Abstract
Microvacuolation is relatively common in the limbic lobe in Lewy body disease (LBD). Similar pathology has also been reported in Alzheimer's disease (AD). Almost all of the studies of microvacuolation in AD, however, antedated the routine application of sensitive immunohistochemical methods to detect Lewy bodies. This raises the possibility that microvacuolation previously reported in AD may have been due to unrecognized LB pathology. To explore this issue, α-synuclein immunohistochemistry was used to evaluate a consecutive series of AD as well as cases with mixed AD and LBD (AD/LBD). Independently, the degree of microvacuolation was graded in the entorhinal cortex and the amygdala of the same cases. The results showed that microvacuolation was more common and more severe in AD/LBD than in pure AD cases. In pure AD cases microvacuolation was related to senile plaque density, especially in the amygdala, where many of the neuropil vacuoles were around dense-cored, neuritic plaques. In contrast, in AD/LBD microvacuolation correlated with LB density in the entorhinal cortex and amygdala. These results suggest that microvacuolation has a different pathogenesis in AD and in AD/LBD. Moreover, when prominent microvacuolation is detected in AD, it is imperative to exclude concurrent LBD.
Keywords: Alzheimer's disease, Lewy body disease, microvacuolation, spongiosis
Introduction
Microvacuolation (MV) is found in the neuropil of a number of neurodegenerative disorders, such as Lewy body disease (LBD) [1], frontotemporal dementia (FTD) [2], cortico-basal degeneration (CBD) [3] and Alzheimer's disease (AD) [4], but its mechanism is unknown. There are several types of MV. “Spongiform degeneration” is characteristic of transmissible spongiform encephalopathies [5]. “Status spongiosis” is less specific and can occur in end-stage degenerative disease of multiple etiologies. It is associated with severe neuronal loss and gliosis. “Superficial spongiosis” is MV in the superficial cortical lamina and is typical of FTD and CBD. MV can also be found in metabolic encephalopathies [6], in hypoxic encephalopathy [7] and even as a tissue-processing artifact.
MV is commonly observed in AD with concurrent LBD (AD/LBD), also known as the Lewy body variant of AD [8], and it is often more widespread in AD/LBD than in pure AD. The distribution of MV in AD/LBD corresponds to that of Lewy bodies [8]. Many AD cases have MV that is almost entirely confined to the medial temporal lobe [4], but previous studies emphasizing this pathologic feature of AD were conducted before routine application of immunohistochemical methods to detect Lewy bodies. It remains uncertain if MV in AD is always due to concurrent LBD.
In this study, we investigated the frequency of MV in pure AD and AD/LBD using α-synuclein immunohistochemistry. The severity of MV and the influence of demographic, genetic and pathologic factors on MV were also investigated in AD and AD/LBD.
Materials and Methods
Histologic sections from a consecutive series of 112 cases of AD and 65 cases of AD with transitional or diffuse LBD (AD/LBD group) were studied. The demographics of these cases are presented in Table 1. Cases were matched for age and sex, as well as for Braak stage and APOE ε4 carrier state and TAU H1/H1 genotype frequency. Neuropathologic diagnoses were rendered based on a standardized dissection, sampling and staining protocol that included quantitative measures of the density of senile plaques (SPs) (X100) and neurofibrillary tangles (NFT) (X400) in multiple brain regions using thioflavine-S fluorescent microscopy. Separate counts were taken from the amygdala in corticomedial (CM) and basolateral (BL) regions. Immunohistochemistry with a polyclonal antibody to α-synuclein was performed to detect Lewy bodies. The pure AD cases had no Lewy body pathology, even in the amygdale [9]. All AD and AD/LBD cases were devoid of concomitant pathologies that might contribute to microvacuolation, in particular FTD and CBD. None of the patients carried a clinical diagnosis of CJD, or had significant metabolic or hypoxic encephalopathy.
Table 1.
