Abstract
The diagnosis of follicular dendritic cell (FDC) sarcoma can be challenging because of its morphologic overlaps with many other spindle cell neoplasms and, therefore, new phenotypic markers will be helpful in its differential diagnosis. Podoplanin is a mucin-type transmembrane glycoprotein that has recently been detected in reactive FDCs. In this study, we investigated the expression patterns of podoplanin using a new mouse monoclonal antibody D2-40, and compared them with CD21, a well-established FDC marker, in a comprehensive panel of cases. The panel included 4 FDC sarcomas, 38 spindle cell neoplasms of other types, 25 reactive lymphoid hyperplasia, and 117 lymphoid and 5 myeloid malignant hematopoietic neoplasms. Our study revealed that D2-40 strongly stained 3 of 4 FDC sarcomas. In contrast, D2-40 stained only 2/38 other spindle cell neoplasms tested. Furthermore, we observed that D2-40 highlighted more FDC meshworks than CD21 in Castleman's disease, follicular lymphoma, nodular lymphocyte predominance Hodgkin lymphoma, and residual reactive germinal centers in a variety of lymphoma types. D2-40 and CD21 stained an equal number of cases of reactive lymphoid hyperplasia, progressively transformed germinal centers and angioimmunoblastic T-cell lymphoma. No expression of podoplanin was detected in normal or neoplastic lymphoid and myeloid cells. We conclude that podoplanin (D2-40) is a sensitive and specific FDC marker, which is superior or equal to CD21 in evaluating both reactive and neoplastic FDCs. In addition, our results suggest that podoplanin (D2-40) can be used to support the diagnosis of FDC sarcoma.
Keywords: Podoplanin, D2-40, follicular dendritic cell, follicular dendritic cell sarcoma
Introduction
Follicular dendritic cells (FDCs) are stromal cells unique to primary and secondary lymphoid follicles. They play important immunologic roles in presenting antigens to B cells and in generating memory B cells and plasma cells. FDCs participate in the germinal center reaction by preventing apoptosis of germinal center B-cells and by stimulating cellular interactions and proliferation [1, 2]. However, the cellular origin of FDCs remains controversial. Some experts believe FDCs are derived from mesenchymal cells [3], while others consider them to be of hematopoietic lineage [4]. Recently, it has been suggested that FDCs are a specialized form of myofibroblast, which arises from bone marrow stromal progenitors [5]. FDCs present antigens mainly in association with CD21 and CD35, which are highly expressed on FDCs. Some other cell types can express these molecules as well, but at much lower levels [2].
FDC tumors/sarcomas are rare neoplasms of FDC lineage. Although most occur in lymph nodes, about one-third of them are located in extranodal sites [6]. Initial misdiagnosis of FDC sarcomas is common because of its morphological overlap with other spindle cell neoplasms [7], and one or more of the FDC makers CD21, CD23, CD35 and clusterin should be included in the immunohistochemical panel. Because FDC neoplasms may not express all FDC markers available, their diagnosis can be very challenging. Therefore, any additional new markers for FDCs should be helpful in improving diagnostic accuracy.
It has recently been suggested that podoplanin, a mucin-type transmembrane glycoprotein, is expressed on FDCs, and human podoplanin can be identified by D2-40, a new mouse monoclonal antibody [8–10]. D2-40 was originally raised against an oncofetal antigen, M2A antigen, which is an O-linked sialoglycoprotein with a simple mucin-type carbohydrate epitope associated with germ cell neoplasms [10]. Recent studies have demonstrated that podoplanin, M2A antigen, and type I alveolar cell marker hT1α-2 are identical proteins [10]. Therefore, we evaluated the utility of D2-40 as a possible immunohistochemical marker for FDC lineage determination and diagnosis of FDC sarcomas.
Materials and Methods
Case Selection
We retrospectively studied 194 benign and malignant biopsy tissue specimens from Albert Einstein College of Medicine/Montefiore Medical Center, Bronx, NY; University of Nebraska Medical Center, Omaha, NE; University of Wisconsin Hospital and Clinics, Madison, WI; and Creighton University Medical Center, Omaha, NE. The diagnoses were FDC sarcoma (N=4), malignant fibrous histiocytoma (N=13), leiomyosarcoma (N=10), liposarcoma (N=6), synovial sarcoma (N=4), fibrosarcoma (N=2), rhabdomyosarcoma (N=1), malignant nerve sheath tumor (N=1), dermatofibrosarcoma protuberans (N=1), reactive lymphoid hyperplasia (RLH) (N=25), progressively transformed germinal centers (PTGCs) (N=3), Castleman's disease (CDs) (N=3), follicular lymphoma (FL) (N=22), chronic lymphocytic leukemia/small lymphocytic lymphoma (N=16), diffuse large B-cell lymphoma (N=11), Burkitt lymphoma (N=5), mantle cell lymphoma (N=4), marginal zone B-cell lymphoma (N=6), plasmacytoma (N=2), classical Hodgkin lymphoma (N=22), nodular lymphocyte predominant Hodgkin lymphoma (NLPHL) (N=11), peripheral T-cell lymphoma (N=8), angioimmunoblastic T-cell lymphoma (AITL) (N=4), anaplastic large cell lymphoma (N=1), precursor B-cell lymphoblastic lymphoma (N=4), precursor T-cell lymphoblastic lymphoma (N=1), and acute myeloid leukemia (N=4). The Institutional Review Boards from each institution approved the use of the material for research.
