Abstract
IL27 is the newest member of the IL6/IL12 family. It consists of Epstein-Barr virus-induced gene 3 (EBI3) and p28 subunits and signals through the IL27 receptor complex formed by WSX-1 and gp130 subunits. IL27-IL27 receptor interaction was initially shown to induce an early signal for the induction of Th1 responses. Recently other studies indicate that IL27 inhibits inflammatory Th17 lineage development. Despite the overall effects observed, the role of individual IL27 subunits remains controversial and is largely unclear. EBI3, while predominately found with p28 to form IL27, has different expression kinetics than p28. P28 has also been shown to signal independently of EBI3. Moreover, EBI3 has other potential binding partners such as the p35 subunit of IL12 and theoretically may regulate the inflammatory response through yet undiscovered mechanisms. Understanding the inflammatory and anti-inflammatory roles of IL27 and its subunits are essential before the immunological regulatory therapies can be developed.
Keywords: IL27, IL6, IL12, inflammation, induction, inhibition
Introduction
Interleukin-27 (IL27) is a recently discovered cytokine belonging to the IL6/IL12 cytokine family [1]. Due to the heterodimeric nature of these cytokines and their receptors, studies on the IL12 family members have generated exciting stories to follow. Early studies of IL12 (p40/p35) based on the elimination of the p40 subunit yielded conflicting results. Not until the discovery that the p19 subunit of IL23 also binds p40 were the conflicting results resolved into a reasonable model [2]. Subsequent research on p19 or p35 knock out mice further helped resolve the conflicting data [3, 4].
IL27 plays a role in the innate as well as the adaptive immunity. In adaptive immunity, IL27 was shown to synergize with IL12 to promote IFNγ production by CD4, CD8 T cells and NKT cells [5–7]. IL27 was also identified as an early initiator of Th1 differentiation [8]. In innate immune, IL27 was demonstrated to induce the production of IL1, TNFα, IL18 and IL12 in monocytes, and IL1 and TNFα in mast cells [9]. Recent studies have revealed that IL27 inhibits differentiation of Th17 cells [10–12]. This review focuses on the subunits of IL27, IL27 receptor and their roles in diseases models.
IL27 (p28/EBI3)
IL27 is a heterodimeric cytokine comprised of p28 and Epstein-Barr virus-induced gene 3 (EBI3) subunits [7] (Figure 1A). EBI3 was initially described as being expressed in B lymphocytes infected by Epstein-Barr virus [13]. It is homologous to IL12p40 subunit with 2 fibronectin-3 motifs, placing it in the hematopoietin receptor family. EBI3 is known to exist in three forms, homodimer [14], p35 heterodimer [15], and p28 heterodimer [7]. EBI3 is expressed in lymphoid blasts of reactive lymphoid organs [17, 18]. In the light zone of germinal centers, EBI3 is secreted where B cells can be found in association with CD3+ T cells [19]. A splice variant of EBI-3 has been recently found and expressed in the spleen, liver and kidney [16]. The expression pattern of EBI3 reveals that EBI3 has important functions in the immune system. It has been suggested that free EBI3 or EBI3/p35 might be an antagonist to IL12 family signaling [20], but this role has yet to be confirmed.
Figure 1.
