Abstract
We investigated a new cryopreservation method using xenon, a clathrate-forming gas, under medium pressure (100psi). The objective of the study was to determine whether this cryostasis protocol could protect cardiac mitochondria at cryogenic temperatures (below 100 degrees Celsius).We analyzed transmission electron microscopy images to obtain information about changes in mitochondrial morphology induced by cryopreservation of the hearts. Our data showed absence of mitochondrial swelling, rupture of inner and outer membranes, and leakage of mitochondrial matrix into the cytoplasm after applying this cryostasis protocol. The electron microscopy results provided the first evidence that a cryostasis protocol using xenon as a clathrate-forming gas under pressure may have protective effects on intracellular membranes. This cryostasis technology may find applications in developing new approaches for long-term cryopreservation protocols.
Keywords: Electron microscopy, cardiac tissue, mitochondria, cryopreservation, cryostasis, xenon, high pressure, clathrates, vitrification, tissue banking, organ banking
Introduction
The cryopreservation of organs became an active area of research in the 1950s as a result of the discovery of the cryoprotective properties of glycerol by Polge et al in 1949 [1]. Over the ensuing four decades of research in this area, the advantages of vitrification, or ice-free cryopreservation, have become apparent [2]. Despite successful vitrification of human ova and blood vessels, this method was not successful for cryopreservation of mammalian internal organs because of its toxicity [3, 4].
Clathrate hydrates are a class of solids in which gas molecules occupy “cages” made up of hydrogen-bonded water molecules. These “cages” are unstable when empty, collapsing into conventional ice crystal structure, but they are stabilized by the inclusion of the gas molecule within them. Most low molecular weight gases (including O2, N2, CO2, CH4, H2S, Ar, Kr, and Xe) will form a hydrate under some pressure-temperature conditions [5].
The idea of using xenon as a clathrate-forming substance for suspended animation is very old and was first described by Prehoda [6]. Rodin at al (1984) studied the possibility of using xenon as a cryopreservative agent in experiments in vitro by analyzing protein structure in the presence of xenon clathrate [7].
The noble gas xenon, which can form clathrates under low pressure (above 40 psi) can be an alternative method for cryopreservation of large biological objects, including internal organs. Preliminary tests of the cryostasis protocol (using a gaseous mixture of xenon-nitrogen-oxygen at +5°C under high pressure) on Planaria showed xenon clathrate formation in the animal tissue and the reversibility of this procedure (unpublished data).
The objective of the present study was to determine whether the cryostasis protocol using xenon under pressure has protective effects on the intracellular membranes of cardiomyocytes.
Material and Methods
Animals
The experiments were performed on 10 albino mice (Mus musculus) (male, 6–8 weeks old; 25±2gm body weight). The animals were euthanized with Halothane (Sigma-Aldrich; Cat. #B4388) according to the procedures recommended by the Panel on Euthanasia of the American Veterinary Association. The hearts were immediately dissected and put into Corning® cryo-vials (without the caps) containing 200μL of oxygenated modified Krebs-Henselite solution (118.4mM NaCl, 25mM NaHCO3, 4.7mM KCl, 1.6mM KH2PO4, 0.6mM MgSO4, 2.5mM CaC12, 11mM glucose) to protect the cardiac tissue from drying.
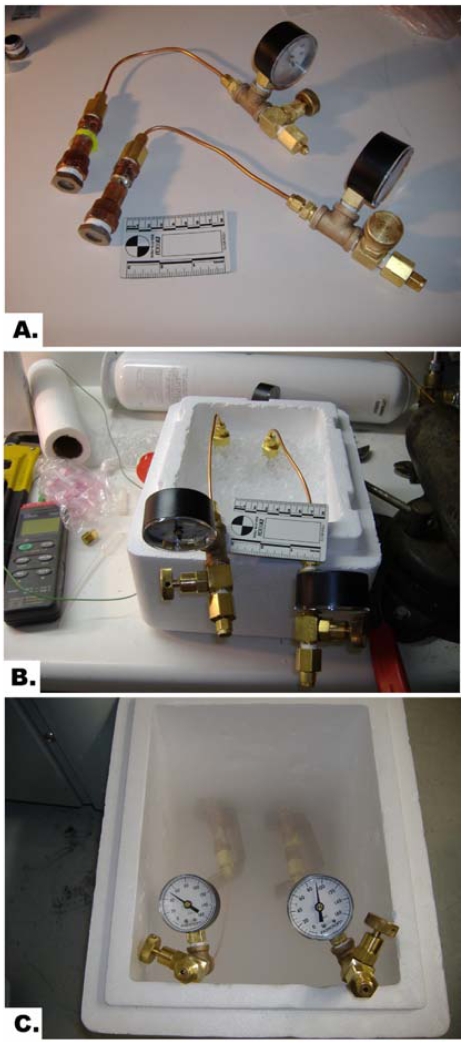
The cryo-vials were put inside the pressure chamber (Figure 1A), pressurized with xenon-oxygen mixture (xenon – 90psi, oxygen – 10psi) and left on ice for 15 minutes as shown in Figure 1B. The control samples were pressurized with nitrogen-oxygen mixture (90psi and 10psi, respectively). Then the pressure chambers were put on stands in a styrofoam container over liquid nitrogen for gradual cooling for 15 minutes and subsequently were immersed in liquid nitrogen for another 15 minutes (Figure 1C).