Demographics and genetic information of patients with AD and AD/LBD
| AD (n=112) | AD/LBD (n=65) | ||
|---|---|---|---|
| Age at death (yrs) | 80.3 ± 0.9 | 79.2 ± 0.8 | n.s. |
| Sex (M:F) | 51:61 | 24:41 | n.s. |
| APOE ε4(+) (%) | 56% | 67% | n.s. |
| H1/H1 (%) | 65% | 64% | n.s. |
| Braak stage | 5.5 ± 0.1 | 5.6 ± 0.0 | n.s. |
n.s., not significant
The following regions were evaluated in coronal sections of the brain stained with hematoxylin and eosin (H&E): the posterior hippocampus at the level of the lateral geniculate nucleus, which included the subiculum, parahippocampal gyrus and the inferior/medial temporal lobe; and the basal forebrain region, which included the amygdala, claustrum and insular cortex. The entorhinal cortex (ERC) and amygdala (Amyg) were selected for grading severity of MV.
Immunohistochemistry
Sections of posterior hippocampus and basal forebrain were cut at 5 µm thickness and used for immunohistochemistry. The deparaffinized and rehydrated sections were microwaved in distilled water at high power setting for 10 min, and then incubated in 0.01 M phosphate buffered saline (PBS; pH 7.4) containing 0.3% hydrogen peroxide for 20 minutes. The sections were treated with 5% normal horse serum for 20 minutes and incubated 2 days with the polyclonal antibody to α-synuclein, NACP [10] (1:1000) at 4°C. After incubation with the primary antibody, the sections were treated with biotinylated anti-rabbit IgG secondary antibody (1:200, Vector Lab., Burlingame, CA) for 2 hours, followed by incubation in the avidin-biotinylated horseradish peroxidase (HRP) complex (ABC Elite; 1:200, Vector Lab.) for 2 hours. Peroxidase labeling was detected by incubation with a solution containing 0.3 mg/mL 3, 3′-diaminobenzidine and 0.03% hydrogen peroxide. After immunostaining, the sections were immersed in hematoxylin briefly for counter staining.
Grading Severity of MV
MV was defined as a microscopic alteration of the neuropil characterized by round, relatively small and evenly distributed vacuoles. Some larger and irregular vacuoles and occasionally confluent microvacuoles were counted, but pericellular and perivascular vacuoles were considered to be processing artifact. The severity of MV was evaluated semi-quantitatively (X100) with a double-headed microscope by the authors from H&E-stained sections, using the following grading system: grade 0, “none”; grade 1, “very-mild”; grade 2, “mild-moderate”, and grade 3, “moderate-severe” (Figure 1). Very-mild cases (grade 1) had sparse small, uniform and non-confluent vacuoles that mostly were present in the superficial cortical layers. Mild-to-moderate cases (grade 2) had many vacuoles that were sometimes irregular and confluent, and usually distributed in multiple cortical layers. Moderate-to-severe cases (grade 3) had numerous, often irregular and confluent MV, distributed in all cortical layers. Spongiosis in this study is defined as at least grade 2, while grade 1 is considered only incidental MV.
Figure 1.