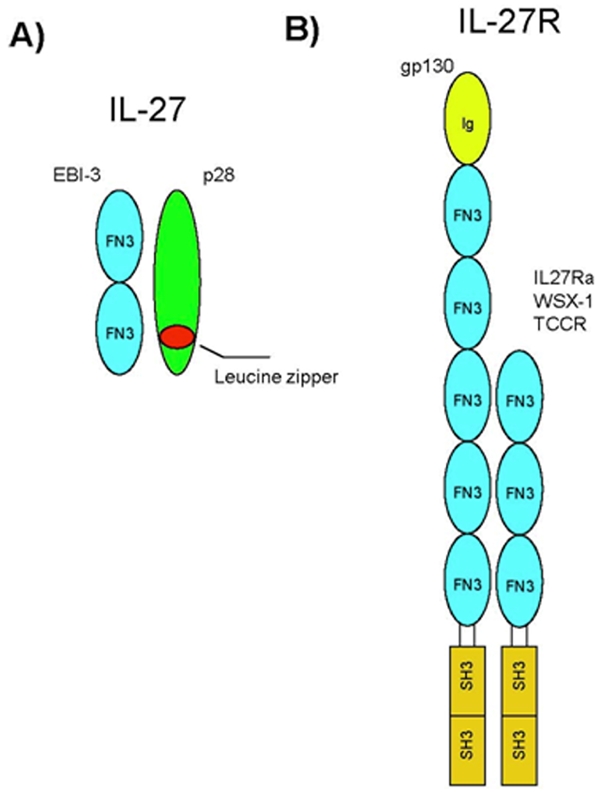
IL27 and IL27 receptor. A. IL27 is comprised of two subunits, EBI-3 and p28. EBI-3 has two fibronectin type 3 domains, and p28 has a leuicine zippper motif. B. IL27R is comprised of gp130 and IL27Rα. gp130 belongs to the IL6 receptor family. IL27Rα is also called WSX-1 or TCCR. Both chains have intracellular signaling domains.
P28 is related to the IL12p40 subunit [7], containing a long-chain four-helix bundle typical of the IL6/IL12 family. P28 contains a leucine zipper motif indicative of homo-or heterodimerization. P28 is normally found to be co-expressed with EBI3 in activated macrophages and dendritic cells, forming a non-covalently linked heterodimer [7, 17, 21]. In the normal central nervous system (CNS), IL27p28 and EBI3 are detected at very low levels if at all [22]. But in the brain during chronic toxoplasmic encephalitis, EBI3 levels were increased by 3 folds while p28 increased 500 folds. Furthermore, in response to lipopolysaccharide (LPS) and IFNγ induction, the expression of p28 increased more than 2000 folds while EBI3 levels remained unchanged [10, 23]. In viral-transformed neoplastic cells, there is a dissociated expression of EBI3 and p28 in which EBI3 is expressed while p28 is not [19, 21]. EBI3 was up-regulated during the induction of septic peritonitis, but returned to normal levels within 20 hours, while p28 remained elevated [24].
IL27 Receptor (gp130/IL27Rα)
Class I cytokine receptors are usually comprised of α and β heterodimers. The α subunit is the primary cytokine binding protein, while the β subunit is for high affinity binding and signal transduction. IL27 receptor (IL27R) is comprised of two signaling molecules, IL27Rα and gp130 (Figure 1B).
IL27Rα, also know as WSX-1 or TCCR, has 2 tyrosine residues that can be phosphorylated in humans. In mice it has 3 tyrosine residues, one of which is conserved. Human IL27Rα has 63% sequence match with murine IL27Rα. Human IL27Rα contains 7 N-linked glycosylation sites, while murine IL27Rα has only 5. IL27Rα is very similar to IL12β1R in that it also lacks an Ig domain, indicating that IL12βR1 might also be a signaling partner with gp130 [25].
GP130 subunit belongs to the IL6 receptor family and is shared by many other cytokine receptor partners such as IL6, IL11, CT-1, CNTF, LIF, and OM [26]. IL6 signaling through gp130 is well understood. IL6 first binds to IL6R and then dimerizes with gp130 to induce signal transduction in leukocytes and hepatocytes [27]. A soluble form of IL6R also exists which can bind IL6 and trans-signal through gp130 on non-immunological cells [28]. Soluble gp130 can effectively block trans-signaling in this IL6 pathway [29]. Experiments that utilized soluble gp130 to block signaling through IL27Rα suggest that IL27Rα is not expressed in a soluble form [30].