Figure 1.
A. High-pressure cryostasis chambers. B. Cooling down the cardiac samples in the pressurized cryostasis chambers on ice. C. Gradual cooling down the cardiac samples in the pressurized cryostasis chambers in vapors of liquid nitrogen.
The pressure chambers with the whole murine hearts were warmed at room temperature for 15 minutes and opened. The temperature of the samples was measured using the Sper Scientific 800024 Multi-Input Thermocouple Thermometers (Cat. #K-94461-35 from Cole-Parmer Instrument Company, Vernon Hills, Illinois) with the flexible Teflon®-insulated-wire thermocouple probe.
Electron Microscopy
The heart tissue specimens (3×3×3mm) were cut from the apex and immediately fixed in cold (+4°C) 2.5% glutaraldehyde in 0.1M Sorensen's sodium buffer (Electron Microscopy Sciences; Cat. #15980). The cardiac samples were thawed at room temperature to 0°C before putting them in a fixative to avoid any fixation artifacts. Samples were post-fixed with 1% osmium tetroxide in the same buffer for one hour at room temperature, and then washed three times with the same buffer and three times with distilled water. Samples were stained en bloc with 0.5% uranyl acetate for two hours at room temperature, then washed three times with distilled water and gradually dehydrated with ethanol before being transferred to acetone. Spurr's epoxy resin was used for infiltration and blocks were polymerized for 48 hours at 60°C. Thin-sections were cut on a Leica Ultracut R microtome and post-stained with uranyl acetate and lead citrate. Samples were observed and recorded at 80kV accelerating voltage on a Philips CM-12 Scanning Transmission Electron Microscope (the Netherlands) at the Electron Microscopy and W.M. Keck Bioimaging Laboratory at Arizona State University.
Images were recorded digitally on a Gatan 791 charge-coupled device camera (Gatan, Inc., Warrendale, PA). Densitometry of the mitochondrial matrix was performed using ImageJ software (http://rsb.info.nih.gov/ij/). Density of the mitochondrial matrix in mitochondria was normalized against the ribosomes of the rough endoplasmic reticulum.
Statistical analysis was done by Student's t-test; values are mean ± SD. A p-value of less than 0.05 was considered statistically significant.
Results
During cooling down the pressurized chambers in liquid nitrogen vapor, the pressure dropped due to gas contraction and minor leakage.
The temperature of the cardiac samples was −94±2.5°C just after opening the chamber.
The hardness of the cardiac tissue in the chamber filled with xenon-oxygen mixture under pressure was different in comparison with the control cardiac samples which were straight frozen in liquid nitrogen. The cardiac samples were firm and were easily cut with a blade, but not stone-hard as in the control sample, which was straight frozen in liquid nitrogen under normal pressure conditions.
To investigate the extent of the damage of the cardiac tissue after application of the cryostasis protocol based on the use of the pressurized mixture of xenon and oxygen and the controls frozen under the pressurized nitrogen-oxygen mixture, transmission electron microscopy of the cardiac tissue was performed.
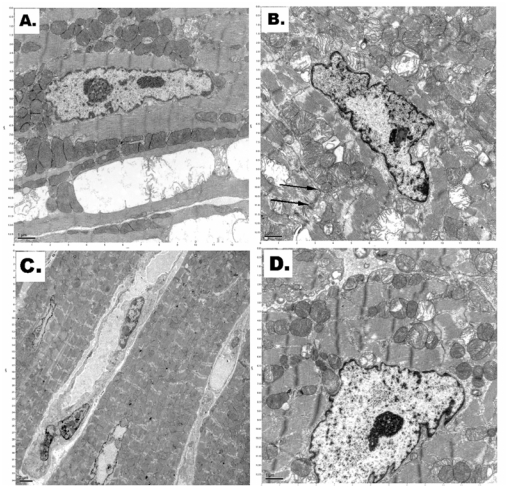
Electron microscopy of the cardiac tissues showed obvious and significant differences in the cardiomyocytes' mitochondria structures from the different cryopreservation protocols as shown in Figure 2.
