Abstract
The existence of adult renal stem cells has long been suspected because the kidney is capable of regeneration in response to injury, such as acute tubular necrosis (ATN), but their location, or niche, has not been fully defined yet. The aim of this study was to identify the niche of adult renal stem cells responsible for the tubular regeneration. The location of label-retaining cells (LRCs) was studied in adult mouse kidneys after administration of a pulse of bromodeoxyuridine (BrdU) during embryonic period. To study regional participation in renal tubular regeneration, the expression of the proliferation marker Ki-67 was examined after induction of unilateral ATN in mouse kidneys. Regional colony-forming capacity was examined using cultured cells derived from normal mouse and human kidneys and their multipotency was examined in human kidneys. LRCs in adult mouse kidneys were mostly tubular epithelial cells and concentrated constantly in the outer stripe of the corticomedullary junction (CMJ). In the ATN model, Ki-67 positive cells were concentrated in the tubular epithelial cells of the outer stripe, not only in the ATN kidneys but also in the contralateral non-ATN kidneys. High colony-forming capacity was noted in the CMJ of mouse and human kidneys. Cultured cells derived from a single human CMJ cell revealed multipotency, differentiating not only into tubular cells but also into glomerular podocytes. These results demonstrate that the CMJ of the kidney contains label-retaining, renal-repairing, highly colony-forming multipotent stem cell-like tubular cells, suggesting the CMJ as the niche of adult renal stem cells.
Keywords: Adult stem cells, kidney, niche, acute renal failure
Introduction
Adult stem cells, the stem cells residing in adult tissue, possess prolonged self-renewal capacity and multipotency, and produce various specialized mature tissue cell types. They play a major role in maintaining homeostasis and repairing damaged adult tissue. Organ-specific adult stem cells usually reside in a special location, or niche, which is characterized by well vascularized stroma and good physical protection. Adult stem cells in the niche own high proliferative potential and proliferate actively in response to regenerative stimuli although they are slow-cycling under normal condition [1].
The kidney has long been speculated to have adult stem cells because it can undergo rapid cell division in response to regenerative stimuli, such as acute tubular necrosis (ATN). These renal-repairing cells are thought to be derived from extrarenal bone marrow stem cells, intrarenal adult stem cells, and/or dedifferentiated tubular cells. Recent evidence suggests that bone marrow-derived cells may not make a significant contribution to the repair of tubular cells, but rather mainly constitute inflammatory cells in the interstitium [2]. Instead, resident cells in the kidney may be the major source for renal regeneration, suggesting the existence of intrarenal adult stem cells [3–5].
The location of adult renal stem cells, however, has not been clearly defined yet although the renal papilla has been proposed [6]. The corticomedullary junction (CMJ) and cortex may also contain adult renal stem cells, as shown by the presence of cells bearing the stem cell specific transcription factor Oct4 in the CMJ, cells bearing the stem cell marker CD133 in the cortex, and CD24/CD133 coexpressing cells in the glomerular parietal epithelium of the cortex [7–9].
In an attempt to clarify the niche of adult renal stem cells, we examined the location of label-retaining cells (LRCs) in adult mouse kidneys to study the slow cycling property, the location of proliferating cells in an ATN model and regional colony-forming capacity to study the high proliferative potential, and multilineage differentiation of cultured renal cells to study multipotency. Our findings show that stem cell-like tubular cells reside in the CMJ of the kidney, suggesting the CMJ as the niche of adult renal stem cells.
Materials and Methods
Mouse and Human Kidneys
CD1 (ICR) mice were supplied by Orient (Sungnam, Republic of Korea). The normal portion of human kidneys was obtained from surgically removed kidneys of four patients (aged 32, 41, 53 and 56 years). The animal study was approved by the Institutional Animal Care and Use Committee of Asan Institute for Life Sciences, Asan Medical Center, Seoul, Republic of Korea, which abides by the Institute of Laboratory Animal Resources guide. The human study was approved by Asan Medical Center Institutional Review Board.
BrdU Labeling of Mice
BrdU (Sigma-Aldrich, St Louis, MO) was diluted in phosphate-buffered saline (PBS) and injected subcutaneously at a dose of 50 µg/g into the posterior neck of pregnant CD1 mice, twice daily for 3 days, beginning on embryonic day (E) 11, or E17. The kidneys were harvested at 2 months and 6 months. At least 3 mice were used at each time point. PBS-injected mice were used as negative controls.