Both IL27Rα and gp130 receptor subunits have signaling domains, but they do not signal independently [31]. IL27Rα expression is found in naïve and memory B cells but not in germinal center B cells [32]. Alternatively activated macrophages, induced by IL4 and IL13, express IL27Rα while classically activated macrophages do not [33]. In naïve T cells, IL27Rα expression is low. Its expression is high in effector and memory T cells. IL2 suppresses the expression of IL27Rα nn activated CD4 T cells in a dose-dependent manner [34]. Resting NK, NKT, and TReg cells express high levels of IL27 receptors. Upon stimulation, naïve T cells greatly enhance the expression of IL27Rα, while NK and NKT reduce IL27Rα expression [34].
IL27 Signal Transduction
Engagement of the IL27 receptor recruits several Jak family kinases, which induces phosphorylation of STAT1 and STAT3 [35]. Some evidence indicates that STAT2, STAT4 and STAT5 are phosphorylated as well [32, 36–38]. In activated T cells, IL27 predominantly signals through STAT3 [23], while in memory B cells it signals predominately through STAT1 [32]. It has been shown that IL27Rα co-precipitates with JAK1 and STAT1 interacts with the conserved Y609 residue [36]. IL27 signaling induces Tbet expression in T cells in a STAT4-independent manner, which results in up-regulation of IL12Rβ2 expression even under Th2-inducing conditions [39]. It is known that IFNγ production through Tbet is modulated by estrogen [40]. IL12 does not synergize with estrogen, but IL27 does to induce IFNγ [41]. IL27 induces granzyme B and perforin production in CD8 T cells in a STAT1-dependent, but Tbet-independent manner [42]. In mature B cells, IL27 induces Tbet expression, IL12Rβ1 up-regulation, and class switch recombination (CSR) [38]. IL27 induces phosphorylation of STAT1 and subsequently blocks IL4-dependent CSR to IgG1, and induces IgG2a independent of IFNγ [38].
In Vivo Functions of IL27-IL27R Interaction in Inflammation
A number of studies support a proinflammatory role of IL27 in pathogen-induced or autoimmune inflammation models. p28 blockade resulted in suppression of ongoing adjuvant-induced arthritis [43] and experimental autoimmune encephalomyelitis (EAE) [44]. However, these results were challenged by later studies using blocking antibodies and IL27Rα−/− mice [1, 11]. WSX-1−/− mice show impaired IFNγ production and are susceptible to intracellular pathogens, such as Leishmania major and Listeria monocytogenes which are best combated by Th1 responses [5, 6]. Th1-mediated clearance of Mycobacterium tuberculosis and resistance to the disease are dependent upon the production of IFNγ and WSX1−/− mice had prolonged bacterial clearance [45]. WSX-1−/− mice show a skewed profile towards Th2 in response to infection by Trichuris muris [9, 46] and exhibit enhanced asthmatic phenotypes [47]. Interestingly, a p28 polymorphism (964A>G) in a Korean population was shown to be associated with susceptibility to asthma [48]. WSX-1−/− mice have also been shown to be less susceptible to experimental autoimmune uveitis [49], another T cell mediated autoimmune disease model.
A number of other studies also reported inhibitory roles of IL27-IL27R interaction in inflammation. Infection of EBI3−/− mice resulted in a massive infiltration of neutrophils and macrophages that resulted in a faster clearance of bacteria in a septic peritonitis model [24]. Toxoplasma gondii infection in WSX-1−/− mice generated a robust Th1 response. However, these mice succumb to an acute lethal CD4 mediated inflammatory disease [10]. WSX-1−/− mice mount a robust Th1 response to Listeria donovani and control parasite burden faster than WT mice; but the mice still suffer significant liver damage and diffuse inflammation [50]. WSX-1 deficient mice are hypersusceptible to EAE [11].