Figure 2.
A. Transmission electron micrograph of typical normal mitochondria in a cardiomyocyte (scale bar – 1.0μm). B. Irreversible cellular membrane damage (cytoplasmic membrane invaginations as indicated by black arrows) and disruption of myofibrils in a cardiomyocyte after straight freezing in liquid nitrogen under normal pressure (scale bar – 1.0μm). C. Decreased density of the mitochondrial matrix in a cardiomyocyte after incubation at high-pressure nitrogen-oxygen gas mixture and cooling in liquid nitrogen (scale bar – 2.0μm). D. Preserved mitochondria in a cardiomyocyte after applied cryostasis (incubation in high-pressure xenon-oxygen mixture followed by cooling in liquid nitrogen) (scale bar – 1.0μm).
As shown in Figure 2, there was the presence of irreversible cellular membrane damage (such as disruption of myofibrils and cytoplasmic membrane invaginations) of the cardiomyocytes, which were straight frozen in liquid nitrogen under normal pressure (Figure 2B).
There was decreased density of the mitochondrial matrix in cardiomyocytes cryopreserved at high pressure nitrogen-oxygen gas mixture and cooling in liquid nitrogen (Figure 2C).
Relatively well-preserved mitochondria were present in cardiomyocytes of the cardiac samples after applied cryostasis protocol (incubation in high-pressure xenon-oxygen mixture followed by cooling in liquid nitrogen) (Figure 2D).
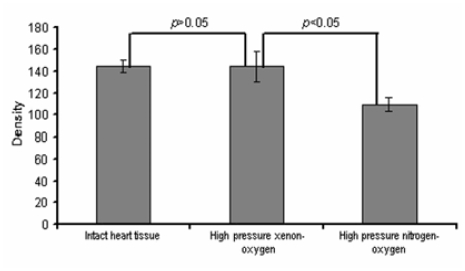
Comparative analysis of structural changes in mitochondria revealed a strong diminution in density of the mitochondrial matrix in the cardiac samples which were straight frozen in liquid nitrogen, compared with the treated cardiac samples (Figure 3).
Figure 3.
Density of the mitochondrial matrix in the cardiac samples cryopreserved under different conditions.
As shown in the histogram, the density of the mitochondrial matrix in the cardiac samples cryopreserved using a pressurized xenon-oxygen mixture (cryostasis protocol) was much higher (almost like in the intact cardiac tissue) than in mitochondria of cardiomyocytes in the samples, which were frozen in the pressurized nitrogen-oxygen mixture. A p-value of <.05 indicated a statistically significant difference between. Approximately 76% of mitochondria of the cardiac samples, which underwent the pressurized xenon cryostasis procedure, had the same density as the reference cardiac samples.
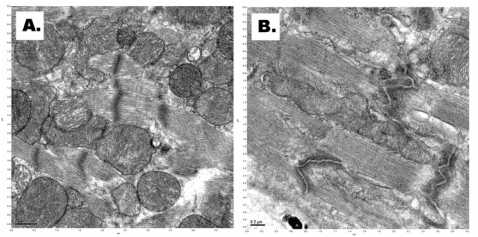
We did not see mitochondrial swelling, rupture of inner and outer membranes, and leakage of mitochondrial matrix into the cytoplasm after applying the cryostasis protocol based on using of pressurized xenon (Figure 4A). In the control cardiac samples (after straight freezing under high pressure in nitrogen-oxygen mixture at 100psi), electron microscopy demonstrated fragmentation of the mitochondrial inner membrane, rupture of inner and outer membranes, and leakage of mitochondrial matrix into the cytoplasm (Figure 4B).
Figure 4.
A. Normal structure of the mitochondria in a cardiomyocyte after cryostasis using pressurized xenon-oxygen mixture (scale bar – 0.5μm). B. Rupture of inner and outer membranes of mitochondria and leakage of mitochondrial matrix into the cytoplasm after incubation at high-pressure nitrogen-oxygen gas mixture and cooling in liquid nitrogen (scale bar – 0.2μm).