Tissue Microarrays
Tissue microarrays (TMA) were constructed using a manual tissue arrayer (Beecher Instruments, Silver Spring, MD). Hematoxylin and eosin-stained sections from each block were used to define diagnostic areas. 2-3 representative 1-mm cores were obtained from formalin-fixed, paraffin-embedded tissue blocks.
Immunohistochemical Studies
All tissues were routinely fixed in 10% buffered formalin and embedded in paraffin. The paraffin sections were cut at 5 µm thickness and placed on positively charged slides, which were baked in a 60°C oven for one hour. Tissue sections were deparaffinized and rehydrated through a series of xylene and graded alcohols. Endogenous peroxidase was blocked in 3% H2O2 for 10 minutes. Antigen retrieval was performed by placing the slides in an Oyster vegetable steamer in Dako Target Retrieval Solution (Dako Cytomation, Carpinteria, CA). The pH of the Target Retrieval Solution for D2-40 was 8.0 (EDTA), and for CD21 the pH was 6.0. The IHC staining procedure was performed in an automatic slide stainer (Dako LV-1 Autostainer, Carpinteria, CA) using the Dako universal staining system. The primary mouse monoclonal antibodies D2-40 (Signet Laboratories, Dedham, MA) and CD21 (Dako Cytomation, Carpinteria, CA) were applied in a dilution of 1:50 for 30 minutes at room temperature. Following this, a secondary antibody, Dako Cytomation Envision+ system-HRP Labeled Polymer, anti–mouse IgG (Dako Cytomation, Carpinteria, CA), was applied for 30 minutes. UltraMarque DAB Substrate kit (Cell Marque, Hot Springs, AR) was used with 3,3’-diaminobenzidine as chromogen. Slides were counterstained with Surgipath Hematoxylin (Richmond, IL), dehydrated through graded alcohols, cleared in xylene, and coverslipped with cytoseal 60 (Richard- Allen Scientific, Kalamazoo, MI). Appropriate positive and negative controls were run concurrently for all antibodies tested. Immunohistochemical staining results were evaluated independently by two pathologists. Positive staining of reactive FDCs by D2-40 and CD21 was defined as membrane staining in a dendritic pattern. Positive staining by D2-40 and CD21 of spindle cell neoplasms was defined as membrane staining in more than 10% of neoplastic cells.
Results
Comparison of D2-40 and CD21 Immunohistochemical Staining in Reactive and Neoplastic Hematopoietic Disorders
The results of immunohistochemical staining in reactive and neoplastic hematopoietic disorders are summarized in Table 1. Both D2-40 and CD21 highlighted FDC network of germinal centers in the majority of RLH cases (22/25 for D2-40, 23/25 for CD21). D2-40 stained the FDCs in all cases of PTGCs (3/3), CD (3/3), FL (22/22), and NLPHL (11/11) (Figure 1). CD21 stained FDCs in 3/3 (100%) PTGCs, 2/3 (67%) CDs, 18/22 (82%) FLs and 7/11 (64%) NLPHLs. Although D2-40 and CD21 equally stained FDC meshworks in most cases (Figure 1), there were many cases in which D2-40 highlighted FDC meshwork better than CD21 (Figures 1 and 2). D2-40 reliably highlighted FDC meshwork when CD21 failed to stain the FDCs in 1/3 CDs, 4/22 FLs, and 4/11 NLPHLs (see examples in Figures 1 and 2). In addition, the lymphatic endothelial cells were strongly stained with D2-40 (Figures 1, 2 and 3). Interdigitating dendritic cells (IDC), which were identified by positive staining for S-100 protein, were negative for D2-40 (Figure 3). No D2-40 positivity was detected in the neoplastic cells in any of 122 cases of malignant hematopoietic disorders (Table 1 and Figure 4).
