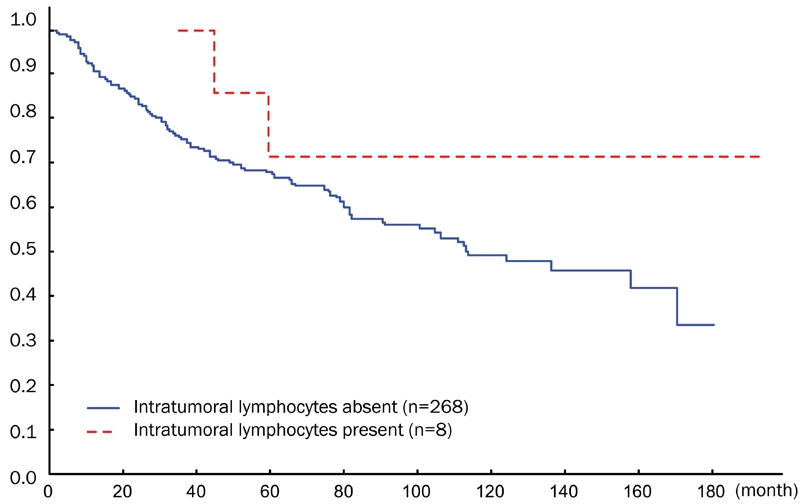
Cumulative proportional survival of SCCAC patients with or without TILs.
Recently we diagnosed an additional case of SCCAC with massive TILs, showing 69 TILs/100 tumour cells (Figure 1). The patient was also a female. The results of the various immunohistochemical stains in this case are illustrated in Figure 3. The TILs expressed CD3 and CD5. They were composed of a mixture of helper (CD4+), cytotoxic (CD8+, Granzym B+, TIA1+) as well as NK cells (Granzym B+). The tumour cells were positive for proteins involved in the regulation of cell cycle entry (p16 and p63), the suprabasal-basal cell marker CK5, and expressed mutated p53 protein. WAF1 was weakly expressed. The tumor cells showed HPV infection both with immunostain and with in situ hybridization (fam 16, high risk, Ventana, Illkirch France). EBV was negative by in situ hybridization (Ventana, Illkirch, France).
Figure 3.
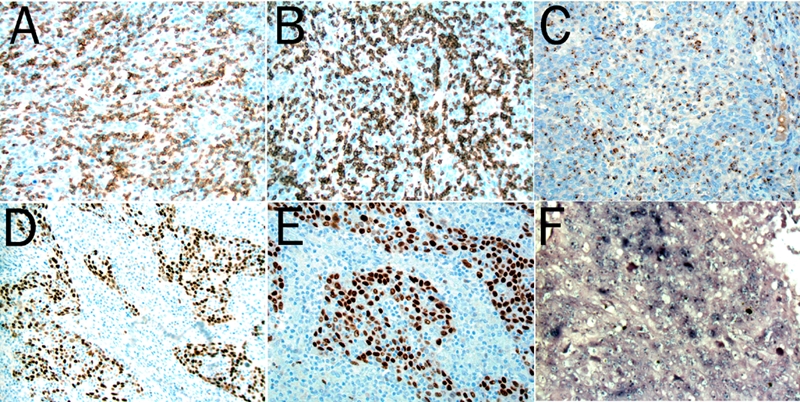
The massive TILs are positive for CD3 (A, ×400), CD8 (B, ×400) and the cytotoxic molecule granzyme B (C, ×400). The SCCAC cells show overexpression of P63 (D, ×250) and P53 (E, ×250). The tumor cells are also positive for HPV (F, HPV fam 16, high risk, ×250).
Discussion
The presence of TILs is regarded as a reaction of the host directed against the growing tumour tissues [12–16]. T cell migration into tumour masses is critical to the process of immunologically-induced tumour regression [12]. Recent studies revealed that the systemic administration of the immunoregulatory lymphokine, interleukin-2 (IL-2) into a number of tumour-mice models, resulted in complete tumour regression [16, 17]. Tumour regression was associated with a massive T cell infiltration into the neoplastic tissue. The results of the immunohistochemical challenge in the recently diagnosed SCCAC case revealed that the intraepithelial lymphocytes are helper, cytotoxic and NK cells, helper and NK cells being regarded as cytotoxic to tumour cells.