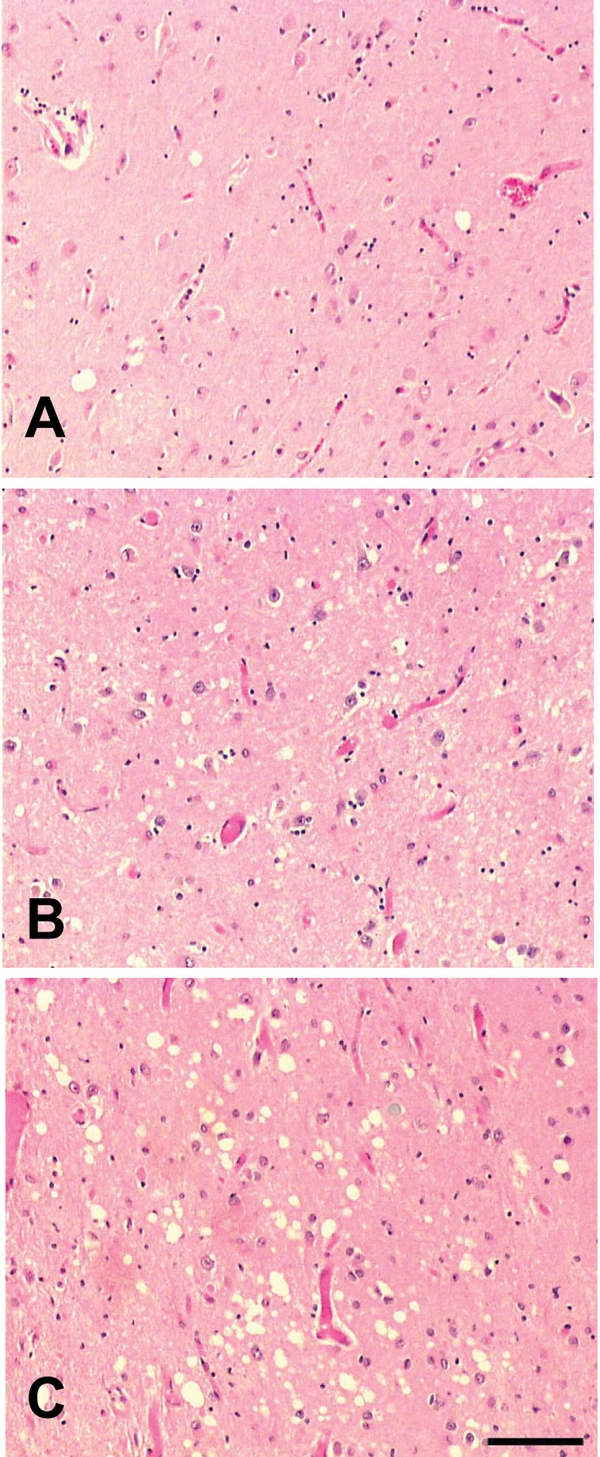
H&E staining in AD/LBD cases. A. Grade 1 “very-mild” MV; B. Grade 2 “mild-moderate” MV; C. Grade 3 “moderate-severe” MV. Bar = 100µm
Statistical Methods
The frequency and severity of MV were evaluated in AD and AD/LBD, and subsequently the frequency of spongiosis and the average MV grade were compared between the two groups. The severity of MV was correlated with average counts of SP and NFT in ERC, Amyg/CM and Amyg/BL to assess the influence of pathologic variables on MV in AD. For AD/LBD, severity of MV was correlated with average Lewy body counts. The APOE ε4 carrier state and TAU H1/H1 genotype frequencies were also evaluated to assess the influence of genetic factors. Genomic DNA extracted from frozen brain and analysed as previously described [11]. Genetic information was available in 111 cases of pure AD and 86 cases of AD/LBD. Mann-Whitney's U test, Chi-square test and Spearman Rank Order Correlation test were used as appropriate. P<0.05 was considered statistically significant.
Results
Frequency of MV in AD and AD/LBD as grouped by grades is presented in Table 2. Among 112 cases of AD, 77 (69%) had some degree of MV. MV in AD was mostly mild and found in superficial cortical layers only. In 17 AD cases (15%), more severe MV consistent with spongiosis was detected (Table 3). No pure AD cases had grade 3 MV. Interestingly, MV was often detected around or sometimes within senile plaques with dense amyloid cores, especially in the amygdala (Figure 2).
Table 2.
Distribution of MV grades
| MV Grade | AD | AD/LBD |
|---|---|---|
| 0 | 35 (31%) | 6 (9%) |
| 1 | 60 (54%) | 13 (20%) |
| 2 | 17 (15%) | 35 (54%) |
| 3 | 0 | 11 (17%) |
Table 3.
Spongiosis in AD and AD/LBD
| AD | AD/LBD | |
|---|---|---|
| spongiosis (+) | 17 (15%) | 46 (71%) |
| spongiosis (−) | 95 (85%) | 19 (29%) |
Figure 2.