Table 1.
Comparison of D2-40 and CD21 immunohistochemical staining in reactive lymph nodes and malignant lymphomas
| Total number | Reactive FDCs | Reactive Lymphocytes/Lymphoma Cells | |||
|---|---|---|---|---|---|
| D2-40 | CD21 | D2-40 | CD21 | ||
| RLH | 25 | 22 (88%) | 23 (92%) | 0 (0%) | 0 (0%) |
| PTGC | 3 | 3 (100%) | 3 (100% | 0 (0%) | 0 (0%) |
| CD | 3 | 3 (100%) | 2 (67%) | 0 (0%) | 0 (0%) |
| FL | 22 | 22 (100%) | 18 (82%) | 0 (0%) | 0 (0%) |
| NLPHL | 11 | 11 (100%) | 7 (64%) | 0 (0%) | 0 (0%) |
| AITL | 4 | 3 (75%) | 3 (75%) | 0 (0%) | 0 (0%) |
| OL | 80 | 11(14%) | 8 (10%) | 0 (0%) | 0 (0%) |
RLH: reactive lymphoid hyperplasia; PTGC: progressively transformed germinal center; CD: Castleman's disease; FL: follicular lymphoma; NLPHL: nodular lymphocyte predominant Hodgkin lymphoma; AITL: angioimmunoblastic T-cell lymphoma; OL: other lymphomas.
Figure 1.
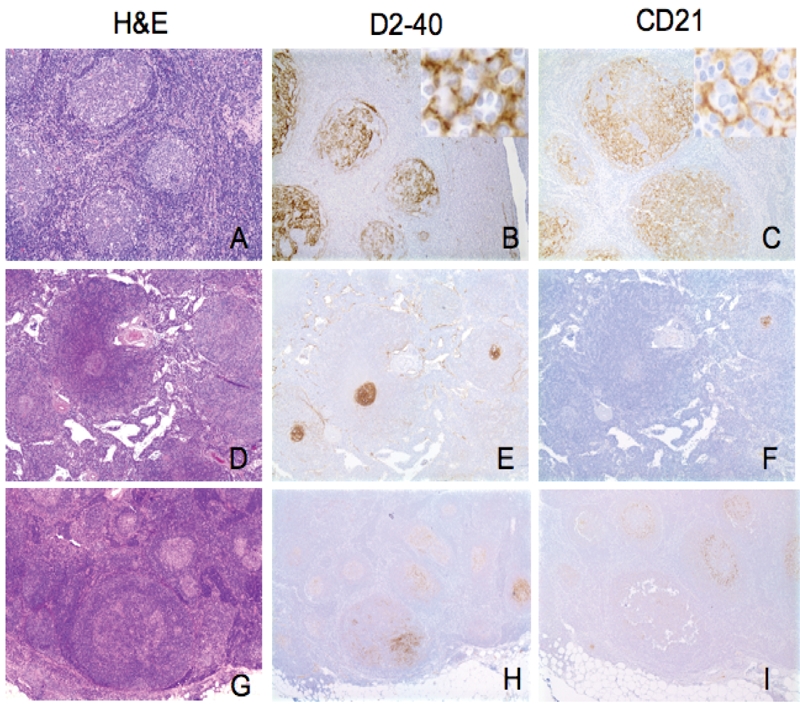
D2-40 immunohistochemical staining pattern in reactive lymph nodes. A-C. Reactive lymphoid hyperplasia (RLH): Several reactive follicles with hyperplastic germinal centers and well-defined mantle zones (H&E)(A). Strong D2-40 immunostaining identifies the follicular dendritic cell (FDC) meshworks in germinal centers (GCs)(B). Identical immunostaining pattern is seen for CD21 (C). D-F. Castleman's disease, hyaline vascular type: H&E staining reveals three atrophic GCs surrounded by onion skin mantle zones (D). D2-40 highlights FDC meshworks in all three atrophic GCs (E). CD21 is weakly positive in only one of the three atrophic GCs (F). G-I. progressively transformed germinal centers: H&E staining reveals an expanded follicle with indistinct GC/mantle zone borders in the lower center (G). Strong immunostaining for D2-40 reveals extensive FDC meshworks in expanded follicle (H). In contrast, CD21 is much weaker in the same expanded follicle as seen in H (I). (Original magnification: A-C ×100; insert, ×400; D-I, ×40).
Figure 2.