In inflammation models such as EAE, the IL17-producing CD4 T cells (Th17) have been identified as being better correlated with the severity of the disease than the Th1 subset [51, 52]. The Th17 subset is characterized by the expression of transcription factor RORγt [53] and cytokines including IL17A, IL17F, IL6, TNF, and GM-CSF, but not IFNγ or IL4. TGFβ and IL6 induce Th17 lineage of cells, which then become responsive to IL23 by expressing IL23 receptor [54, 55]. Recent studies suggest that Th17 response during inflammation is critically suppressed by IL27 [10–12] (Figure 2). We have recently found that EAE can be induced in EBI3−/− mice; however we did not observe significant difference between wild type and EBI3−/− mice in Th17 response (our unpublished observations). Thus, considerable conflicting data still exists in the field and more research is required to delineate the role of IL27 in inflammation.
Figure 2.
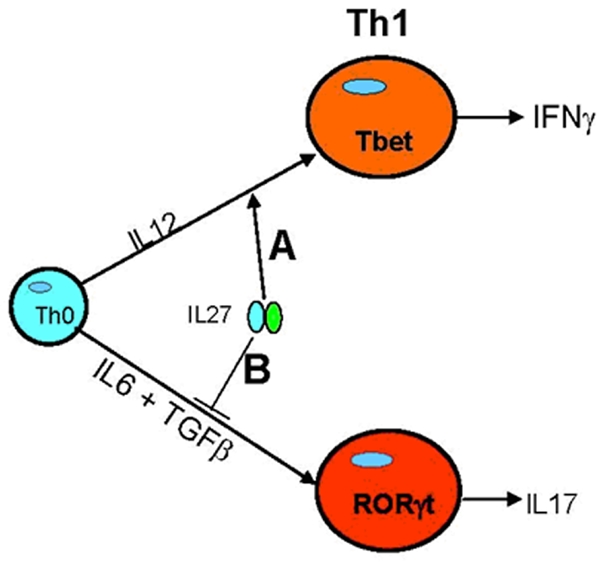
IL27 promotes Th1 but inhibits Th17 cell differentiation. A. IL27 promotes näive T cells (Th0) to differentiate into Th1 effectors characterized by expression of transcription factor Tbet and IFNγ. B. IL27 inhibits CD4 T cell differentiation into Th17 effectors characterized by expression of transcription factor RORγt and IL17. This model is largely based on the results obtained from IL27Rα−/− mice.
Concluding Remarks and Future Perspective
IL27 is comprised of p28 and EBI3 subunits that functions through interaction with IL27R. IL27 has been shown to have both proinflammatory and anti-inflammatory properties. Recent studies revealed that IL27 signaling inhibits the generation of Th17 lineages of cells. Since p28 and EBI3 can be independently expressed (Figure 3), one should be careful in interpreting the results of IL27 studies. For instance, opposing results were obtained when studying IL27 subunit P28 and its receptor subunit WSX-1, suggesting different partners may have different functions as shown in the IL12/IL23 case. Future experiments using P28 or EBI3 knockout mice are required to resolve these conflicting results.
Figure 3.
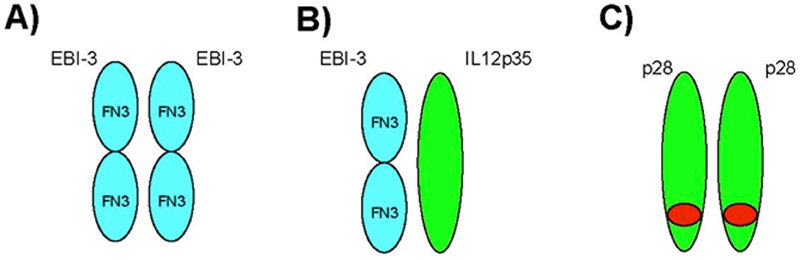
EBI3 and p28 associations other than IL27. A. EBI3 exists as a homodimer but its function is not yet known. B. EBI3 is associated with IL12p35 and is expressed during human pregnancy by syncytial trophoblasts. C. p28 has a leucine zipper motif and can homodimerize with each other.