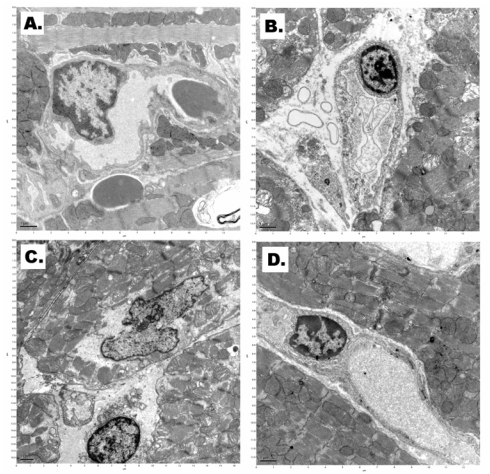
Electron microscopy showed diffuse endothelial cell damage marked perivascular space edema in the cardiac samples after straight freezing in liquid nitrogen under normal pressure (Figure 5B). Extensive capillary damage was in cardiac tissue after incubation at high-pressure nitrogen-oxygen gas mixture and cooling in liquid nitrogen as well (Figure 5C). Structure of capillary endothelial cells was relatively well preserved after applied cryostasis (incubation in high-pressure xenon-oxygen mixture followed by cooling in liquid nitrogen (Figure 5D).
Figure 5.
A. Transmission electron micrograph of typical cardiac capillary (scale bar – 1.0μm). B. Perivascular space edema in the cardiac sample after straight freezing in liquid nitrogen under normal pressure (scale bar – 1.0μm). C. Capillary damage after incubation at high-pressure nitrogen-oxygen gas mixture and cooling in liquid nitrogen (scale bar – 1.0μm). D. Intact capillary endothelial cell after applied cryostasis (incubation in high-pressure xenon-oxygen mixture followed by cooling in liquid nitrogen) (scale bar – 1.0μm).
Discussion
Living tissues cooled below the freezing point of water are damaged by the dehydration of the cells as ice is formed between the cells. The mechanism of freezing damage in living tissues has been elucidated by Renfret (1968) and Mazur (1984) [8, 9]. Ice formation begins in the intercellular spaces. The vapor pressure of the ice is lower than the vapor pressure of the solute water in the surrounding cells and as heat is removed at the freezing point of the solutions, the ice crystals grow between the cells, extracting water from them.
As the ice crystals grow, the volume of the cells shrinks, and the cells are crushed between the ice crystals. Additionally, as the cells shrink, the solutes inside the cells are concentrated in the remaining water, increasing the intracellular ionic strength and interfering with the organization of the proteins and other intracellular structures. Eventually, the solute concentration inside the cells reaches the eutectic and freezes. The final state of frozen tissues is pure ice in the former extracellular spaces, and inside the cell membranes a mixture of concentrated cellular components in ice and bound water. In general, this process is not reversible to the point of restoring the tissues to life, although there are occasional exceptions observed in nature (vitrifying polyols (i.e., insects, amphibians), thermal hysteresis proteins (insects, fish) [10, 11].
Most lesions occur during re-warming and reperfusion of the cryopreserved biological tissues/organs and the process of its development is time-consuming [8]. These changes included condensation of chromatin, large lipid droplets, and partly disrupted plasma membrane and can be seen on electron microscopy (which can be a consequence of the osmotic excursions incurred during a freeze-thaw cycle; leakage of mitochondrial matrix can trigger apoptosis as well). Damage by freezing is caused, besides temperature stress owing to decrease in temperature itself, through the following processes: irreversible change of biological membrane by dehydration from the cells and surface of the membranes caused by freezing process, destruction by loss in selective permeability or physical deformation and death of cell. Light microscopy doesn't show early freezing damage to the cells.
The cryostasis technology is based on the concept of forming clathrates (xenon, sulfur hexafluoride, Freon-23, etc.) within the cells, thus reducing dehydration. Biological tissues can be saturated with the clath rate-forming gas(es) by diffusion or perfusion at the appropriate pressure in the range 1–50 bars at a temperature above clath rate-forming temperature. After saturation, the tissue is cooled, first below the clathrate-forming temperature, but above the freezing point of water, then to a temperature where the clathrates are metabolically stable at ambient pressure, and the pressure allowed to go to ambient. The tissue is then gradually cooled down to some appropriate temperature at normal atmospheric pressure and stored an indefinite time.