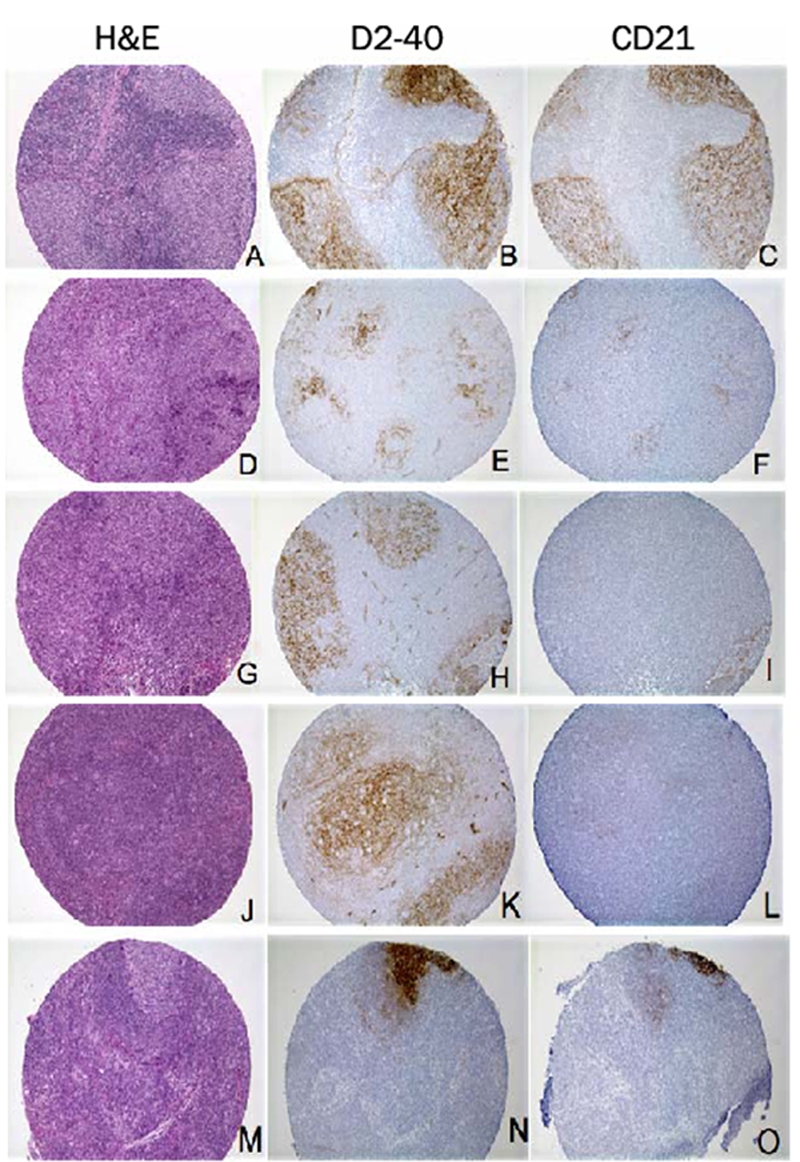
D2-40 immunohistochemical staining pattern in lymphomas. A-I. Follicular lymphoma: H&E staining reveals tightly apposed follicles (A, D and G). D2-40 (B) and CD21 (C) show identical staining patterns and intensity of the FDC meshworks. D2-40 (E) immunostaining is much stronger in FDC meshworks than CD21 (F). D2-40 (H) is positive in this disrupted FDC meshwork, while CD21 (I) fails to highlight the same FDC meshwork. J-L. Nodular lymphocyte predominant Hodgkin lymphoma: H&E staining reveals markedly expanded follicle (J). D2-40 (K) immunostaining is much stronger in the markedly expanded FDC meshworks than CD21 (L). M-O. Diffuse large B-cell lymphoma (DLBCL): H&E staining reveals diffuse area with focal residual germinal center (M). D2-40 (N) immunostaining is much stronger in residual FDC meshwork than CD21 (O). (Original magnification: A-O, ×100)
Figure 3.
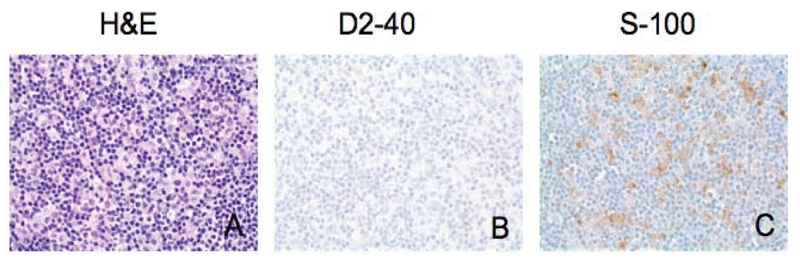
D2-40 immunohistochemical staining in interdigitating dendritic cells (IDC). H&E staining of dermatopathic lymphadenopathy reveals expanded paracortical region with a mixture of small lymphocytes, IDCs, and histiocytes (A). IDCs are positive for S-100 (C), but negative for D2-40 (B). (Original magnification × 400)
Figure 4.