Histology and Immunohistochemistry
Kidney specimens were fixed in 10% buffered formalin, embedded in paraffin, and cut into 4 μm thick sections. Immunohistochemical staining was performed using anti-BrdU (Amersham, Piscataway, NJ) and anti-Ki-67 (Neomarkers, Fremont, CA) antibodies with a universal secondary antibody kit (iView™DAB detection kit, Ventana Medical Systems, Inc. Montmorency, Australia). Diaminobenzidine was used as a chromogen and the tissues were counterstained with hematoxylin or Periodic acid-Schiff (PAS) staining. The numbers of LRCs in ATN and age-matched normal kidneys were counted in 8 consecutive CMJ areas adjacent to arcuate vessels at × 400 magnification. The number of 8 was the least number of arcuate vessels in the largest coronal section of the kidneys.
Transient Ischemic Acute Tubular Necrosis
BrdU- or PBS-injected 2-month-old CD1 mice were anesthetized by intraperitoneal injection of 80 mg/g ketamine and 16 mg/g xylazine. The left renal pedicle was clamped for 45 minutes to induce unilateral transient ischemic ATN and then the mice were returned to the cages. Two days later, both right and left kidneys were harvested. The non-clamped right kidneys were used as controls. The experiment was repeated two times and at least 4 mice were used at each experiment.
Cell Culture
The kidneys of four 2-month-old CD1 mice and adult human were grossly dissected into three portions (cortex, CMJ including medulla, and renal papilla) and four portions (cortex, CMJ, medulla, and renal papilla), respectively. Cells were cultured using a previously described procedure with modifications [6]. Briefly, the dissected tissues were placed in HBSS containing penicillin/streptomycin on wet ice, minced, and washed. Each sample was digested with 2 mg/mL collagenase I (Worthington Biochemical Corp., Lakewood, NJ) while being shaken at 175 rpm in a 37°C shaker for 1 hour. The samples were filtered through 20µM nylon mesh (Spectrum, Gardena, CA) to exclude glomeruli and prepare single dispersed tubular epithelial cells. The dispersed cells were plated on plastic culture dishes, containing DMEM/Ham F12 medium, penicillin/streptomycin, and 10% fetal bovine serum and maintained at 37°C with 5% CO2. The dispersed cells were plated on 60 mm plastic culture dishes at a density of 1 × 105 cells for the study of colony-forming activity. For the study of multilineage differentiation, cells were plated as single cells on 96 well plates and then cells of one colony were subcultured.
Immunocytochemistry
Cells were fixed in methanol for 20 minutes and treated with 5% skim milk for 1 hour. The cells were incubated with primary antibodies overnight at 4°C and then with FITC- or TRITC-conjugated secondary antibodies for 1 hour at room temperature. The following primary antibodies were used: anti-aquaporin-5 (Santa Cruz Biotechnology, Santa Cruz, CA), anti- cytokeratin-multi (Neomarkers), and anti-podocin (Sigma-Aldrich). After mounting with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI)-containing mounting media, fluorescent signals were detected with a fluorescence microscope (Olympus, Center Valley, PA).
Statistics
Data was reported as mean ± standard deviation of triplicate determinations. Statistical comparisons were based on one-way analysis of variance. A probability value of less than 0.05 was considered statistically significant.
Results
The Distribution Patterns of LRCs in Adult Kidneys
To label the DNA of prospective adult renal stem cells, we injected a pulse of the thymidine analogue BrdU into mouse embryos during metanephric nephrogenesis from E17 to E19. As differentiating cells undergo multiple cell divisions during renal development and growth, their BrdU is diluted and undetectable in the adult. In contrast, since adult stem cells are slow cycling, they retain BrdU for a long time and present as LRCs in the adult. After the BrdU administration during embryonic periods, we waited up to 6 months and then studied the location of LRCs in mouse kidneys.