This cryostasis method may protect tissues by retention of water inside the cells by clathrate formation of the water with the introduced gases, limiting the formation of ice outside the cells.
Xenon is a nearly ideal clathrate-forming gas, forming clathrates above 0°C under relatively low pressure (two atmospheres) [12, 13]. Xenon has high skin permeability as well [14]. Even an early explanation for xenon anesthesia given by Pauling (1961) and Miller (1961) suggested that clathrate hydrate structures encapsulating the rare gas atoms are formed near synapses, impeding interneuronal transmission [15, 16].
Using xenon under high pressure (which is highly permeable) may prevent cells from freezing damage caused by intracellular dehydration. Our results also showed absence of mitochondrial swelling, rupture of inner and outer membranes, and leakage of mitochondrial matrix into the cytoplasm after applying the cryostasis protocol in comparing with freezing under high pressure of nitrogen. Intercellular freezing causes damage, not so much during the freezing process but rather in the course of thawing, during which re-crystallization can occur [17]. Xenon clathrates are easily broken as the pressure drops, without intracellular water recrystallization (compared to damage after intracellular ice crystal formation).
Cardiomyocytes serve as the best model to study dynamics of mitochondrial damage in clinical and experimental pathology.
Safiulina et al (2006) demonstrated that loss of mitochondrial membrane potential leads to mitochondrial swelling [18]. It is well known that permanent ischemia causes loss of matrix density, and this is associated with mitochondrial swelling [19]. Another important sign of severe cellular damage is invagination of the cytoplasmic membrane. This was seen in our control cardiac samples after straight freezing in liquid nitrogen. This morphologic finding is especially seen after applying the cryopreservation procedures and indicates membrane damage [20]. Mitochondrial volume homeostasis is a housekeeping function that is essential for maintaining the structural integrity of the organelle [21]. Mitochondrial swelling is also one of the key players in cytochrome c release associated with apoptotic cell death.
Our study used electron microscopy of the cryopreserved cardiac samples because this method can easily detect any ultrastructural morphologic changes after applying cryopreservation protocols. We did not compare our work with vitrification protocols. Vitrification requires the impregnation of biological tissues with high concentrations of cryoprotective chemicals that promote the vitreous state, but these are somewhat toxic. While vitrification prevents the hazardous effect of ice formation, new potential mechanisms of injury associated with the amorphous state have been identified [22]. Devitrification (ice formation during warming) is another major obstacle to successful organ vitrification.
We hypothesized that xenon clathrates could protect cardiomyocyte mitochondria from freezing damage. Although xenon clathrate formation can be accomplished above zero Celsius, we wanted to see the extent of intracellular membrane damage after exposure to cryogenic temperatures. We showed that the mitochondrial membranes were less damaged after the applied cryostasis protocol compared to the control cardiac samples, which were straight frozen in liquid nitrogen. Cryopreservation under a pressurized nitrogen-oxygen mixture also did not have any benefits. Measuring the concentration of cardiolipin (the cellular distribution of which is restricted to mitochondria; it serves as a sign of severe mitochondrial membrane damage according to Virag et al (1998) during testing the cryostasis protocols can be easily done and is warranted in the future study of this cryostasis procedure [23].
Acknowledgments
We acknowledge Dr. Robert Roberson (School of Life Sciences, Arizona State University, Tempe, AZ) for electron microscopy. This research project was supported by Innovative Biological Preservation Technologies LLC, Scottsdale, AZ 85262.