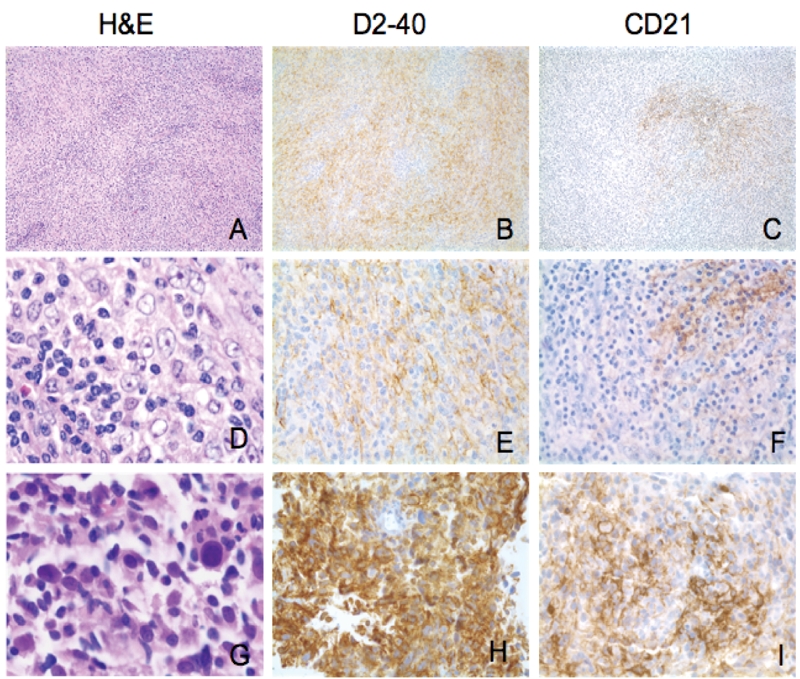
Morphologic and immunophenotypic features of FDC sarcomas. A-F. Case No. 1: A and D show classic morphology of FDC sarcoma/tumor. Individual tumor cells have ovoid nuclei and vesicular chromatin. They tend to grow as fascicles in a whirling pattern. Note occasional multinucleated cells (D). D2-40 is strongly and diffusely positive in tumor cells (B, E). CD21 show focal weak positivity (C, F). G-I. Case No. 2: Marked nuclear pleomorphism and high mitotic count are seen in this case. D2-40 (H) and CD21 (I) are strongly and diffusely positive in tumor cells. D2-40 and CD21 reveal a vague whirling pattern (Original magnification: A-C, ×100; D-I, ×400).
Clinicopathological Features of FDC Sarcomas
Four patients diagnosed with FDC sarcoma were studied including three males and one female. None of the cases were previously reported. The ages ranged from 58 to 63 years. Tumors were located in lymph node (2 cases); right thigh soft tissue (1 case); or multi-focal in lymph node, spleen, and liver (1 case) (Table 2).
Table 2.
Clinical features and immunophenotypic profiles of follicular dendritic cell sarcomas
| Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|
| Age (yr) | 59 | 60 | 58 | 63 |
| Sex | F | M | M | M |
| Anatomic Site | Soft tissue, thigh | LN, spleen, liver | LN, neck | LN, supraclavicular |
| D2-40 | + | + | + (Focal) | − |
| CD21 | + (Focal) | + (Focal) | − | − |
| CD23 | + (Focal) | − | + | − |
| CD35 | ND | + | ND | ND |
| Clusterin | + | + | − | + |
| CD3 | − | − | − | − |
| CD20 | − | − | − | − |
| CD30 | − | ND | − | − |
| CD68 | − | ND | − | − |
| S-100 | − | + (Focal) | − | − |
| EMA | + (Focal) | ND | + (Focal) | − |
| AE1/3 | − | ND | − | − |
| Vimentin | + | ND | + (Focal) | ND |
+, positive; + (Focal), focal positive; −, negative; ND, not done; LN, lymph node
Histologically, FDC sarcomas from the four patients revealed two major growth patterns. The first pattern (cases 2, 3, 4; Figures 4A and 4D) consisted of tumor cells growing in sheets and fascicles, with focal storiform and whirling areas, which were intermixed with many small lymphocytes. The tumor cells were oval to spindle in shape, with oval or elongated vesicular nuclei, inconspicuous to small eosinophilic nucleoli, and eosinophilic cytoplasm with indistinct cytoplasmic borders. Nuclear pleomorphism was minimal. Occasionally, binucleated and multinucleated cells were present. In case 2 (Figure 4A), perivascular lymphocytic cuffing was prominent. The second pattern was seen in case 1. The tumor cells were more pleomorphic with focal necrosis and many mitotic figures. There were few lymphocytes (Figure 4G).