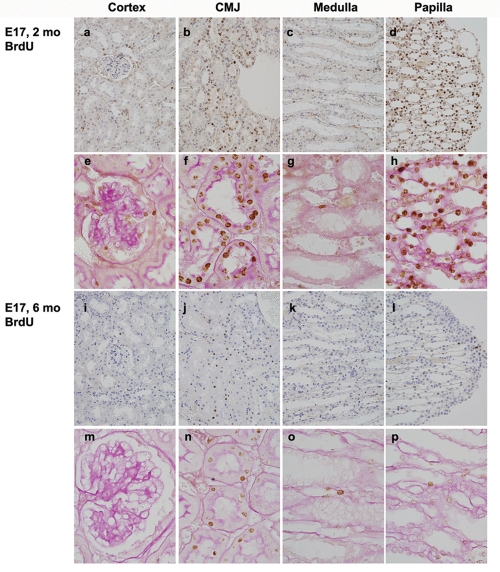
The kidneys of 2-month-old mouse littermates revealed a concentration of LRCs in the outer stripe of the CMJ and the renal papilla (Figures 1a, f, d and h). Most of the LRCs in the CMJ were tubular cells, among which most showed abundant cytoplasm with prominent PAS-positive brush borders and the others with inconspicuous brush borders. In contrast, most cells in the renal papilla including epithelial cells, endothelial cells, and interstitial cells were present as LRCs. LRCs were also scattered in the interstitium, glomerular mesangium and visceral and parietal epithelia of Bowman's capsule, and tubules of the cortex and medulla (Figures 1a, c, e and g).
Figure 1.
Distribution patterns of LRCs in adult mouse kidneys. After injection of BrdU for 3 days beginning on E17, the location of BrdU-positive LRCs was examined immunohistochemically in 2-month-old (a-h) and 6-month-old (i-p) mouse kidneys. The second (e-h) and fourth (m-p) rows are counterstained by Periodic acid-Schiff (PAS) staining and represent high magnifications of the first and third rows, respectively. Original magnifications were × 400 (a-d and i-l) or × 1000 (the others).
The kidneys of 6-month-old littermates revealed a similar distribution pattern of LRCs but with marked decrease in their number and intensity, especially in the renal papilla. LRCs were concentrated in tubules of the outer stripe of the CMJ (Figures 1j and n) whereas only a few LRCs were noted in the renal papilla (Figures 1l and p). Most tubular cells in the CMJ had prominent PAS-positive brush borders and the others with inconspicuous brush borders (Figure 1n). Only a few LRCs were scattered in the cortex and medulla (Figures 1i, k, m, and o). These results show that LRCs were concentrated constantly in the CMJ and most of them were tubular epithelial cells.
Distribution Patterns of Proliferating Cells and LRCs in ATN- and Non-ATN Kidneys
In ATN-induced kidneys, adult renal stem cells may actively proliferate to repair the injured tubules. To study the regional proliferating activity during renal repair, unilateral transient ATN was induced by clamping left renal vessels of four 2-month-old mice. They had been injected with BrdU at the beginning of metanephric nephrogenesis (E11∼E13) in an attempt to label adult renal stem cells more specifically. Bilateral kidneys were harvested 2 days later after the clamping and then the distribution patterns of Ki-67-positive proliferating cells and LRCs were studied.
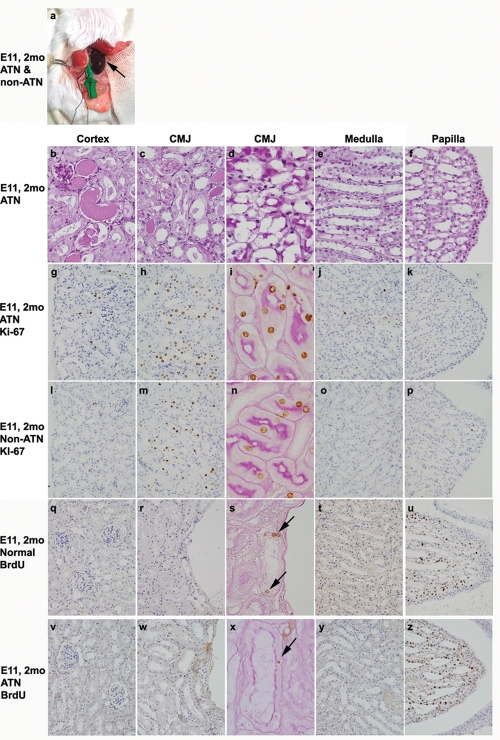
The left ATN kidney was markedly congested macroscopically compared to the contralateral right non-ATN kidney (Figure 2a). On microscopic examination, the renal tubular cells demonstrated pathologic features of ATN, including diffuse tubular epithelial degeneration, necrosis, and detachment from the underlying basement membrane. These features were more prominent in the CMJ and cortex than the medulla and renal papilla (Figures 2b-f).