In a recent publication, Grabenbauer et al [17] reported 38 cases of SCCAC, with a much lower number of TILs than in the present work, namely 3.8 TILs/100 tumour cells (mean, range 0–17). Paradoxically, patients having SCCACs with tumor infiltrating lymphocytes had a worse prognosis than cases without TILs.
In the present work, we found that SCCAC cases with massive TILs were in a more advanced clinical stage than control SCCAC cases, particularly at stage III. However, SCCAC patients with massive TILs had a better long-term survival (15 years follow up, 180 months in Figure 2) than control SCCAC patients. It should be mentioned that in the report by Grabenbauer et al [17], the mean follow up was only 2.1 years.
Interestingly, all 9 SCCAC patients with massive TILs (the 8 having clinical follow up as well as the additional case recently diagnosed in this department) occurred in females. The question arises as to whether this gender predominance was merely coincidental or whether estrogens played a crucial role in the accumulation of lymphocytes in the SCCAC tumoral area.
It is concluded that despite patients with SCCAC showing massive TILs had a more advanced clinical stage than SCCAC controls, the former patients had a longer 15 years survival rate than controls, with no deaths after 5 years. The search via proteomic methodology for the lymphocyte-attracting tumor-protein, might bring forward a novel co-adjuvant therapy, capable to increase prolonged survival time in patients having SCCAC without massive TILs.
References
- 1.Fenger C, Frisch M, Marti M, Parc C. Tumours of the anal canal. In: Hamilton S, Aaltonen, editors. WHO Pathology and Genetics. Tumours of the Digestive System. Lyon: IARC Press; 2000. pp. 147–155. [Google Scholar]
- 2.Alexander J, Watanabe T, Wu TT, Rashid A, Li S, Hamilton SR. Histopathological identification of colon cancer with micro-satellite instability. Am J Pathol. 2001;158:527–535. doi: 10.1016/S0002-9440(10)63994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Staib L, Gottwald T, Lehnert T, Ruf G, Sturm J, Becker HD, Farthmann E, Herfarth C, Post S, Trede M, Beger HG. Sphincter-saving treatment in epidermoid anal cancer: cooperative analysis of 142 patients in five German university surgical centers. Int J Colorectal Dis. 2000;15:282–290. doi: 10.1007/s003840000246. [DOI] [PubMed] [Google Scholar]
- 4.Fenger C, Frisch M, Jass J, Williams G, Hilden J. Anal cancer subtype reproducibility study. Virchows Arch. 2000;436:229–233. doi: 10.1007/s004280050035. [DOI] [PubMed] [Google Scholar]
- 5.Chetty R, Serra S, Hsieh E. Basaloid squamous carcinoma of the anal canal with an adenoid cystic pattern: histologic and immunohistochemical reappraisal of an unusual variant. Am J Surg Pathol. 2005;29:1668–1672. doi: 10.1097/01.pas.0000172193.32918.92. [DOI] [PubMed] [Google Scholar]
- 6.Frisch M, Fenger C, van den Brule AJ, Sorensen P, Meijer CJ, Walboomers JM. Variants of squamous cell carcinoma of the anal canal and perianal skin and their relation to human papillomaviruses. Cancer Res. 1999;59:753–757. [PubMed] [Google Scholar]