The results of immunostaining with a panel of Antibodies including D2-40, CD21, CD23, CD35, clusterin, CD45, CD3, CD20, CD30, S-100, CD1a, CD68, AE1/AE3 and EMA are summarized in Table 2. Three of four FDC sarcomas stained positively for D2-40. Two of four FDC sarcomas focally expressed CD21 and CD23. One case examined expressed CD35. Three of four cases expressed clusterin. D2-40 highlighted the delicate, interconnecting, cell processes of the tumor cells, which formed whorls and complex networks, consistent with their FDC lineage (Figure 4B).
D2-40 Immunohistochemical Staining in Spindle Cell Soft Tissue Sarcoma/Neoplasm
Podoplanin expression was studied in a variety of other types of spindle cell soft tissue sarcomas (Table 3). Among 38 spindle cell soft tissue sarcomas other than FDC sarcoma, strong membrane staining was seen only in two cases (5.3%): 1/13 (7.7%) of malignant fibrous histiocytomas and 1/10 (10%) of leiomyosarcomas. Intracytoplasmic staining, which is nonspecific, was detected in one case each of malignant fibrous histiocytoma, liposarcoma and rhabdomyosarcoma.
Table 3.
D2-40 expression in spindle cell sarcomas
| Diagnosis | No. of tumors studied | No. of D2-40 positive cases (%) |
|---|---|---|
| MFH | 13 | 1 (7.7) |
| Leiomyosarcoma | 10 | 1 (10) |
| Liposarcoma | 6 | 0 |
| Synovial sarcoma | 4 | 0 |
| Fibrosarcoma | 2 | 0 |
| Rhabdomyosarcoma | 1 | 0 |
| DFSP | 1 | 0 |
| MPNST | 1 | 0 |
| Total | 38 | 2 (5.3) |
MFH: malignant fibrous histiocytoma; DFSP: dermatofibrosarcoma protuberans; MPNST: malignant peripheral nerve sheath tumor. If more than 10% of neoplastic spindle cells expressed podoplanin (D2-40), cases were scored as positive.
Discussion
Because CD21 has been considered one of the most reliable FDC markers [11, 12], pathologists have routinely used CD21 immunohistochemical stain on paraffin-embedded tissue sections to highlight the atrophic, expanded, or disrupted FDC meshworks in benign and neoplastic conditions, such as CDs, PTCGs, FLs, NLPHLs, and AITLs. In our study, however, we found that CD21 immunostaining occasionally failed to stain FDCs. Therefore, when searching for FDC differentiation in a diagnostic work-up, it is prudent to use more than one FDC markers.
We confirmed that podoplanin, as detected by the D2-40 monoclonal antibody, is expressed in FDCs. In fact, D2-40 detected FDCs more often than CD21 in almost all the reactive conditions. The biological function of podoplanin is poorly understood. In vitro studies indicated that podoplanin is involved in mediating cell motility by promoting rearrangement of the actin cytoskeleton [13]. Further studies are needed to determine whether podoplanin has a similar function in FDCs. Strong podoplanin expression has been detected in myoepithelial cells of the breast and salivary glands, as well as in myofibroblasts of the prostate [8]. Therefore, positive staining of FDCs by D2-40 may help support the hypothesis that FDCs are derived from stromal myofibroblasts.
FDC sarcomas are rare neoplasms that were first reported by Monda and their associates in 1986 [14]. They have been increasingly recognized to occur at a variety of anatomic sites. The morphology of our cases reported here was typical for FDC sarcoma. Two cases were located in lymph nodes, one in extranodal soft tissue, and one with multi-focal involvement in lymph node, liver and spleen. The hepatic and splenic involvement in case 2 could represent metastases from the lymph node. Because of the histologic overlap, either a panel of immunohistochemical stains and/or electron microscopy has been required to distinguish FDC sarcoma from other low-grade, spindle cell neoplasms. Most FDC sarcoma/tumor cases are positive for one or more FDC-associated antigens, such as CD21, CD23, or CD35 [11, 15–17]. However, many cases showed only focal and/or weak staining for these markers [17, 18].