References
- 1.Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–242. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- 2.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M, Gorman D, Wagner J, Zurawski S, Liu Y, Abrams JS, Moore KW, Rennick D, dewaal-Malefyt R, Hannum C, Bazan JF, Kastelein RA. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 3.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Weikowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 4.Gran B, Zhang GX, Yu S, Li J, Chen XH, Ventura ES, Kamoun M, Rostami A. IL-12p35-deficient mice are susceptible to experimental autoimmune encephalomyelitis: evidence for redundancy in the IL-12 system in the induction of central nervous system autoimmune demyelination. J Immunol. 2002;169:7104–7110. doi: 10.4049/jimmunol.169.12.7104. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida H, Hamano S, Senaldi G, Covey T, Faggioni R, Mu S, Xia M, Wakeham AC, Nishina H, Potter J, Potter J, Saris CJ, Mak TW. WSX-1 is required for the initiation of Th1 responses and resistance to L. major infection. Immunity. 2001;15:569–578. doi: 10.1016/s1074-7613(01)00206-0. [DOI] [PubMed] [Google Scholar]
- 6.Chen Q, Ghilardi N, Wang H, Baker T, Xie MH, Gurney A, Grewal IS, de Sauvage FJ. Development of Th1-type immune responses requires the type I cytokine receptor TCCR. Nature. 2000;407:916–920. doi: 10.1038/35038103. [DOI] [PubMed] [Google Scholar]
- 7.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E, Blumenschein WM, Mattson JD, Wagner JL, To W, Zurawski S, McClanahan TK, Gorman DM, Bazan JF, de Waal Malefyt R, Rennick D, Kastelein RA. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 8.Owaki T, Asakawa M, Morishima N, Hata K, Fukai F, Matsui M, Mizuguchi J, Yoshimoto T. A role for IL-27 in early regulation of Th1 differentiation. J Immunol. 2005;175:2191–2200. doi: 10.4049/jimmunol.175.4.2191. [DOI] [PubMed] [Google Scholar]
- 9.Artis D, Villarino A, Silverman M, He W, Thornton EM, Mu S, Summer S, Covey TM, Huang E, Yoshida H, Koretzky G, Goldschmidt M, Wu GD, de Sauvage F, Miller HR, Saris CJ, Scott P, Hunter CA. The IL-27 receptor (WSX-1) is an inhibitor of innate and adaptive elements of type 2 immunity. J Immunol. 2004;173:5626–5634. doi: 10.4049/jimmunol.173.9.5626. [DOI] [PubMed] [Google Scholar]
- 10.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, Saris CJ, O’Shea JJ, Hennighausen L, Ernst M, Hunter CA. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 11.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 12.Colgan J, Rothman P. All in the family: IL-27 suppression of T(H)-17 cells. Nat Immunol. 2006;7:899–901. doi: 10.1038/ni0906-899. [DOI] [PubMed] [Google Scholar]
- 13.Devergne O, Hummel M, Koeppen H, Le Beau MM, Nathanson EC, Kieff E, Birkenbach M. A novel interleukin-12 p40-related protein induced by latent Epstein-Barr virus infection in B lymphocytes. J Virol. 1996;70:1143–1153. doi: 10.1128/jvi.70.2.1143-1153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nieuwenhuis EE, Neurath MF, Corazza N, Iijima H, Trgovcich J, Wirtz S, Glickman J, Bailey D, Yoshida M, Galle PR, Kronenberg M, Birkenbach M, Blumberg RS. Disruption of T helper 2-immune responses in Epstein-Barr virus-induced gene 3-deficient mice. Proc Natl Acad Sci USA. 2002;99:16951–16956. doi: 10.1073/pnas.252648899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devergne O, Birkenbach M, Kieff E. Epstein-Barr virus-induced gene 3 and the p35 subunit of interleukin 12 form a novel heterodimeric hematopoietin. Proc Natl Acad Sci USA. 1997;94:12041–12046. doi: 10.1073/pnas.94.22.12041. 