Abstract
We studied the effect of freezing and inserting meniscal plugs in lesions generated in the avascular area of sheep menisci maintained in vitro, and whether the healing process can be improved by adding growth factors TGF-β1 and IGF-1. Thirty six menisci obtained from healthy 3 months-old sheep were cultured in 6 well plates and holes were perforated in the avascular area. Meniscal plugs, either fresh or frozen at −20°C for 1 month, were used to fill in the lesions, and then cultured in the presence or absence of TGF-β1 or IGF-1 for 8 weeks. Samples stained with Massons trichrome were analyzed to evaluate the attachment of the plug and the cell density of the tissue. BrdU immunohistochemistry was performed to identify the proliferation of meniscal cells. Both growth factors improved considerably the cell density of implanted plugs. TGF-β1 increased significantly the attachment of both fresh and frozen plugs, but it had no effect on meniscal cell proliferation. In contrast, IGF-1 had no effect on the attachment, but did increase significantly the number of proliferating cells in the surface of the host meniscus and the inserted plug. In conclusion, frozen plugs can survive if treated with either TGF-β1 or IGF-1. The combination of TGF-β1 and IGF-1 could aid in the repairing of the avascular meniscal injuries, as they are capable of promoting the attachment of tissue, and increasing the proliferation of meniscal cells.
Keywords: TGF-β1, IGF1, meniscus, avascular injury, sheep, tissue engineering
Introduction
The periphery of the meniscus is vascular, while the middle and inner zones are avascular [1, 2]. Lesions in the avascular zone are common and have the poorest prognosis. [3–6]. There is a need for new strategies for repairing lesions in the menisucs in order to improve techniques commonly used in orthopaedic surgery. Tissue engineering brings us the possibility of trying new methods to treat these lesions [7].
Factors like neo-vascularization as well as the size and shape of the lesion may affect the healing processes [6]. Common techniques to repair meniscal lesions include debriding, synovial abrasion, fibrin clots or fibrin glue, trefine, or even laser, as well as the more classical sutures and fixation with bioabsorbable materials [8–12]. Nevertheless, none of these methods achieve complete healing of lesions when they are localized in the avascular area [6, 10].
Recently, tissue engineering techniques have been applied to meniscal repair, trying to combine the effects of meniscal cell implantation with growth factor treatments and the use of different types of scaffolds [7]. Among growth factors, the more commonly used are TGF-β1 and IGF-1 [13–15]. The capacity of these two growth factors to promote meniscal and cartilage cell proliferation and differentiation has been demonstrated [16–21]. However, all these observations have not yet been translated into clinical trials.
The material used for the development of scaffolds is also an important issue, and the constructs should be designed so that the specific mechanical and biological needs of the meniscus are satisfied [22]. Some biological tissues as well as synthetic polymer based scaffolds have been used. Among these, meniscal tissue itself could be the better option, as its geometry, biochemical and mechanical properties fit exactly those of the altered tissue [23]. Nevertheless, the availability and the integration of meniscal grafts are issues that still must be overcome by additional research. We hypothesize that storage of meniscal allografts in a frozen state will solve the lack of available grafts, while growth factor treatment will help to improve its integration. We have therefore employed an in vitro model of meniscal culture, and used it to investigate the repair of lesions in the avascular area of the tissue using frozen and fresh meniscal plugs. Moreover, we have addressed the influence of addition of growth factors TGF-β1 and IGF-1 on these repair processes.
Materials and Methods
Meniscal Explants
We used 36 menisci from 3 months aged healthy sheep, weighting about 35 kg. The study was performed according to the policies and principles established by the Animal Welfare Act, the NIH Guide for Care and Use of Laboratory Animals, and the national animal welfare guidelines, and approved by The Animal Care and Use Committee of the University of Navarra.
Animals were euthanized by administration of sodium thiopental (Penthotal Sodium, 0,5 g; Abbot, Chicago, IL) and intravenous injection of 20 mL of KCl (2 mEq/mL), after which the complete knee joint was removed and processed under sterile conditions.
Experimental Design
The excised menisci, including the capsule and synovial membrane, were divided in 9 groups as indicated in Table 1. Menisci from all groups were perforated in the avascular area using a 3 mm diameter punch biopsy to create a hole, and were then placed in culture dishes. Control menisci from groups 1, 2 and 3 were placed directly in culture dishes. Lesions in menisci groups 4, 5 and 6 were press-fit with the cylinders extracted with the punch and then placed in the dishes, while those in groups 7, 8 and 9 were press-fit with cylinders that were previously placed at −20°C for one month with no additives. Before insertion into the meniscal holes, plugs were placed in culture medium for 1 day.