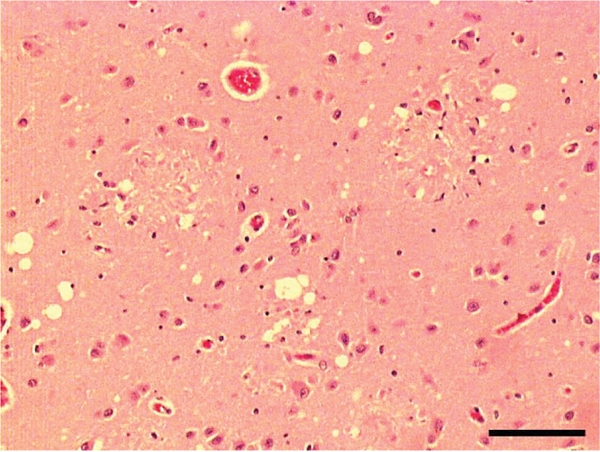
H&E staining in an AD case showing MV around a senile plaque. Bar = 100µm
Among the 65 cases of AD/LBD group, 59 (91%) had various degree of MV. MV limited to superficial cortical layers was present in only 6 (9%) cases while significant spongiosis was present in 46 (71%) of the cases. The frequency of MV in AD/LBD was significantly higher than that in pure AD (χ2=10.0, p<0.01). The frequency of spongiosis (MV grades 2 and 3) in AD/LBD was even greater compared to pure AD (χ2 = 32.3, p<0.001) (Table 3).
The average MV grade in both the ERC and Amyg was significantly greater in AD/LBD than in pure AD (Figure 3) (p<0.001; Mann-Whitney's U test). The severity MV did not differ in the ERC compared to the Amyg for either pure AD or AD/LBD.
Figure 3.
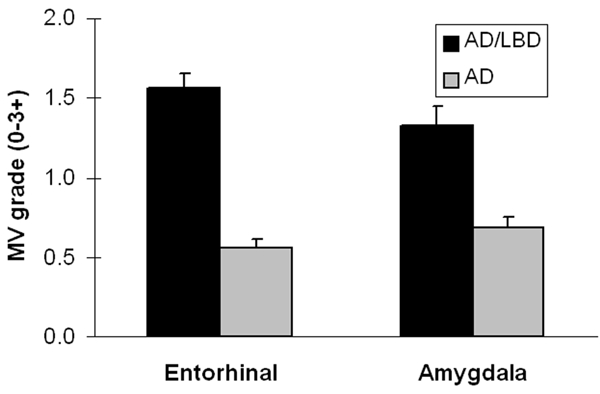
The MV grade in both the ERC and Amygdala was significantly greater in AD/LBD than in pure AD (p<0.001: Mann-Whitney's U test).
Spearman Rank Order Correlation analysis showed that MV correlated with SP in the Amyg/CM and the Amyg/BL in pure AD cases (Table 4). MV did not correlate with NFT counts in any region. For AD/LBD cases, MV correlated with Lewy body counts in ERC and Amyg, but showed no correlation with either SP or NFT in any region.
Table 4.
Correlation between MV and pathologic variables
| AD | r | p | |
|---|---|---|---|
| ERC | SP | −0.11 | n.s. |
| NFT | 0.04 | n.s. | |
| Amyg | SP/CM | 0.26 | p<0.01 |
| SP/BL | 0.19 | p<0.05 | |
| NFT/CM | 0.11 | n.s. | |
| NFT/BL | 0.13 | n.s. | |
| AD/LBD | r | p | |
| ERC | SP | 0.14 | n.s. |
| NFT | 0.10 | n.s. | |
| LB | 0.39 | p<0.01 | |
| Amyg | SP/CM | 0.06 | n.s. |
| SP/BL | 0.22 | n.s. | |
| NFT/CM | 0.04 | n.s. | |
| NFT/BL | 0.06 | n.s. | |
| LB | 0.34 | p<0.01 | |
SP, spongiosis; NFT, neurofibrillary tangle; CM, corticomedial; BL, basolateral; LB, Lewy body; Ad, Alzheimer disease; LBD, Lewy body disease; ERC, entorhinal cortex; Amyg, amygdala; n.s., not significant
Frequencies of the APOE ε4 carrier state and Tau H1/H1 genotype are presented in Table 5. The APOE ε4 carrier state was greater in AD cases with spongiosis than in cases without spongiosis, reflecting the greater overall severity of Alzheimer type pathology in the APOE ε4 positive cases [12]. There was no difference in APOE ε4 frequency in AD/LBD cases with and without spongiosis. No differences were detected in frequency of Tau H1/H1 genotype for AD or AD/LBD cases with and without spongiosis.