CD21 has been considered the most reliable FDC marker for diagnosing FDC sarcoma [11, 12]. In our study, however, only 2 of the 4 FDC sarcomas expressed CD21, and in both cases, the staining was focal. Clusterin, a relatively new FDC marker, has shown excellent sensitivity for FDC sarcomas [19, 20]. One immunohistochemical study reported that 12/12 FDC sarcomas/tumors expressed clusterin [19]. In our study, 3/4 FDC sarcomas expressed clusterin. D2-40 was also positive in 3/4 FDC sarcomas. In our limited experience in diagnosing FDC sarcomas, D2-40 appears to be more sensitive than CD21 or CD23, and at least as sensitive as clusterin. Since only one out of four FDC sarcomas was tested for CD35, we cannot compare the sensitivity of these antibodies.
Although D2-40 appears to be highly sensitive for the diagnosis of FDC sarcoma, it is well known that D2-40 also stains several other types of spindle cell sarcomas, such as angiosarcoma and Kaposi's sarcoma [21, 22]. However, the unique morphology of these tumors should readily separate them from FDC sarcoma/tumor.
To our knowledge, we are the first to demonstrate that D2-40 could be useful in confirming the diagnosis of FDC sarcoma. Further studies with more cases are needed to confirm our findings. In addition, we have also shown that D2-40 is superior or equal to CD21 for evaluating atrophic, expanded, or disrupted reactive FDC meshworks in a variety of lymphocytic disorders, such as FL, PTGC, CD, and NLPHL. In conclusion, D2-40 is a new marker that appears to be both sensitive and specific for reactive and neoplastic FDCs.
Note Added
During the publication process, a paper was published by Yu et al [23], who analyzed D2-40 in follicular dendritic cell tumors.
References
- 1.Park CS, Choi YS. How do follicular dendritic cells interact intimately with B cells in the germinal centre? Immunology. 2005;114:2–10. doi: 10.1111/j.1365-2567.2004.02075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Nierop K, de Groot C. Human follicular dendritic cells: Function, origin and development. Semin Immunol. 2002;14:251–257. doi: 10.1016/s1044-5323(02)00057-x. [DOI] [PubMed] [Google Scholar]
- 3.Lindhout E, de Groot C. Follicular dendritic cells and apoptosis: Life and death in the germinal centre. Histochem J. 1995;27:167–183. [PubMed] [Google Scholar]
- 4.Kapasi ZF, Qin D, Kerr WG, Kosco Vilbois MH, Shultz LD, Tew JG, Szakal AK. Follicular dendritic cell (FDC) precursors in primary lymphoid tissues. J Immunol. 1998;160:1078–1084. [PubMed] [Google Scholar]
- 5.Munoz Fernandez R, Blanco FJ, Frecha C, Martin F, Kimatrai M, Abadia Molina AC, Garcia Pacheco JM, Olivares EG. Follicular dendritic cells are related to bone marrow stromal cell progenitors and to myofibroblasts. J Immunol. 2006;177:280–289. doi: 10.4049/jimmunol.177.1.280. [DOI] [PubMed] [Google Scholar]
- 6.Biddle DA, Ro JY, Yoon GS, Yong YW, Ayala AG, Ordonez NG, Ro J. Extranodal follicular dendritic cell sarcoma of the head and neck region: Three new cases, with a review of the literature. Mod Pathol. 2002;15:50–58. doi: 10.1038/modpathol.3880489. [DOI] [PubMed] [Google Scholar]
- 7.Shia J, Chen W, Tang LH, Carlson DL, Qin J, Guillem JG, Nobrega J, Wong WD, Klimstra DS. Extranodal follicular dendritic cell sarcoma: Clinical, pathologic, and histogenetic characteristics of an underrecognized disease entity. Virchows Arch. 2006;449:148–158. doi: 10.1007/s00428-006-0231-4. [DOI] [PubMed] [Google Scholar]
- 8.Schacht V, Dadras SS, Johnson LA, Jackson DG, Hong YK, Detmar M. Up-regulation of the lymphatic marker podoplanin, a mucin-type transmembrane glycoprotein, in human squamous cell carcinomas and germ cell tumors. Am J Pathol. 2005;166:913–921. doi: 10.1016/S0002-9440(10)62311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ordonez NG. Podoplanin: A novel diagnostic immunohistochemical marker. Adv Anat Pathol. 2006;13:83–88. doi: 10.1097/01.pap.0000213007.48479.94. [DOI] [PubMed] [Google Scholar]