Table 1.
Treatment of excised menisci with TGF-β1 or IGF-1 in vitro
| Group | Implant | Treatment | n | Time of evolution (weeks) |
|---|---|---|---|---|
| 1 | - | - | 4 | 8 |
| 2 | - | TGF-β1 | 4 | 8 |
| 3 | - | IGF-1 | 4 | 8 |
| 4 | Fresh | - | 4 | 8 |
| 5 | Fresh | TGF-β1 | 4 | 8 |
| 6 | Fresh | IGF-1 | 4 | 8 |
| 7 | Frozen | - | 4 | 8 |
| 8 | Frozen | TGF-β1 | 4 | 8 |
| 9 | Frozen | IGF-1 | 4 | 8 |
Meniscal Explant Culture
All menisci were cultured in 6 well plates, containing culture DMEM supplemented with 10% fetal bovine serum, 1 mM L-cysteine, 100 U/mL penicillin, 100 μg/mL streptomycin and 10mM Hepes buffer (Gibco-BRL, Gaithersburg, MD). They were all cultured in the presence of 5-bromo-2-deoxyuridine (BrdU, Sigma, St. Louis, MO) in order to study its uptake (indicating DNA synthetic activity) by meniscal cells. They were also cultured in the presence and absence of TGF-β1 and IGF-1 (Peprotech, Rozky Hill, NJ). Based on previously published studies, concentration was set at 50 ng/mL [15, 16, 24]. They were cultured for 8 weeks, renewing the culture medium with freshly added growth factors every 2–3 days. At 8 weeks, menisci were fixed in 4% formaldehyde and embedded in paraffin.
Analysis of Plug Attachment
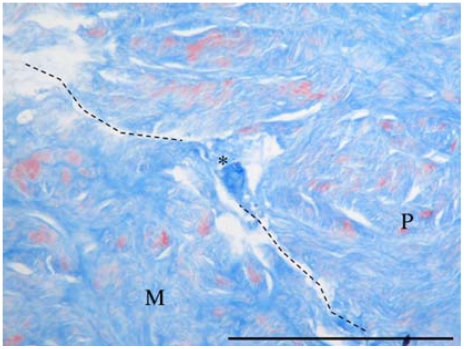
Sections, 4 μm thick, were stained using Massons trichrome staining and analyzed in light microscope to evaluate the state of the lesion. To estimate the grade of attachment of the inserted material, we looked for sites in which the edges of the meniscus and the filling tissue were so tightly bound that fibers of matrix could be observed spanning the crevice between the plug and the meniscus tissue. The sections were evaluated for the following properties to eliminate the possibility of sectioning artifacts: 1) direct contact between both edges, 2) fibers spanning the crevice, and 3) these properties to be also observed in the following and previous sections (Figure 1).
Figure 1.
Analysis of plug adhesion (Masson's Trichrome, ×20) showing a point of tissue union indicated by the asterisk * (M = meniscus, P = plug. Barr: 100 μm).
The total number of tight contact points between meniscus lesions and the plug were counted in all contact surface of 2 different non-consecutive sections per sample (n=4). Samples were finally photographed using a Digital Sight 2S-2M camera (Nikon, Tokyo, Japan).
Assessment of Cell Density
To estimate the cell viability of the explants, we analyzed stained samples under light microscope and counted the number of nuclei present in 10 random fields per sample (n=4), dividing them into avascular, inserted plug (if any) and vascular areas. The total number was divided by the area of the measured field (0,031 mm2) to determine the cell density (cells/mm2). They were also compared with the cell density of fresh and frozen meniscal cylinders processed in the same way.