Figure 2.
Regional proliferating activity and LRCs in ATN and non-ATN kidneys. After induction of unilateral transient ATN by clamping the left renal vessels of 2-month-old mice which were injected with BrdU for 3 days started on E11, the macroscopic findings (a), histologic features (PAS staining, b-f), and the distribution patterns of Ki-67 positive cells (g-p) and BrdU-positive LRCs (q-z) were studied in ATN (a-k and v-z) and non-ATN (a, l-p) kidneys and age-matched normal kidney (q-u). The left ATN kidney (a) and LRCs (s and x) are indicated by arrows. Original magnifications were × 1000 (d, i, n, s, and x) or × 400 (the others).
Two of the four left ATN kidneys showed a concentration of Ki-67-positive tubular cells as a broad band in the outer stripe of the CMJ (Figures 2h and i), with occasional Ki-67- positive cells in the cortex (Figure 2g). The other two left ATN kidneys showed high Ki-67 expression both in the CMJ and cortex (data not shown). Only a few Ki-67-positive cells were present in the medulla and none in the renal papilla of all four ATN kidneys (Figures 2j and k). Interestingly, the right non-ATN kidneys also demonstrated a Ki-67 expression pattern similar to those of the left ATN kidneys, i.e., a concentration of proliferating cells as a broad band in the outer stripe of the CMJ (Figures 2m and n). The mean number of Ki-67-positive cells in the right non-ATN kidneys was 48% of those in contralateral left ATN kidneys. These results demonstrate that tubular cells of the outer stripe of the CMJ participate in the renal repair through active cell proliferation.
A few LRCs were present in the tubules of CMJ adjacent to the arcuate vessels and many in the renal papilla in 2-month-old normal mouse littermates on which vascular clamping procedure was not performed (Figures 2q-u). Both left ATN and right non-ATN kidneys of age-matched mice also showed residual LRCs in the tubules of CMJ (Figures 2w and x). The numbers of LRCs in the CMJ tended to be decreased in the ATN kidneys compared to those of age-matched normal kidneys (80.7± 28.9 versus 102.3 ± 50.0), but this difference was not statistically significant. In contrast, LRCs in the renal papilla of the ATN kidneys were similar or increased (Figures 2u and z).
We performed double immunostaining of Ki-67 and BrdU on the ATN kidneys to investigate a co-localization of proliferating cells and LRCs. Except for a few cells, most Ki-67-positive cells did not co-localize to the LRCs (data not shown).
Regional Colony-forming Capacity of the Kidneys
Adult stem cells, including epidermal stem cells of the skin, possess high colony-forming capacity in cell culture [10].
To determine regional colony-forming capacity, mouse kidneys were dissected macroscopically into 3 portions: cortex, CMJ and medulla, and renal papilla (Figure 3a). On histologic examination, the CMJ and medullar portion included the juxtamedullary cortex, the outer and inner stripes of the outer medulla, and the inner medulla (data not shown). Tubular epithelial cells were isolated from each portion and plated at the same cell density. Most of these cells were polygonal in shape and grew into well-formed colonies (Figure 3b). The CMJ and medullar portion formed higher numbers of colonies than the other portions (Figure 3c, p = 0.03). Moreover, the CMJ and medullar portion retained well-formed colonies as late as 3 months after the plating, whereas those derived from the other portions vanished due to senescence (data not shown).
Figure 3.