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuetze N, Schoeneberger S, Mueller U, Freudenberg MA, Alber G, Straubinger RK. IL-12 family members: differential kinetics of their TLR4-mediated induction by Salmonella enteritidis and the impact of IL-10 in bone marrow-derived macrophages. Int Immunol. 2005;17:649–659. doi: 10.1093/intimm/dxh247. [DOI] [PubMed] [Google Scholar]
- 17.Larousserie F, Pflanz S, Coulomb-L'Hermine A, Brousse N, Kastelein R, Devergne O. Expression of IL-27 in human Th1-associated granulomatous diseases. J Pathol. 2004;202:164–171. doi: 10.1002/path.1508. [DOI] [PubMed] [Google Scholar]
- 18.Gehlert T, Devergne O, Niedobitek G. Epstein-Barr virus (EBV) infection and expression of the interleukin-12 family member EBV-induced gene 3 (EBI3) in chronic inflammatory bowel disease. J Med Virol. 2004;73:432–438. doi: 10.1002/jmv.20109. [DOI] [PubMed] [Google Scholar]
- 19.Larousserie F, Bardel E, Coulomb L'Hermine A, Canioni D, Brousse N, Kastelein RA, Devergne O. Variable expression of Epstein-Barr virus-induced gene 3 during normal B-cell differentiation and among B-cell lymphomas. J Pathol. 2006;209:360–368. doi: 10.1002/path.1995. [DOI] [PubMed] [Google Scholar]
- 20.Holscher C. The power of combinatorial immunology: IL-12 and IL-12-related dimeric cytokines in infectious diseases. Med Microbiol Immunol. 2004;193:1–17. doi: 10.1007/s00430-003-0186-x. [DOI] [PubMed] [Google Scholar]
- 21.Larousserie F, Bardel E, Pflanz S, Arnulf B, Lome-Maldonado C, Hermine O, Bregeaud L, Perennec M, Brousse N, Kastelein R, Devergne O. Analysis of interleukin-27 (EBI3/p28) expression in Epstein-Barr virus- and human T-cell leukemia virus type 1-associated lymphomas: heterogeneous expression of EBI3 subunit by tumoral cells. Am J Pathol. 2005;166:1217–1228. doi: 10.1016/S0002-9440(10)62340-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Gran B, Zhang GX, Rostami A, Kamoun M. IL-27 subunits and its receptor (WSX-1) mRNAs are markedly up-regulated in inflammatory cells in the CNS during experimental autoimmune encephalomyelitis. J Neurol Sci. 2005;232:3–9. doi: 10.1016/j.jns.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Guan X, Ma X. Regulation of IL-27 p28 gene expression in macrophages through MyD88- and interferon-gamma-mediated pathways. J Exp Med. 2007;204:141–152. doi: 10.1084/jem.20061440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wirtz S, Tubbe I, Galle PR, Schild HJ, Birkenbach M, Blumberg RS, Neurath MF. Protection from lethal septic peritonitis by neutralizing the biological function of interleukin 27. J Exp Med. 2006;203:1875–1881. doi: 10.1084/jem.20060471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sprecher CA, Grant FJ, Baumgartner JW, Presnell SR, Schrader SK, Yamagiwa T, Whitmore TE, O'Hara PJ, Foster DF. Cloning and characterization of a novel class I cytokine receptor. Biochem Biophys Res Commun. 1998;246:82–90. doi: 10.1006/bbrc.1998.8576. [DOI] [PubMed] [Google Scholar]