Assessment of Cell Proliferation
Immunohistochemistry using a specific antibody to BrdU was performed to analyze the proliferation rate of meniscal cells, as it is taken up by cells in S phase of cell cycle. Briefly, after one hour treatment with 4N HCl followed by trypsin treatment (0.1% at 37°C for 1 hour) and deparaffinization, endogenous peroxidase was blocked by placing the sections in 3% hydrogen peroxide solution for 30 min in darkness. They were then incubated in the following reagents with appropriate Tris-buffered-saline (TBS: 0.55M Tris, 50 mM NaCl, pH 7.36) washes: normal rabbit serum for 30 min, primary antibody (anti-BrdU diluted 1:100, Sigma) over night, biotinylated anti-rabbit antibody for 30 min, and avidin-biotin complex (ABC, DAKO, Copenhagen, Germany) for 30 min. The reaction was visualized with chromogen substrate solution (0.3 mg/mL diaminobenzidine and hydrogen peroxide), and sections were then counterstained with Harris' hematoxylin, dehydrated, and mounted. Normal serum was used as negative control. Positive cells were counted under a light microscope in ten random fields of vascular area of specimens (n=4). Distance of the deepest positive cell to the surface was measured in every area counted, using software for Digital Sight 2S-2M camera (Nikon).
Statistical Analysis
The data were analyzed using the nonparametric Kruskal-Wallis and Mann-Whitney U- tests, with SPSS 9.0 for Windows. Significance was set at p≤0.05.
Results
Cell Density
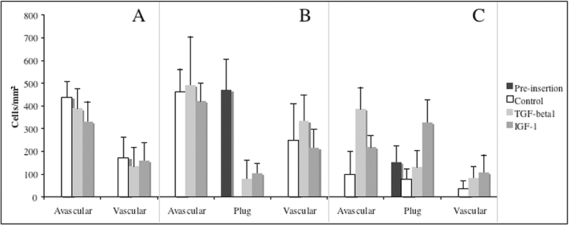
Analysis of total cell nuclei in stained samples under light microscope revealed significant differences between the cell density of cultured explants (p<0.001). Cell density measurement showed the presence of cells in both vascular and avascular areas of menisci with no filling (Figure 2). There were no significant differences between growth factor treatment and the control groups (p=0.550 for avascular and p=0.549 for vascular areas).
Figure 2.
Analysis of cell density of explants (number of nuclei/mm2 of stained tissue). Samples were divided into vascular, avascular areas and graft (if any).
In explants filled with fresh tissue (Figure 2), we could also detect nuclei in both areas of menisci (avascular and vascular), without significant differences between growth factor treatment and control groups (p=0.517 for avascular and p=0.087 for vascular areas). Fresh plugs before implantation showed a cell density value with no significant differences between avascular area from which they were originated (p=0.730). The cell density was zero after 8 weeks of culture without growth factor treatment. After treatment with either TGF-ß1 or IGF-1, the explants used to fill up the lesions showed the presence of cells without significant differences between both treatments (p=0.353).
On the other hand, explants inserted with frozen tissue (Figure 2) showed a significantly less quantity of cell nuclei in the avascular and vascular areas (p<0,001). Plugs processed after frozen storage did show a cell density significantly lower than fresh cylinders (p<0,001), and that decrease correlated significantly (p<0.05) with the time of the study. The frozen plugs maintained cells inside, even in untreated ones. After the treatment with TGF-β1 we did not observe significant differences in the number of viable cells inside the frozen plug (p=0,063), but we could observe differences after the treatment with IGF-1 (p<0.001).
Finally, the number of cells in avascular area of explants filled up with frozen was significantly higher in meniscal explants treated with both TGF-ß1 (p<0,001) and IGF-1 (p<0,05), and the same occurred with cells in vascular area (p<0.05 for both TGF-β1 and IGF-1).
Plug Attachment
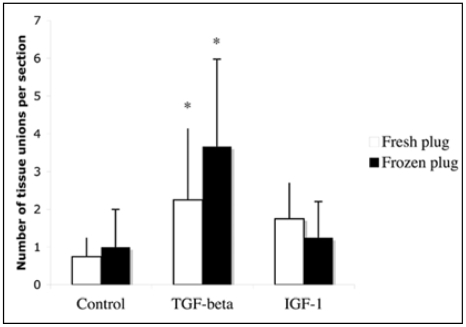
Light microscopy analysis of stained samples allowed us to locate sites of union between the inserted plug and the meniscus. The total count of these points for samples of every group is shown in Figure 3.
Figure 3.
Quantification of tissue unions between the injured meniscus and the plug for grafted groups.
The comparison of fresh and frozen plugs for every treatment used allowed us to detect no significant differences in the number of unions (p=0.753), indicating that the preservation had no effect on the attachment. On the other hand, we could observe a significant increase in the number of unions in menisci cultured with TGF-β1 (p=0.039), but not with IGF-1 (p=0.155) for both fresh and frozen plugs. Thus, TGF -β 1 but not IGF-1 at the concentrations studied did appear to enhance the process of adhesion of both plugs to the meniscus and thus integration.