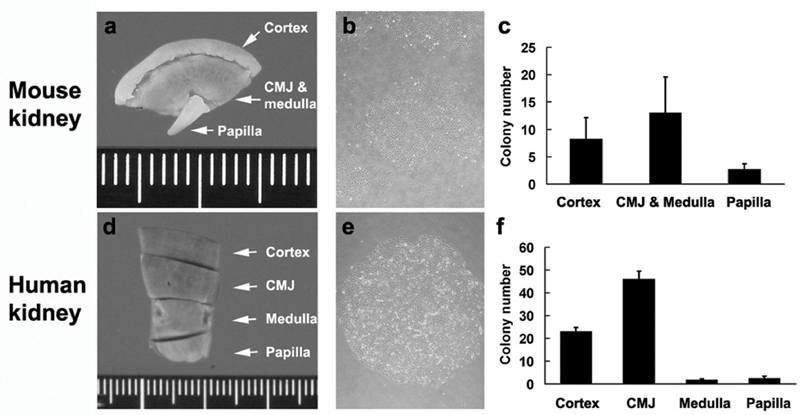
Regional colony-forming capacity of mouse (a-c) and human (d-f) kidneys. After macroscopic dissection of adult mouse (a-c) and human (d-f) kidneys into 3 portions (cortex, CMJ and medulla, and renal papilla) and 4 portions (cortex, CMJ, medulla, and renal papilla), respectively, regional tubular cells were plated at the same cell density, and then the morphology (b, e) and colony numbers (c, f) were studied. Results shown are representative of mouse experiments performed in triplicate and human experiments performed in quadruplicate. Original magnifications were × 40 (b and e).
To extend these findings into the human, we dissected normal adult human kidneys into four portions: the cortex, CMJ, medulla, and renal papilla (Figure 3d). Tubular epithelial cells were isolated from each portion and cultured at the same cell density. The CMJ included the juxtamedullary cortex and the outer and inner stripes of the outer medulla on microscopic examination (data not shown). Similar to the mouse kidneys, the CMJ portion of human kidneys, on average, formed higher numbers of colonies than the other portions (Figure 3f, p = 1.5 × 10−8). These findings indicate that the CMJ of adult mouse and human kidneys possesses high colony-forming cells.
Multipotency of CMJ Cells
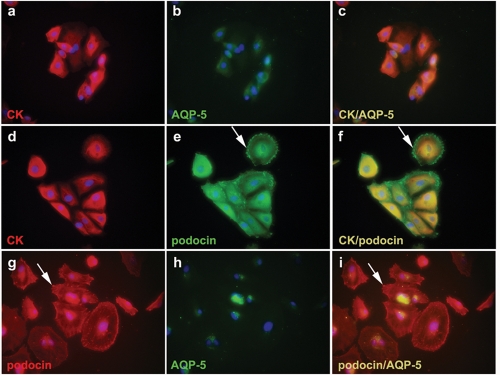
One of the important characteristics of adult stem cells is multipotency. We therefore studied the expression of various differentiation markers, an epithelial marker cytokeratin, a distal tubule marker aquaporin-5, and a glomerular podocyte marker podocin, on cultured cells derived from a single human CMJ cells. As expected the cells expressed cytokeratin strongly and co-expressed aquaporin-5 (Figures 4 a-c). The cytokeratin positive cells also co-expressed podocin. Similar to the normal kidney, in which it is expressed in the slit diaphragm of the podocyte cell membrane, the podocin was expressed not only in the cytoplasm but also in the cell membrane as a dot-like pattern (Figures 4d-f and g-i). Cells also co-expressed podocin and aquaporin-5 (Figures 4g-i). They did not express smooth muscle actin, a marker of smooth muscle cells and fibroblasts and NeuN, a neuronal marker (data not shown). Cultured human renal papilla cells also expressed cytokeratin, aquaporin 5, and podocin (data not shown). These results demonstrate that tubular epithelial cells of the CMJ are capable of multilineage differentiation.
Figure 4.
Multipotency of tubular epithelial cells of the CMJ. Cells derived from a single human CMJ cells were double immunostained with cytokeratin (CK) and aquaporin 5 (AQP-5) (a-c), cytokeratin and podocin (d-f), and podocin and aquaporin 5 (g-i). Nuclei were stained with DAPI (blue). The images of the third column are merged images of the first and second columns. Arrows indicate granular membranous staining of podocin (e, f, g, i). Original magnifications were × 400 (a-i).
Discussion
Here we demonstrate that the CMJ of the kidney contains label-retaining, renal-repairing, highly colony-forming, multipotent stem cell-like tubular cells. Our results suggest that the CMJ might be the niche of adult renal tubular stem cells in mouse and human.