References
- 1.Polge C, Smith AU, Parkes AS. Revival of spermatozoa after vitrification and dehydration at low temperatures. Nature. 1949;164:666. doi: 10.1038/164666a0. [DOI] [PubMed] [Google Scholar]
- 2.Fahy GM, Wowk B, Wu J, Phan J, Rasch C, Chang A, Zendejas E. Cryopreservation of organs by vitrification: perspectives and recent advances. Cryobiology. 2004;48:157–178. doi: 10.1016/j.cryobiol.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Song YC, Khirabadi BS, Lightfoot FG, Brockbank KGM, Taylor MJ. Vitreous cryopreservation maintains the function of vascular grafts. Nat Biotechnol. 2000;18:296–299. doi: 10.1038/73737. [DOI] [PubMed] [Google Scholar]
- 4.Karlsson JO. Cryopreservation: freezing and vitrification. Science. 2002;296:655–656. doi: 10.1126/science.296.5568.655d. [DOI] [PubMed] [Google Scholar]
- 5.Englezos P. Clathrate hydrates. Ind Eng Chem Res. 1993;32:1251–1274. [Google Scholar]
- 6.Prehoda RW, editor. Suspended Animation. Philadelphia: Chilton Book Co.; 1969. pp. 81–86. [Google Scholar]
- 7.Rodin VV, Isangalin FSH, Volkov VYA. Structure of protein solutions in a presence of xenon clathrate. Navukova Domka, Kiev: Cryobiology & Cryo-Medicine; 1984. pp. 3–7. (in Russian) [Google Scholar]
- 8.Renfret AP. Cryobiology: some fundamentals in surgical context. In: Rand RW, Renfret AP, von Leden H, editors. Cryosurgery. Springfield, IL: 1968. pp. 112–115. [Google Scholar]
- 9.Mazur P. Freezing of living cells: mechanisms and implications. Am J Physiol. 1984;247:125–142. doi: 10.1152/ajpcell.1984.247.3.C125. [DOI] [PubMed] [Google Scholar]
- 10.Fletcher GL, Hew CL, Davies PL. Antifreeze proteins of teleost fishes. Annu Rev Physiol. 2001;63:359–590. doi: 10.1146/annurev.physiol.63.1.359. [DOI] [PubMed] [Google Scholar]
- 11.Graham LA, Liou YC, Walker VK, Davies PL. Hyperactive antifreeze protein from beetles. Nature. 1997;388:727–728. doi: 10.1038/41908. [DOI] [PubMed] [Google Scholar]
- 12.Istomin VA, Yakushev VS. Gas Hydrates in Nature. Moscow, Russia: Nedra; 1992. p. 32. (in Russian) [Google Scholar]
- 13.Ohgaki K, Sugahara T, Suzuki M, Jindai H. Phase behavior of xenon hydrate system. Fluid Phase Equilibria. 2000;175:1–6. [Google Scholar]
- 14.Pauling L. A molecular theory of general anesthesia. Science. 1961;134:15–21. doi: 10.1126/science.134.3471.15. [DOI] [PubMed] [Google Scholar]
- 15.Miller S. A theory of gaseous anesthetics. Proc Natl Acad Sci USA. 1961;47:1515–1524. doi: 10.1073/pnas.47.9.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mirtov AV, Tarashukhin VR, Peschanskii KI. [Permeability of the skin for xenon absorption] Med Radiol (Mosk) 1973;18:26–28. (in Russian) [PubMed] [Google Scholar]
- 17.Acker JP, McGrann LE. Protective effect of intracellular ice during freezing? Cryobiology. 2003;46:197–202. doi: 10.1016/s0011-2240(03)00025-7. [DOI] [PubMed] [Google Scholar]
- 18.Safiulina D, Veksler V, Zharkovsky A, Kaasik A. Loss of mitochondrial membrane potential is associated with increase in mitochondrial volume: physiological role in neurons. J Cell Physiol. 2006;206:347–353. doi: 10.1002/jcp.20476. [DOI] [PubMed] [Google Scholar]
- 19.Schild L, Huppelsberg J, Kahlert S, Keilhoff G, Reiser G. Brain mitochondria are primed by moderate Ca2+ rise upon hypoxia/reoxygenation for functional breakdown and morphological disintegration. J Biol Chem. 2003;278:25454–25460. doi: 10.1074/jbc.M302743200. [DOI] [PubMed] [Google Scholar]
- 20.Popelková M, Chrenek P, Pivko J, Makarevich AV, Kubovicová E, Kacmárik J. Survival and ultrastructure of gene-microinjected rabbit embryos after vitrification. Zygote. 2005;13:283–293. doi: 10.1017/S096719940500331X. [DOI] [PubMed] [Google Scholar]
- 21.Kaasik A, Safiulina D, Zharkovsky A, Veksler V. Regulation of mitochondrial matrix volume. Am J Physiol Cell Physiol. 2007;292:C157–163. doi: 10.1152/ajpcell.00272.2006. [DOI] [PubMed] [Google Scholar]
- 22.Fahy GM. Biological effects of vitrification and devitrification. In: Pegg DE, Karow AM Jr, editors. The Biophysics of Organ Preservation. New York: Plenum Publishing Corp.; 1987. pp. 265–297. [Google Scholar]
- 23.Virag L, Salzman AL, Szabo C. Poly(ADP-ribose) synthetase activation mediates mitochondrial injury during oxidant-induced cell death. J Immunol. 1998;161:3753–3759. [PubMed] [Google Scholar]