Cell Proliferation
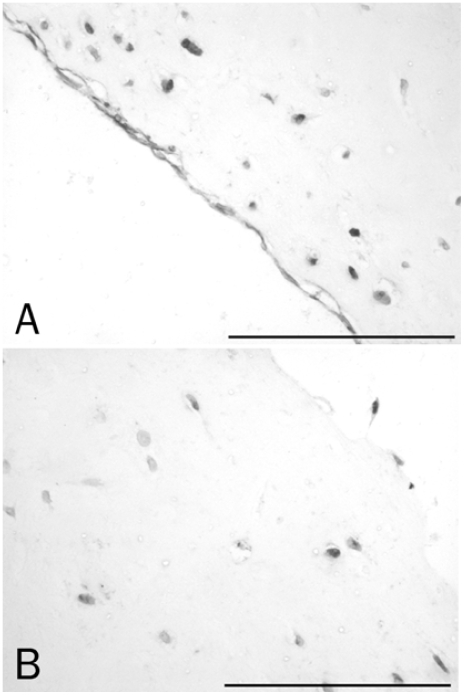
BrdU immunohistochemistry allowed us to detect cells with DNA synthetic activity, thus in the S-phase. We could observe stained cells spread over all the surface of the menisci (Figure 4), but no positive cells were observed inside the tissue. We found no positive cells in untreated or in fresh or frozen plugs treated with TGF-β1, proving no induction of proliferation exerted by this growth factor. On the other hand, plugs treated with IGF-1 did show the presence of cells with DNA synthetic processes.
Figure 4.
Immunohistochemistry detection of BrdU. A. Vascular area of an untreated and unfilled explant showing cells in proliferation (×20). B. Frozen plug treated with IGF-1 (×20).
For the comparison of growth factor effect on the proliferation rate of meniscal cells, we quantified the number of positive cells in the vascular area of the meniscal explant under all treatments. The presence of IGF-1 in the culture medium did increase significantly the number of proliferating cells (p=0.001). On the contrary, treatment with TGF-β1 did not affect the number of proliferating cells in the surface of the tissue (p=0.257). Finally, measurement of the distance of the deepest stained cell to the surface of the meniscus showed that treatment with either IGF-1 or TGF-β1 increased significantly the depth of responding cells (p=0.003 and 0.009, respectively) (Figure 5).
Figure 5.
Quantification of positive cells (A) and the distance to the deepest positive cell founded (B).
Discussion
In our study, we have employed culture of meniscal explant to develop a model of using fresh or frozen allografts combined with the use of growth factors TGF-β1 and IGF-1. Our main goal was to evaluate the use of frozen allografts to heal meniscal lesions, and to improve this healing by using growth factors such as TGF-β1 or IGF-1.
We showed here that meniscal explants did survive in culture for 8 weeks, maintaining cells inside, agreeing with results previously published by Webber et al [25, 26]. Fresh plugs, on their own, did not survive in the culture conditions used. The addition of IGF-1 or TGF-β1 to the culture medium supported the maintenance of living cells, and thus supported its activity of meniscal cells.
Low temperature storage, as well as the freezing and thawing are responsible for cell death in meniscal and cartilage samples [27]. This process is facilitated by the release of proteolytic enzymes that may lead to degeneration of meniscal structure as it has been observed in other previously published works [22, 27–29]. This degeneration may be avoided or slowed by improving the freezing process in order to avoid loosing cell viability [27, 30], or by treating cells or meniscal tissue with growth factors after the thawing of the tissue as suggested by this work. We have observed the presence of cells that survived the freezing and thawing process. These cells presumably die subsequently in culture in non-treated explants. This population of cells might have been prompted by growth factors to stay alive or to continue their expression of matrix molecules that might lead to the correct preservation of meniscal structure. In fact, we have observed that both growth factors had a positive effect on the cell density of fresh and frozen tissues. This data supports the use of these factors as strong inducers of meniscal cell survival. In addition, the implantation of frozen, but not fresh plugs, decreased the cell density of avascular and vascular areas of the meniscus in which they were inserted. This suggests that changes in frozen tissue that affect the protease content and thus, matrix integrity, not only affect frozen plug, but also the adjacent tissues. Signals produced by the death of frozen cells could translocate to the surrounding tissue and affect the cells of the host meniscus. This is not produced by freshly inserted plugs that, on the other hand did show a loss of cells during the time of the study. Changes occurred in these plugs are dissimilar (in terms of intensity or nature) to those produced by the freezing or thawing processes, as they do not affect surrounding meniscal tissue. Further research in this area would allow us to maintain the correct structure of meniscal tissue, eliminating one of the major hurdles for the use of allografts, i.e., the availability of fresh tissue to implant.