The renal papilla has been suggested as a niche of adult renal stem cells based on the identification of LRCs in the renal papilla of adult mice and rats after injection of BrdU into 3-day-old mouse and rat pups [6]. The previous study showed that LRCs were mostly interstitial cells of renal papilla and observed as late as six months. We also noted abundant LRCs in the renal papilla of 2-month-old mouse littermates, some of which were interstitial cells. However, LRCs in the renal papilla were markedly decreased in the 6-month-old mouse littermates whereas many LRCs were remained in the tubules of the CMJ in our study. The discrepancy might be attributed to the BrdU injection time: late metanephric nephrogenesis in the previous study but early in our study. The metanephros, the anlage of adult mammalian kidney, originates during the 5th week of human gestation and during E11 in mouse development. Metanephric renal development accompanies morphological and functional maturation, forming urine as early as the 5th week of human gestation. The renal papilla is shown to be well formed early in embryonic kidneys and similar to the state of differentiation of mature kidneys by 13th to 14th weeks of human gestation [11, 12]. Because differentiated renal tubular epithelial cells do not actively proliferate under normal conditions, we assumed that tubular LRCs in adult kidneys may represent stem cells or differentiated cells derived from long-lived committed progenitors present at the time of the BrdU injection. We therefore injected a pulse of BrdU at the beginning of (E11 – E13) and during early metanephric nephrogenesis (E17 – E19). Our study showed that tubular LRCs resided in the CMJ constantly as late as 6 months whereas those of renal papilla were abundant in 2-month-old mouse kidneys but markedly decreased in 6-month-old mouse kidneys. The findings suggest that the CMJ is the niche of adult renal tubular stem cells, which is also supported by the concentration of proliferating tubular cells in the CMJ of ATN kidneys and its high colony-forming capacity.
It has been well known that active proliferation occurs in the outer stripe of the CMJ in ATN kidneys. Since the proliferating cell nuclear antigen (PCNA), a G1-S transition marker, is detected primarily in the S3 proximal tubules in this area, it has been speculated that surviving cells of the outer medulla after an ischemic injury undergo active cell division and suggested that the S3 epithelial cells are the progenitor cells [13, 14]. In addition, c-Fos, an immediate-early response gene protein, and p21, a cyclin kinase inhibitor maximally expressed in G1 phase, are expressed in the outer stripe of the outer medulla [13, 15]. In agreement with these reports, we found that Ki-67 expression, indicative of proliferating activity, was most prominent in the outer stripe of the CMJ.
The CMJ of contralateral non-ATN kidneys also showed high proliferating activity in ATN-induced mice. This finding strongly suggests that adult renal stem cells reside in the CMJ. This also indicates that cells of the CMJ can respond to endocrine-induced regenerative stimuli even without direct injury. Growth factors, such as epidermal growth factor (EGF), insulin-like growth factor (IGF), and hepatocyte growth factor (HGF), are shown to enhance renal tubular regeneration and accelerate recovery of ATN kidneys [16–18]. HGF levels are also known to be increased in uninjured tissues like liver, lung, spleen and contralateral kidney and in plasma as well [19]. These growth factors may act as the endocrine mediators. Interestingly, the mean number of proliferating cells in non-ATN kidneys was about half that of ATN kidneys, suggesting that the proliferating capacity of the CMJ is augmented by direct stimuli like ATN.
Because adult stem cells may actively proliferate to repair injured tissue, we expected a decrease of LRCs in the CMJ of ATN kidneys due to dilution of BrdU and a co-localization of Ki-67 positive cells to LRCs. The LRCs tended to be decreased in the CMJ of ATN kidneys compared to those of age-matched normal kidneys; however, the decrease was not statistically significant. Most of the Ki-67 positive cells did not co-localize to the LRCs. The LRCs were present in the outer stripe of the CMJ especially adjacent to arcuate vessels whereas the Ki-67 positive cells were present as a broad band in the outer stripe of the CMJ. The lack of co-localization may represent dilution of BrdU due to antecedent cell divisions because the kidneys were harvested 2 days after the ATN induction. It may also be explained by the migratory property of stem cells. For example, corneal stem cells in the limbus undergo centripetal migration to the center of the cornea [20]. Epidermal stem cells in the hair bulge also migrate upward to replenish the epidermis and sebaceous glands and downward to generate hair [10]. The migration of tubular epithelial cells has been reported when tubular necrosis was induced [21]. Therefore, further studies using a more number of mice and examination of kidneys at multiple time points after ATN induction might explain the discrepancies.