- 26.Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- 27.Kishimoto T. Interleukin-6: from basic science to medicine–40 years in immunology. Annu Rev Immunol. 2005;23:1–21. doi: 10.1146/annurev.immunol.23.021704.115806. [DOI] [PubMed] [Google Scholar]
- 28.Rose-John S, Heinrich PC. Soluble receptors for cytokines and growth factors: generation and biological function. Biochem J. 1994;300(Pt 2):281–290. doi: 10.1042/bj3000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jostock T, Mullberg J, Ozbek S, Atreya R, Blinn G, Voltz N, Fischer M, Neurath MF, Rose-John S. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur J Biochem. 2001;268:160–167. doi: 10.1046/j.1432-1327.2001.01867.x. [DOI] [PubMed] [Google Scholar]
- 30.Scheller J, Schuster B, Holscher C, Yoshimoto T, Rose-John S. No inhibition of IL-27 signaling by soluble gp130. Biochem Biophys Res Commun. 2005;326:724–728. doi: 10.1016/j.bbrc.2004.11.098. [DOI] [PubMed] [Google Scholar]
- 31.Pflanz S, Hibbert L, Mattson J, Rosales R, Vaisberg E, Bazan JF, Phillips JH, McClanahan TK, de Waal Malefyt R, Kastelein RA. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol. 2004;172:2225–2231. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- 32.Larousserie F, Charlot P, Bardel E, Froger J, Kastelein RA, Devergne O. Differential effects of IL-27 on human B cell subsets. J Immunol. 2006;176:5890–5897. doi: 10.4049/jimmunol.176.10.5890. [DOI] [PubMed] [Google Scholar]
- 33.Ruckerl D, Hessmann M, Yoshimoto T, Ehlers S, Holscher C. Alternatively activated macrophages express the IL-27 receptor alpha chain WSX-1. Immunobiology. 2006;211:427–436. doi: 10.1016/j.imbio.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 34.Villarino AV, Larkin J, 3rd, Saris CJ, Caton AJ, Lucas S, Wong T, de Sauvage FJ, Hunter CA. Positive and negative regulation of the IL-27 receptor during lymphoid cell activation. J Immunol. 2005;174:7684–7691. doi: 10.4049/jimmunol.174.12.7684. [DOI] [PubMed] [Google Scholar]
- 35.Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev Immunol. 2005;5:521–531. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- 36.Takeda A, Hamano S, Yamanaka A, Hanada T, Ishibashi T, Mak TW, Yoshimura A, Yoshida H. Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol. 2003;170:4886–4890. doi: 10.4049/jimmunol.170.10.4886. [DOI] [PubMed] [Google Scholar]
- 37.Hibbert L, Pflanz S, De Waal Malefyt R, Kastelein RA. IL-27 and IFN-alpha signal via Stat1 and Stat3 and induce T-Bet and IL-12Rbeta2 in naive T cells. J Interferon Cytokine Res. 2003;23:513–522. doi: 10.1089/10799900360708632. [DOI] [PubMed] [Google Scholar]
- 38.Yoshimoto T, Okada K, Morishima N, Kamiya S, Owaki T, Asakawa M, Iwakura Y, Fukai F, Mizuguchi J. Induction of IgG2a class switching in B cells by IL-27. J Immunol. 2004;173:2479–2485. doi: 10.4049/jimmunol.173.4.2479. [DOI] [PubMed] [Google Scholar]
- 39.Lucas S, Ghilardi N, Li J, de Sauvage FJ. IL-27 regulates IL-12 responsiveness of naive CD4+ T cells through Stat1-dependent and -independent mechanisms. Proc Natl Acad Sci USA. 2003;100:15047–15052. doi: 10.1073/pnas.2536517100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawana K, Kawana Y, Schust DJ. Female steroid hormones use signal transducers and activators of transcription protein-mediated pathways to modulate the expression of T-bet in epithelial cells: a mechanism for local immune regulation in the human reproductive tract. Mol Endocrinol. 2005;19:2047–2059. doi: 10.1210/me.2004-0489. [DOI] [PubMed] [Google Scholar]