Previous studies [3, 4, 25, 26, 31] have suggested that avascular zone has the potential to heal, but lacks the factors to initiate the repairing process. We have tried to provide those factors in our in vitro model of meniscal culture by adding TGF-β1 and IGF-1 to the culture medium. In our study, TGF-β1 improved the attachment of the inserted plugs that may eventually lead to integration. Despite the previously described capacity to induce matrix formation in meniscal cells [24, 32–34], IGF-1 on its own was unable to induce the increase in the number of tissue unions in our model. Nevertheless, it did have a strong stimulatory activity on the proliferation of meniscal cells even in frozen plugs used to fill in the lesion, which was not seen in TGF-β1 alone in our culture conditions. Thus, it appears that TGF-β1 and IGF-1 have different effects. While TGF-β1 induced the attachment of the plug, IGF-1 increased the proliferation rate of meniscal cells, making them ideal to be further studied as a combination in the development of treatments for meniscal lesions. Nevertheless, this dual activity has some common effects, as both of them are capable of promoting the survival of meniscal cells of extracted tissue fragments. IGF-1 treatment did induce a higher number of proliferating cells in frozen tissue that may be related to a higher cell density found in frozen tissue after 8 weeks of evolution. Changes mentioned above, i.e., the disruption of extracellular matrix after the release of proteolytic enzymes, may have permitted a better diffusion of IGF-1 and so, a higher effect in frozen tissue, explaining, at least in part, this effect found in frozen plugs.
This combination of activities was also found for both growth factors in cartilage cells [16]. Our group described a high induction of proliferation exerted by IGF-1, not shared with TGF-β1, and on the contrary a strong induction of matrix synthesis by TGF-β1 and to a lesser extent, by IGF-1. Other results agree strongly with our findings in this model of meniscal lesions, in which the integration of the implant could be related to the induction of matrix synthesis. Others [15, 33] have also proposed IGF-1 as a promoter of matrix formation. However, the work by Tumia and Johnstone [33] studied the effect of IGF-1 in monolayer sheep meniscal cells while Pangborn and Athanasiou [15] used rabbit cells in poly-glycolic scaffolds. Our work on the contrary uses cells in the whole meniscus, which could be more similar to its natural environment, and what may have been responsible for the lack of effect for IGF-1 in the attachment of the implant. In the same way, the restriction of the effect of IGF-1 to the superficial layer of meniscal cells could be related with the incapacity of the growth factor to diffuse into the inner parts of the tissue, or even with the incapacity of BrdU itself to penetrate inside the tissue, and label proliferating cells. Both restrictions could have been prevented by TGF-β1. The capacity of TGF-β1 to induce the production of matrix degrading enzymes has been well described. Therefore, TGF-β1 could have facilitated the diffusion of both IGF-1, BrdU or even TGF-β1 itself, explaining the increase in the depth of appearance of BrdU stained cells after the treatment with TGF-β1 found in our work. This might also be due to the induction of expression of some cytokines by meniscal cells that could act in a paracrine way and induce neighboring cells to proliferate, achieving proliferation of cells in the inner zones of the meniscus, but without affecting their total number. In any case, the presence of living cells inside the tissue, and the induction of survival by growth factors used in the plugs inserted, allow us to suggest that culture media and growth factors can diffuse effectively inside the tissue. Then, superficial cells would constitute a population of particularly responsive cells.
In our model, delivery of both factors must be improved. We have used growth factors added directly to the culture medium and renewed every 2–3 days. It is widely accepted that the half life of IGF-1 is quite short [14, 16], which could also be an explanation for the poor effect in the attachment of fresh and frozen plugs in our study. Nevertheless concentration reached in our model seems to be enough to induce the proliferation of superficial cells. Indeed, the half life of TGF-β1 is also quite short, and is able to achieve effects on the plug attachment at the concentration used. Then, in a similar way we suggest, as other authors [15, 24, 32, 33, 35], that improving the concentration and delivery of growth factors could provide a more efficient activity for them and thus we promote greatly the research in this area. In fact, despite of the many basic research studies focusing in the potential of many different growth factors, only BMP-2 and 7 have reached the clinical level [14]. This may be due to the lack of an effective delivery system that achieves an efficient concentration at the site where they are needed, and to the many effects that those factors may have. Active research in this are could lead these factors into clinical use.