Table 5.
Correlation of spongiosis with genetic factors in AD and AD/LBD
| APOE | ε4 (+) | ε4 (−) | |
|---|---|---|---|
| AD | |||
| Spongiosis (+) | 11 (69%) | 5 (31%) | χ2 = 17.9; p<0.01 |
| Spongiosis (−) | 44 (52%) | 41 (48%) | |
| AD/LBD | |||
| Spongiosis (+) | 30 (70%) | 13 (30%) | χ2 = 0.05; n.s. |
| Spongiosis (−) | 12 (63%) | 7 (37%) | |
| TAU | H1/H1 | non-H1/H1 | |
| AD | |||
| Spongiosis (+) | 11 (69%) | 5 (31%) | χ2 = 0.01; n.s |
| Spongiosis (−) | 55 (66%) | 28 (34%) | |
| AD/LBD | |||
| Spongiosis (+) | 28 (67%) | 14 (33%) | χ2 = 0.05; n.s. |
| Spongiosis (−) | 13 (59%) | 9 (41%) | |
Discussion
The present study demonstrated that the frequency of MV is significantly greater in AD cases with concurrent LBD and that spongiosis is almost always associated with concurrent LBD. The frequency of spongiosis in AD was only 15% compared to 71% for AD/LBD. Concurrently, this study showed the severity of MV, as assessed by MV grade in both the ERC and amygdala, was significantly greater in AD/LBD than in pure AD. These findings suggest that spongiosis is a characteristic feature of AD with concomitant Lewy body pathology, but not pure AD.
Hansen and coworker first reported that MV was more common in the temporal lobe in AD/LBD than in AD [13]; however, α-synuclein immunohistochemistry was not used to detect Lewy bodies and no effort was made to grade the severity of MV or to distinguish spongiosis from superficial cortical microvacuolation. The present study confirms the previous observations and further emphasizes the importance of considering Lewy body pathology in AD cases in which spongiosis is detected.
Concomitant Lewy body pathology is often detected in AD [9, 14]. While some previous studies have shown differences in clinical presentation of AD/LBD and pure AD [15–19], this holds only for cases in which AD pathology is not advanced. When AD pathology is advanced, the clinical presentation of AD/LBD is indistinguishable from pure AD [16, 18, 19]. Therefore, the detection of Lewy body pathology needs to be considered even in AD cases with a typical clinical syndrome. Given the present findings, the histologic presence of spongiosis in the limbic lobe should be a clue that Lewy body pathology may coexist even if Lewy bodies are not obvious with routine methods of histologic analysis.
While it is reasonably well accepted that spongiosis is a feature of LBD [1, 8], there have been few studies that address the pathogenesis of this histologic finding. Iseki and coworkers suggested that α-synuclein pathology begins in distal neuronal processes and that this induces a form of “dying back” degeneration of affected neurons [20]. Moreover, they suggested that spongiosis in LBD may derive from degeneration of terminal axons of the large pyramidal neurons [20]. The present findings of a strong correlation between spongiosis and Lewy body counts in AD/LBD would support the pathogenic link between α-synuclein pathology and spongiosis as suggested by Iseki and coworkers. The pathogenesis of microvacuolation in pure AD is different, and the present correlative studies suggest a relationship between vacuolation and SP in pure AD. Moreover, vacuolation often appeared around plaques with dense amyloid cores. Mancardi and coworkers showed MV in AD may derive from enlarged post-synaptic dendrites [21]. These findings further support the hypothesis that MV in AD is related to neuritic dystrophy.