The locations of adult stem cells are generally characterized by good physical protection from external injury and the presence of well vascularized stroma like the hair bulge and corneal limbus of skin and cornea stem cells, respectively [1]. The CMJ, which is located in the mid portion of the kidney, may be physically well protected from external injury such as vesicoureteral reflux. It has been reported that the outer medulla of the CMJ is a major target of hypoxic injury due to regional hypoxia and the presence of tubules vulnerable to hypoxia, including the S3 proximal tubules and the thick ascending limb. Protection from hypoxic injury, however, has been reported in tubules surrounding the arcuate and interlobular arteries in the outer medulla of the CMJ [22]. We found that LRCs were concentrated in the outer stripe of the outer medulla of the CMJ, especially in the area surrounding the arcuate vessels, where both good physical protection and vascular supply might be provided.
The co-expressions of cytokeratin, aquaporin 5, and podocin but the absence of expression of smooth muscle actin and NeuN in the CMJ-derived cells suggest that their differentiation potential is restricted to nephron components in the conventional culture condition. The colonies derived from a single CMJ cells were morphologically polygonal and immunocytochemically strongly positive for cytokeratin but negative for smooth muscle actin. This finding supports the idea that the colonies were derived from tubular epithelial cells and exclude participation of glomerular and stromal cells in the colony formation.
In summary, our results suggest that adult renal stem cell-like tubular cells reside in the CMJ, especially in the outer stripe. However, further studies are required for their confirmation as stem cells: isolation of viable LRCs from the CMJ and demonstration of their self-renewal capacity, asymmetric replication, and multipotency. We hope additional studies may lead to the identification of specific surface marker(s) and eventually their clinical applications.
Acknowledgments
We thank Jae Y. Ro, Se Jin Jang and Jene Choe for helpful discussions, Hanjong Ahn for providing the human kidney samples, Su-Kil Park for technical advice on cell culture, and Ronald Osborne for proofreading the manuscript. K. Kim and K. Lee contributed equally to this work. This study was supported by a grant (2006-388) from the Asan Institute for Life Sciences, Seoul, Korea.
References
- 1.Lavker RM, Sun TT. Epidermal stem cells: properties, markers, and location. Proc Natl Acad Sci USA. 2000;97:13473–13475. doi: 10.1073/pnas.250380097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duffield JS, Park KM, Hsiao L-L, Kelley VR, Scadden DT, Ichimura T, Bonventre JV. Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest. 2005;115:1743–1755. doi: 10.1172/JCI22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin F, Moran A, Igarashi P. Intrarenal cells, not bone marrow-derived cells, are the major source for regeneration in postischemic kidney. J Clin Invest. 2005;115:1756–1764. doi: 10.1172/JCI23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duffield JS, Bonventre JV. Kidney tubular epithelium is restored without replacement with bone marrow-derived cells during repair after ischemic injury. Kidney Int. 2005;68:1956–1961. doi: 10.1111/j.1523-1755.2005.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dekel B, Shezen E, Even-Tov-Friedman S, Katchman H, Margalit R, Nagler A, Reisner Y. Transplantation of human hematopoietic stem cells into ischemic and growing kidneys suggests a role in vasculogenesis but not tubulogenesis. Stem Cells. 2006;24:1185–1193. doi: 10.1634/stemcells.2005-0265. [DOI] [PubMed] [Google Scholar]