- 7.Nilsson PJ, Svensson C, Goldman S, Ljungqvist O, Glimelius B. Epidermoid anal cancer: a review of a population-based series of 308 consecutive patients treated according to prospective protocols. Int J Radiat Oncol Biol Phys. 2005;61:92–102. doi: 10.1016/j.ijrobp.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 8.Frisch M, Smith E, Grulich A, Johansen C. Cancer in a population-based cohort of men and women in registered homosexual partnerships. Am J Epidemiol. 2003;157:966–972. doi: 10.1093/aje/kwg067. [DOI] [PubMed] [Google Scholar]
- 9.Varnai AD, Bollmann M, Griefingholt H, Speich N, Schmitt C, Bollmann R, Decker D. HPV in anal squamous cell carcinoma and anal intraepithelial neoplasia (AIN). Impact of HPV analysis of anal lesions on diagnosis and prognosis. Int J Colorectal Dis. 2006;21:135–142. doi: 10.1007/s00384-005-0777-7. [DOI] [PubMed] [Google Scholar]
- 10.Daling JR, Madeleine MM, Johnson LG, Schwartz SM, Shera KA, Wurscher MA, Carter JJ, Porter PL, Galloway DA, McDougall JK. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer. 2004;101:270–280. doi: 10.1002/cncr.20365. [DOI] [PubMed] [Google Scholar]
- 11.Frisch M. On the etiology of anal squamous carcinoma. Dan Med Bull. 2002;49:194–209. [PubMed] [Google Scholar]
- 12.Tachibana T, Onodera H, Tsuruyama T, Mori A, Nagayama S, Hiai H, Imamura M. Increased intratumor V{alpha}24-positive natural killer T cells: a prognostic factor for primary colorectal carcinomas. Clin Cancer Res. 2005;11:7322–7327. doi: 10.1158/1078-0432.CCR-05-0877. [DOI] [PubMed] [Google Scholar]
- 13.Schumacher K, Haensch W, Roefzaad C, Schlag PM. Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res. 2004;61:3932–3936. [PubMed] [Google Scholar]
- 14.Grabenbauer GG, Lahmer G, Distel L, Niedobitek G. Tumor-infiltrating cytotoxic T cells but not regulatory T cells predict outcome in anal squamous cell carcinoma. Clin Cancer Res. 2006;12:3355–3360. doi: 10.1158/1078-0432.CCR-05-2434. [DOI] [PubMed] [Google Scholar]
- 15.Ogawa M, Tsutsui T, Zou JP, Mu J, Wijesuriya R, Yu WG, Herrmann S, Kubo T, Fujiwara H, Hamaoka T. Enhanced induction of very late antigen 4/lymphocyte function-associated antigen 1-dependent T-cell migration to tumor sites following administration of interleukin 12. Cancer Res. 1997;57:2216–2222. [PubMed] [Google Scholar]
- 16.Ogawa M, Umehara K, Yu WG, Uekusa Y, Nakajima C, Tsujimura T, Kubo T, Fujiwara H, Hamaoka T. A critical role for a peritumoral stromal reaction in the induction of T-cell migration responsible for interleukin-12-induced tumor regression. Cancer Res. 1999;59:1531–1538. [PubMed] [Google Scholar]
- 17.Zou JP, Yamamoto N, Fujii T, Takenaka H, Kobayashi M, Herrmann SH, Wolf SF, Fujiwara H, Hamaoka T. Systemic administration of rIL-12 induces complete tumor regression and protective immunity: response is correlated with a striking reversal of suppressed IFN-γ production by anti-tumor T cells. Int Immunol. 1995;7:1135–1145. doi: 10.1093/intimm/7.7.1135. [DOI] [PubMed] [Google Scholar]
- 18.Nilsson PJ, Rubio CA, Lenander C, Auer G, Glimelius B. Tumour budding detected by laminin-5γ2-chain immunohistochemistry is of prognostic value in epidermoid anal cancer. Ann Oncol. 2005;16:893–898. doi: 10.1093/annonc/mdi179. [DOI] [PubMed] [Google Scholar]
- 19.Mangano MM, Antonioli DA, Schnitt SJ, Wang HH. Nature and significance of cells with irregular nuclear contours in esophageal mucosal biopsies. Mod Pathol. 1992;5:191–196. [PubMed] [Google Scholar]
- 20.Sobin LH, Wittekind C. UICC International Union Against Cancer: TNM classification of malignant tumours. 6th edition. New York: Wiley-Liss; 2002. [Google Scholar]