The increased frequencies of the APOE ε4 carrier state in AD cases with MV can be most easily interpreted as reflecting the fact that ε4 carrier state is associated with more marked Alzheimer type pathology, in terms of SP and NFT density [12]. On the other hand, TAU H1/H1 genotype has not been shown to influence Alzheimer type pathology [11]. Thus, it is not surprising that TAU variants had no relationship to presence of vacuolation in AD.
In conclusion, the present study demonstrates that spongiosis in limbic lobe structures is uncommon in AD in the absence of concurrent LBD. Consequently, when spongiosis is detected in AD, it is important to exclude concurrent Lewy body pathology. The present correlative studies also suggest that the pathogenesis of neuropil vacuolation in AD probably differs from that in LBD.
Acknowledgments
The authors thank Virginia Phillips and Linda Rousseau for their histologic support and Natalie Thomas for genetic analyses. Most of the cases in this study were derived from The State of Florida Alzheimer Disease Initiative brain bank. Supported by NIH grants: P50 AG16574, P01 AG17216, P50 NS40256 and P01 AG03949.
References
- 1.McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, Salmon DP, Lowe J, Mirra SS, Byrne EJ, Lennox G, Quinn NP, Edwardson JA, Ince PG, Bergeron C, Burns A, Miller BL, Loverstone S, Collerton D, Jansen EN, Ballard C, de Vos RA, Wilcock GK, Jellinger KA, Perry RH. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 2.Brun A, Passant U. Frontal lobe degeneration of non-Alzheimer type. Structural characteristics, diagnostic criteria and relation to other frontotemporal dementias. Acta Neurol Scand Suppl. 1996;168:28–30. [PubMed] [Google Scholar]
- 3.Boeve BF, Maraganore DM, Parisi JE, Ahlskog JE, Graff-Radford N, Caselli RJ, Dickson DW, Kokmen E, Petersen RC. Pathologic heterogeneity in clinically diagnosed cortico-basal degeneration. Neurology. 1999;53:795–800. doi: 10.1212/wnl.53.4.795. [DOI] [PubMed] [Google Scholar]
- 4.Smith TW, Anwer U, DeGirolami U, Drachman DA. Vacuolar change in Alzheimer's disease. Arch Neurol. 1987;44:1225–1228. doi: 10.1001/archneur.1987.00520240007003. [DOI] [PubMed] [Google Scholar]
- 5.Budka H, Aguzzi A, Brown P, Brucher JM, Bugiani O, Gullotta F, Haltia M, Hauw JJ, Ironside JW, Jellinger K, et al. Neuropathological diagnostic criteria for Creutzfeldt-Jakob disease (CJD) and other human spongiform encephalopathies (prion diseases) Brain Pathol. 1995;5:459–466. doi: 10.1111/j.1750-3639.1995.tb00625.x. [DOI] [PubMed] [Google Scholar]
- 6.Cottrell DA, Ince PG, Blakely EL, Johnson MA, Chinnery PF, Hanna M, Turnbull DM. Neuropathological and histochemical changes in a multiple mitochondrial DNA deletion disorder. J Neuropathol Exp Neurol. 2000;59:621–627. doi: 10.1093/jnen/59.7.621. [DOI] [PubMed] [Google Scholar]
- 7.Van Reempts J. The hypoxic brain: histological and ultrastructural aspects. Behav Brain Res. 1984;14:99–108. doi: 10.1016/0166-4328(84)90177-3. [DOI] [PubMed] [Google Scholar]
- 8.Hansen LA, Masliah E, Terry RD, Mirra SS. A neuropathological subset of Alzheimer's disease with concomitant Lewy body disease and spongiform change. Acta Neuropathol (Berl) 1989;78:194–201. doi: 10.1007/BF00688209. [DOI] [PubMed] [Google Scholar]