- 41.Karpuzoglu E, Phillips RA, Gogal RM, Jr, Ansar Ahmed S. IFN-gamma-inducing transcription factor, T-bet is upregulated by estrogen in murine splenocytes: role of IL-27 but not IL-12. Mol Immunol. 2007;44:1808–1814. doi: 10.1016/j.molimm.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morishima N, Owaki T, Asakawa M, Kamiya S, Mizuguchi J, Yoshimoto T. Augmentation of effector CD8+ T cell generation with enhanced granzyme B expression by IL-27. J Immunol. 2005;175:1686–1693. doi: 10.4049/jimmunol.175.3.1686. [DOI] [PubMed] [Google Scholar]
- 43.Goldberg R, Wildbaum G, Zohar Y, Maor G, Karin N. Suppression of ongoing adjuvant-induced arthritis by neutralizing the function of the p28 subunit of IL-27. J Immunol. 2004;173:1171–1178. doi: 10.4049/jimmunol.173.2.1171. [DOI] [PubMed] [Google Scholar]
- 44.Goldberg R, Zohar Y, Wildbaum G, Geron Y, Maor G, Karin N. Suppression of ongoing experimental autoimmune encephalomyelitis by neutralizing the function of the p28 subunit of IL-27. J Immunol. 2004;173:6465–6471. doi: 10.4049/jimmunol.173.10.6465. [DOI] [PubMed] [Google Scholar]
- 45.Pearl JE, Khader SA, Solache A, Gilmartin L, Ghilardi N, deSauvage F, Cooper AM. IL-27 signaling compromises control of bacterial growth in mycobacteria-infected mice. J Immunol. 2004;173:7490–7496. doi: 10.4049/jimmunol.173.12.7490. [DOI] [PubMed] [Google Scholar]
- 46.Bancroft AJ, Humphreys NE, Worthington JJ, Yoshida H, Grencis RK. WSX-1: a key role in induction of chronic intestinal nematode infection. J Immunol. 2004;172:7635–7641. doi: 10.4049/jimmunol.172.12.7635. [DOI] [PubMed] [Google Scholar]
- 47.Miyazaki Y, Inoue H, Matsumura M, Matsumoto K, Nakano T, Tsuda M, Hamano S, Yoshimura A, Yoshida H. Exacerbation of experimental allergic asthma by augmented Th2 responses in WSX-1-deficient mice. J Immunol. 2005;175:2401–2407. doi: 10.4049/jimmunol.175.4.2401. [DOI] [PubMed] [Google Scholar]
- 48.Chae SC, Li CS, Kim KM, Yang JY, Zhang Q, Lee YC, Yang YS, Chung HT. Identification of polymorphisms in human interleukin-27 and their association with asthma in a Korean population. J Hum Genet. 2007;52:355–361. doi: 10.1007/s10038-007-0123-8. [DOI] [PubMed] [Google Scholar]
- 49.Sonoda KH, Yoshimura T, Takeda A, Ishibashi T, Hamano S, Yoshida H. WSX-1 plays a significant role for the initiation of experimental autoimmune uveitis. Int Immunol. 2007;19:93–98. doi: 10.1093/intimm/dxl125. [DOI] [PubMed] [Google Scholar]
- 50.Rosas LE, Satoskar AA, Roth KM, Keiser TL, Barbi J, Hunter C, de Sauvage FJ, Satoskar AR. Interleukin-27R (WSX-1/T-cell cytokine receptor) gene-deficient mice display enhanced resistance to leishmania donovani infection but develop severe liver immunopathology. Am J Pathol. 2006;168:158–169. doi: 10.2353/ajpath.2006.050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McKenzie BS, Kastelein RA, Cua DJ. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 2006;27:17–23. doi: 10.1016/j.it.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 53.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 54.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 55.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]