- 6.Oliver JA, Maarouf O, Cheema FH, Martens TP, Al-Awqati Q. The renal papilla is a niche for adult kidney stem cells. J Clin Invest. 2004;114:795–804. doi: 10.1172/JCI20921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta S, Verfaillie C, Chmielewski D, Kren S, Eidman K, Connaire J, Heremans Y, Lund T, Blackstad M, Jiang Y, Luttun A, Rosenberg ME. Isolation and characterization of kidney-derived stem cells. J Am Soc Nephrol. 2006;17:3028–3040. doi: 10.1681/ASN.2006030275. [DOI] [PubMed] [Google Scholar]
- 8.Bussolati B, Bruno S, Grange C, Buttiglieri S, Deregibus MC, Cantino D, Camussi G. Isolation of renal progenitor cells from adult human kidney. Am J Pathol. 2005;166:545–555. doi: 10.1016/S0002-9440(10)62276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, Ronconi E, Meini C, Gacci M, Squecco R, Carini M, Gesualdo L, Francini F, Maggi E, Annunziato F, Lasagni L, Serio M, Romagnani S, Romagnani P. Isolation and characterization of multipotent progenitor cells from the Bowman's capsule of adult human kidneys. J Am Soc Nephrol. 2006;17:2443–2456. doi: 10.1681/ASN.2006010089. [DOI] [PubMed] [Google Scholar]
- 10.Oshima H, Rochat A, Kedzia C, Kobayashi K, Barrandon Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell. 2001;104:233–245. doi: 10.1016/s0092-8674(01)00208-2. [DOI] [PubMed] [Google Scholar]
- 11.Mussap M, Fanos V, Piccoli A, Zaninotto M, Padovani EM, Plebani M. Low molecular mass proteins and urinary enzymes in amniotic fluid of healthy pregnant women at progressive stages of gestation. Clin Biochem. 1996;29:51–56. doi: 10.1016/0009-9120(95)02006-3. [DOI] [PubMed] [Google Scholar]
- 12.Osathanondh V, Potter EL. Development of Human Kidney as Shown by Microdissection. Ii. Renal Pelvis, Calyces, and Papillae. Arch Pathol. 1963;76:277–289. [PubMed] [Google Scholar]
- 13.Witzgall R, Brown D, Schwarz C, Bonventre JV. Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J Clin Invest. 1994;93:2175–2188. doi: 10.1172/JCI117214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park SK, Kang MJ, Kim W, Koh GY. Renal tubule regeneration after ischemic injury is coupled to the up-regulation and activation of cyclins and cyclin dependent kinases. Kidney Int. 1997;52:706–714. doi: 10.1038/ki.1997.386. [DOI] [PubMed] [Google Scholar]
- 15.Price PM, Megyesi J, Safirstein RL. Cell cycle regulation: repair and regeneration in acute renal failure. Semin Nephrol. 2003;23:449–459. doi: 10.1016/s0270-9295(03)00087-1. [DOI] [PubMed] [Google Scholar]
- 16.Humes HD, Cieslinski DA, Coimbra TM, Messana JM, Galvao C. Epidermal growth factor enhances renal tubule cell regeneration and repair and accelerates the recovery of renal function in postischemic acute renal failure. J Clin Invest. 1989;84:1757–1761. doi: 10.1172/JCI114359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding H, Kopple JD, Cohen A, Hirschberg R. Recombinant human insulin-like growth factor-I accelerates recovery and reduces catabolism in rats with ischemic acute renal failure. J Clin Invest. 1993;91:2281–2287. doi: 10.1172/JCI116456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller SB, Martin DR, Kissane J, Hammerman MR. Hepatocyte growth factor accelerates recovery from acute ischemic renal injury in rats. Am J Physiol. 1994;266:F129–134. doi: 10.1152/ajprenal.1994.266.1.F129. [DOI] [PubMed] [Google Scholar]
- 19.Igawa T, Matsumoto K, Kanda S, Saito Y, Nakamura T. Hepatocyte growth factor may function as a renotropic factor for regeneration in rats with acute renal injury. Am J Physiol. 1993;265:F61–69. doi: 10.1152/ajprenal.1993.265.1.F61. [DOI] [PubMed] [Google Scholar]
- 20.Cotsarelis G, Cheng SZ, Dong G, Sun TT, Lavker RM. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57:201–209. doi: 10.1016/0092-8674(89)90958-6. [DOI] [PubMed] [Google Scholar]
- 21.Kartha S, Toback FG. Adenine nucleotides stimulate migration in wounded cultures of kidney epithelial cells. J Clin Invest. 1992;90:288–292. doi: 10.1172/JCI115851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shanley PF, Rosen MD, Brezis M, Silva P, Epstein FH, Rosen S. Topography of focal proximal tubular necrosis after ischemia with reflow in the rat kidney. Am J Pathol. 1986;122:462–468. [PMC free article] [PubMed] [Google Scholar]