The main limitation of our model is the young age of sheep from which menisci were extracted. Three month-old animals are not yet fully mature, and this could have influenced the vascularization pattern as well as the sensitivity of cells to respond to growth factors. In a previous study, we have observed that vascularization of sheep reaches its final structure at 2 months of age, showing the correct pattern of vascular, outer and avascular, inner layers [36]. The results validate our model, which however, could have some other minor limitations, as the young state of cells, and its capacity to respond to external stimuli. On the other hand, a low oxygen level is the normal environment in the knee, which has been described to be inducer, by itself alone, of the matrix formation and differentiation of meniscal cells [37, 38]. In our work, oxygen levels are those commonly used for cell culture, with no hypoxia, which could also have had influence in the state, responsiveness or the pattern of gene expression of cells in meniscal explants used. These factors must be further experimented in order to offer new data about the effect of growth factors in meniscal culture.
In summary, the results presented here allow us to offer new data about the effect of using meniscal frozen plugs in the repairing of meniscal lesions in vitro. The combination of IGF-1 and TGF-β1 may be a very useful tool that should be taken into account, but their delivery must be further investigated. All these data could contribute to the development of new strategies for the repairing of lesions produced in the avascular zone of the meniscus in the future.
Acknowledgments
This work was supported by the MAPFRE foundation.
References
- 1.Arnoczky SP. Building a meniscus – biologic considerations. Clin Orthop. 1999;367(suppl):S244–S253. [PubMed] [Google Scholar]
- 2.McDevitt CA, Webber RJ. The ultrastructure and biochemistry of meniscal cartilage. Clin Orthop. 1990;252:8–18. [PubMed] [Google Scholar]
- 3.Ghadially FN, Wedge JH, Lalonde JM. Experimental methods of repairing injured menisci. J Bone Joint Surg (Br) 1986;68B:106–110. doi: 10.1302/0301-620X.68B1.3753606. [DOI] [PubMed] [Google Scholar]
- 4.Guisasola I, Vaquero J, Forriol F. Knee immobilization on meniscal healing after suture: an experimental study in sheep. Clin Orthop. 2002;395:227–233. doi: 10.1097/00003086-200202000-00027. [DOI] [PubMed] [Google Scholar]
- 5.Roeddeker K, Muennich U, Nagelschmidt M. Meniscal healing: a biomechanical study. J Surg Res. 1994;56:20–27. doi: 10.1006/jsre.1994.1004. [DOI] [PubMed] [Google Scholar]
- 6.Stone KR. Current and future directions for meniscus repair and replacement. Clin Orthop. 1999;367(suppl):S273–S280. doi: 10.1097/00003086-199910001-00026. [DOI] [PubMed] [Google Scholar]
- 7.Buma P, Ramrattan NN, van Tienen TG, Veth RP. Tissue engineering of the meniscus. Biomaterials. 2004;25:1523–1532. doi: 10.1016/s0142-9612(03)00499-x. [DOI] [PubMed] [Google Scholar]
- 8.Biedert RM. Treatment of intrasubstance meniscal lesions: a randomized prospective study of four different methods. Knee Surg Sports Traumatol Arthrosc. 2000;8:104–108. doi: 10.1007/s001670050195. [DOI] [PubMed] [Google Scholar]
- 9.Barber FA, McGarry JE. Meniscal repair techniques. Sports Med Arthrosc. 2007;15:199–207. doi: 10.1097/JSA.0b013e3181595bed. [DOI] [PubMed] [Google Scholar]
- 10.Rodeo SA. Arthroscopic meniscal repair with use of the outside-in technique. Instr Course Lect. 2000;49:195–206. [PubMed] [Google Scholar]