- 10.Marks A, Sutherland DR, Bailey D, Iglesias J, Law J, Lei M, Yeger H, Banerjee D, Baumal R. Characterization and distribution of an oncofetal antigen (M2A antigen) expressed on testicular germ cell tumours. Br J Cancer. 1999;80:569–578. doi: 10.1038/sj.bjc.6690393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perez Ordonez B, Erlandson RA, Rosai J. Follicular dendritic cell tumor: Report of 13 additional cases of a distinctive entity. Am J Surg Pathol. 1996;20:944–955. doi: 10.1097/00000478-199608000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Perez Ordonez B, Rosai J. Follicular dendritic cell tumor: Review of the entity. Semin Diagn Pathol. 1998;15:144–154. [PubMed] [Google Scholar]
- 13.Schacht V, Ramirez MI, Hong YK, Hirakawa S, Feng D, Harvey N, Williams M, Dvorak AM, Dvorak HF, Oliver G, Detmar M. T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J. 2003;22:3546–3556. doi: 10.1093/emboj/cdg342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monda L, Warnke R, Rosai J. A primary lymph node malignancy with features suggestive of dendritic reticulum cell differentiation. A report of 4 cases. Am J Pathol. 1986;122:562–572. [PMC free article] [PubMed] [Google Scholar]
- 15.Pileri SA, Grogan TM, Harris NL, Banks P, Campo E, Chan JK, Favera RD, Delsol G, De Wolf Peeters C, Falini B, Gascoyne RD, Gaulard P, Gatter KC, Isaacson PG, Jaffe ES, Kluin P, Knowles DM, Mason DY, Mori S, Muller Hermelink HK, Piris MA, Ralfkiaer E, Stein H, Su IJ, Warnke RA, Weiss LM. Tumours of histiocytes and accessory dendritic cells: An immunohistochemical approach to classification from the international lymphoma study group based on 61 cases. Histopathology. 2002;41:1–29. doi: 10.1046/j.1365-2559.2002.01418.x. [DOI] [PubMed] [Google Scholar]
- 16.Andriko JW, Kaldjian EP, Tsokos M, Abbondanzo SL, Jaffe ES. Reticulum cell neoplasms of lymph nodes: A clinicopathologic study of 11 cases with recognition of a new subtype derived from fibroblastic reticular cells. Am J Surg Pathol. 1998;22:1048–1058. doi: 10.1097/00000478-199809000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Cheuk W, Chan JK, Shek TW, Chang JH, Tsou MH, Yuen NW, Ng WF, Chan AC, Prat J. Inflammatory pseudotumor-like follicular dendritic cell tumor: A distinctive low-grade malignant intra-abdominal neoplasm with consistent Epstein-Barr virus association. Am J Surg Pathol. 2001;25:721–731. doi: 10.1097/00000478-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Chan JK, Fletcher CD, Nayler SJ, Cooper K. Follicular dendritic cell sarcoma. Clinicopathologic analysis of 17 cases suggesting a malignant potential higher than currently recognized. Cancer. 1997;79:294–313. [PubMed] [Google Scholar]
- 19.Grogg KL, Lae ME, Kurtin PJ, Macon WR. Clusterin expression distinguishes follicular dendritic cell tumors from other dendritic cell neoplasms: Report of a novel follicular dendritic cell marker and clinicopathologic data on 12 additional follicular dendritic cell tumors and 6 additional interdigitating dendritic cell tumors. Am J Surg Pathol. 2004;28:988–998. doi: 10.1097/01.pas.0000112536.76973.7f. [DOI] [PubMed] [Google Scholar]
- 20.Grogg KL, Macon WR, Kurtin PJ, Nascimento AG. A survey of clusterin and fascin expression in sarcomas and spindle cell neoplasms: Strong clusterin immunostaining is highly specific for follicular dendritic cell tumor. Mod Pathol. 2005;18:260–266. doi: 10.1038/modpathol.3800294. [DOI] [PubMed] [Google Scholar]
- 21.Breiteneder Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, Diem K, Weninger W, Tschachler E, Alitalo K, Kerjaschki D. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: Podoplanin as a specific marker for lymphatic endothelium. Am J Pathol. 1999;154:385–394. doi: 10.1016/S0002-9440(10)65285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kahn HJ, Bailey D, Marks A. Monoclonal antibody D2-40, a new marker of lymphatic endothelium, reacts with kaposi's sarcoma and a subset of angiosarcomas. Mod Pathol. 2002;15:434–440. doi: 10.1038/modpathol.3880543. [DOI] [PubMed] [Google Scholar]
- 23.Yu H, Gibson JA, Pinkus GS, Hornick JL. Podoplanin (D2-40) is a novel marker for follicular dentritic cell tumors. Am J Clin Pathol. 2007;128:776–782. doi: 10.1309/7P8U659JBJCV6EEU. [DOI] [PubMed] [Google Scholar]