- 9.Uchikado H, Lin WL, DeLucia MW, Dickson DW. Alzheimer disease with amygdala Lewy bodies: a distinct form of alpha-synucleinopathy. J Neuropathol Exp Neurol. 2006;65:685–697. doi: 10.1097/01.jnen.0000225908.90052.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gwinn-Hardy K, Mehta ND, Farrer M, Maraganore D, Muenter M, Yen SH, Hardy J, Dickson DW. Distinctive neuropathology revealed by alpha-synuclein antibodies in hereditary parkinsonism and dementia linked to chromosome 4p. Acta Neuropathol (Berl) 2000;99:663–672. doi: 10.1007/s004010051177. [DOI] [PubMed] [Google Scholar]
- 11.Liu WK, Le TV, Adamson J, Baker M, Cookson Hardy J, Hutton M, Yen SH, Dickson DW. Relationship of the extended tau haplotype to tau biochemistry and neuropathology in progressive supranuclear palsy. Ann Neurol. 2001;50:494–502. doi: 10.1002/ana.1159. [DOI] [PubMed] [Google Scholar]
- 12.Schmechel DE, Saunders AM, Strittmatter WJ, Crain BJ, Hulette CM, Joo SH, Pericak-Vance MA, Goldgaber D, Roses AD. Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:9649–9653. doi: 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen LA. The Lewy body variant of Alzheimer disease. J Neural Transm Suppl. 1997;51:83–93. doi: 10.1007/978-3-7091-6846-2_7. [DOI] [PubMed] [Google Scholar]
- 14.Hamilton RL. Lewy bodies in Alzheimer's disease: a neuropathological review of 145 cases using alpha-synuclein immunohistochemistry. Brain Pathol. 2000;10:378–384. doi: 10.1111/j.1750-3639.2000.tb00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heyman A, Fillenbaum GG, Gearing M, Mirra SS, Welsh-Bohmer KA, Peterson B, Pieper C. Comparison of Lewy body variant of Alzheimer's disease with pure Alzheimer's disease: Consortium to Establish a Registry for Alzheimer's Disease, Part XIX. Neurology. 1999;52:1839–1844. doi: 10.1212/wnl.52.9.1839. [DOI] [PubMed] [Google Scholar]
- 16.Hohl U, Tiraboschi P, Hansen LA, Thal LJ, Corey-Bloom J. Diagnostic accuracy of dementia with Lewy bodies. Arch Neurol. 2000;57:347–351. doi: 10.1001/archneur.57.3.347. [DOI] [PubMed] [Google Scholar]
- 17.Olichney JM, Galasko D, Salmon DP, Hofstetter CR, Hansen LA, Katzman R, Thal LJ. Cognitive decline is faster in Lewy body variant than in Alzheimer's disease. Neurology. 1998;51:351–357. doi: 10.1212/wnl.51.2.351. [DOI] [PubMed] [Google Scholar]
- 18.Lopez OL, Wisniewski S, Hamilton RL, Becker JT, Kaufer DI, DeKosky ST. Predictors of progression in patients with AD and Lewy bodies. Neurology. 2000;54:1774–1779. doi: 10.1212/wnl.54.9.1774. [DOI] [PubMed] [Google Scholar]
- 19.Stern Y, Jacobs D, Goldman J, Gomez-Tortosa E, Hyman BT, Liu Y, Troncoso J, Marder K, Tang MX, Brandt J, Albert M. An investigation of clinical correlates of Lewy bodies in autopsy-proven Alzheimer disease. Arch Neurol. 2001;58:460–465. doi: 10.1001/archneur.58.3.460. [DOI] [PubMed] [Google Scholar]
- 20.Iseki E, Marui W, Kosaka K, Akiyama H, Ueda K, Iwatsubo T. Degenerative terminals of the perforant pathway are human alpha-synuclein-immunoreactive in the hippocampus of patients with diffuse Lewy body disease. Neurosci Lett. 1998;258:81–84. doi: 10.1016/s0304-3940(98)00856-8. [DOI] [PubMed] [Google Scholar]
- 21.Mancardi GL, Mandybur TI, Liwnicz BH. Spongiform-like changes in Alzheimer's disease. An ultrastructural study. Acta Neuropathol (Berl) 1982;56:146–150. doi: 10.1007/BF00690586. [DOI] [PubMed] [Google Scholar]