- 11.Tetik O, Kocabey Y, Johnson DL. Synovial abrasion for isolated, partial thickness, undersurface, medial meniscus tears. Orthopedics. 2002;25:675–678. doi: 10.3928/0147-7447-20020601-19. [DOI] [PubMed] [Google Scholar]
- 12.Zhang ZN, Tu KY, Zhang WM, Liu ZT, Ou SH. Treatment of longitudinal injuries in avascular area of meniscus in dogs by trephination. Arthroscopy. 1988;4:151–159. doi: 10.1016/s0749-8063(88)80019-7. [DOI] [PubMed] [Google Scholar]
- 13.Collier S, Ghosh P. Effects of transforming growth factor beta on proteoglycan synthesis by cell and explant cultures derived from the knee joint meniscus. Osteoarthritis and Cartilage. 1995;3:127–138. doi: 10.1016/s1063-4584(05)80045-7. [DOI] [PubMed] [Google Scholar]
- 14.Gautschi OP, Frey SP, Zellweger R. Bone morphogenetic proteins in clinical applications. ANZ J Surg. 2007;77:626–631. doi: 10.1111/j.1445-2197.2007.04175.x. [DOI] [PubMed] [Google Scholar]
- 15.Pangborn CA, Athanasiou KA. Growth factors and fibrochondrocytes in scaffolds. J Orthop Res. 2005;23:1184–1189. doi: 10.1016/j.orthres.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 16.Acosta CA, Izal I, Ripalda P, Douglas-Price AL, Forriol F. Gene expression and proliferation analysis in young, aged, and osteoarthritic sheep chondrocytes effect of growth factor treatment. J Orthop Res. 2006;24:2087–2094. doi: 10.1002/jor.20245. [DOI] [PubMed] [Google Scholar]
- 17.McQuillan DJ, Handley CJ, Campbell MA, Bolis S, Milway VE, Herington AC. Stimulation of proteoglycan synthesis by serum and insulin-like growth factor-I in cultured bovine articular cartilage. Biochem J. 1986;240:424–430. doi: 10.1042/bj2400423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schalkwijk J, Joosten LAB, van den Berg WB, van de Putte LB. Insulin-like growth factor stimulation of chondrocyte proteoglycan synthesis by human synovial fluid. Arthritis Rheum. 1989;32:66–71. doi: 10.1002/anr.1780320111. [DOI] [PubMed] [Google Scholar]
- 19.Takigawa M, Okawa T, Pan H, Aoki C, Takahashi K, Zue T, Suzuki F, Kinoshita A. Insulin-like growth factors I and II are autocrine factors in stimulation of proteoglycan synthesis, a marker of differentiated chondrocytes, acting through their respective receptors on a clonal human chondrosarcoma derived cell line HCS-2/8. Endocrinology. 1997;138:4390–4400. doi: 10.1210/endo.138.10.5265. [DOI] [PubMed] [Google Scholar]
- 20.Fortier LA, Nixon AJ, Mohammed HO, Lust G. Altered biological activity of equine chondrocytes cultured in a three-dimensional fibrin matrix and supplemented with transforming growth factor beta-1. Am J Vet Res. 1997;58:66–70. [PubMed] [Google Scholar]
- 21.Reed MJ, Vernon RB, Abrass IB, Sage EH. TGF-β1eta-1 induces the expression of type I collagen and SPARC, and enhances contraction of collagen gels, by fibroblasts from young and aged donors. J Cell Phys. 1994;158:169–179. doi: 10.1002/jcp.1041580121. [DOI] [PubMed] [Google Scholar]
- 22.Arnoczky SP, Di Carlo EF, O'Brien SJ, Warren RF. Cellular repopulation of deep-frozen meniscal autografts: an experimental study in the dog. Arthroscopy. 1992;8:428–436. doi: 10.1016/0749-8063(92)90003-t. [DOI] [PubMed] [Google Scholar]
- 23.Kambic HE, Futani H, McDevit CA. Cell, matrix changes and alpha-smooth muscle actin expression in repair of the canine meniscus. Wound Repair Regen. 2000;8:554–561. doi: 10.1046/j.1524-475x.2000.00554.x. [DOI] [PubMed] [Google Scholar]
- 24.Imler SM, Doshi AN, Levenston ME. Combined effects of growth factors and static mechanical compression on meniscus explant biosynthesis. Osteoarthritis and Cartilage. 2004;12:736–744. doi: 10.1016/j.joca.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Webber RJ, York L, Vanderschilden JL, Hough AJ. An organ culture model for assaying wound repair of the fibrocartilaginous knee joint meniscus. Am Orthop Soc Sports Med. 1989;17:393–400. doi: 10.1177/036354658901700314. [DOI] [PubMed] [Google Scholar]
- 26.Webber RJ, Zitaglio T, Hough AJ. Serum-free culture of rabbit meniscal fibrochondrocytes: proliferative response. J Orthop Res. 1988;6:13–23. doi: 10.1002/jor.1100060103. [DOI] [PubMed] [Google Scholar]
- 27.Arnoczky SP, McDevitt CA, Schmidt MB, Mow VC, Warren RF. The effect of cryopreservation in canine menisci: a biochemical morphologic and biochemical evaluation. J Orthop Res. 1988;6:1–12. doi: 10.1002/jor.1100060102. [DOI] [PubMed] [Google Scholar]
- 28.Jackson DW, Simon T. Biology of meniscal allograft. In: Mow VC, Arnoczky SP, Jackson DW, editors. Knee meniscus: basic and clinical foundations. New York: Raven Press; 1992. pp. 141–152. [Google Scholar]
- 29.Schmid A, Schmid F, Tiling T. Electron microscopical studies on human meniscal tissue preserved for transplantation. In: Muller W, Hacken-Bruch H, editors. Surgery and arthroscopy of the knee. Berlin Heidelberg New York: Springer; 1988. pp. 703–707. [Google Scholar]
- 30.Fabbriciani C, Lucania L, Milano G, Schiavone Panni A, Evangelisti M. Meniscal allografts: cryopreservation vs deep-frozen technique. An experimental study in goats. Knee Surg Sports Traumatol Arthrosc. 1997;5:124–134. doi: 10.1007/s001670050038. [DOI] [PubMed] [Google Scholar]
- 31.Trommel van MF, Simonian PT, Potter HG, Wickiewicz TL. Arthroscopic meniscal repair with fibrin clot of complete radial tears of the lateral meniscus in the avascular zone. Arthroscopy. 1998;14:360–365. doi: 10.1016/s0749-8063(98)70002-7. [DOI] [PubMed] [Google Scholar]
- 32.Stewart K, Pabbruwe M, Dickinson S, Sims T, Hollander AP, Chaudhuri JB. The effect of growth factor treatment on meniscal chondrocyte proliferation and differentiation on polyglycolic acid scaffolds. Tissue Eng. 2007;13:271–280. doi: 10.1089/ten.2006.0242. [DOI] [PubMed] [Google Scholar]
- 33.Tumia NS, Johnstone AJ. Regional regenerative potential of meniscal cartilage exposed to recombinant insulin-like growth factor-I in vitro. J Bone Joint Surg (Br) 2004;86-B:1077–1081. doi: 10.1302/0301-620x.86b7.13747. [DOI] [PubMed] [Google Scholar]
- 34.Bhargava MM, Attia ET, Murrell GA, Dolan MM, Warren RF, Hannafin JA. The effect of cytokines on the proliferation and migration of bovine meniscal cells. Am J Sports Med. 1999;27:636–643. doi: 10.1177/03635465990270051601. [DOI] [PubMed] [Google Scholar]
- 35.Steinert AF, Palmer GD, Capito R, Hofstaetter JG, Pilapil C, Ghivizzani SC, Spector M, Evans CH. Genetically enhanced engineering of meniscus tissue using ex vivo delivery of transforming growth factor-β1 complementary deoxyribonucleic acid. Tissue Eng. 2007;13:2227–2237. doi: 10.1089/ten.2006.0270. [DOI] [PubMed] [Google Scholar]
- 36.Gahr B, Ripalda P, Forriol F. Vascularización meniscal con la edad. Estudio experimental en corderos. Rev Ortop Traumatol. 2000;44:561–565. [Google Scholar]
- 37.Adesida AB, Grady LM, Khan WS, Hardingham The matrix-forming phenotype of cultured human meniscus cells is enhanced after culture with fibroblast growth factor 2 and is further stimulated by hypoxia. Arthritis Res Ther. 2006;8:R61. doi: 10.1186/ar1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adesida AB, Grady LM, Khan WS, Millward-sadler SJ, Salter DM, Hardingham Human meniscus cells express hypoxia inducible factor-1alpha and increased SOX9 in response to low oxygen tension in cell aggregate culture. Arthritis Res Ther. 2007;9:R69. doi: 10.1186/ar2267. [DOI] [PMC free article] [PubMed] [Google Scholar]