Abstract
It has been documented that some tissues, such as salivary gland, liver, cardiac and skeletal muscles and kidney, have high level endogenous biotin or endogenous avidin binding activity (EABA). Limited data is available on EABA in renal cell neoplasms. A tissue microarray (TMA) was constructed that included oncocytoma (n=30), chromophobe renal cell carcinoma (RCC) (n=18), clear cell RCC (n=45), clear cell RCC with granular/eosinophilic (G/E) features (n=19), papillary RCC (n=21), papillary RCC with G/E features (n=29) and benign renal tissues (n=31). The TMA slides were stained with or without biotin blocker and analyzed using the automated cellular imaging system (ACIS®). Without biotin blocker, a high positive rate of EABA was found in oncocytoma (56/60, 93%) and normal renal tubules (46/60, 77%). A moderate positive rate of EABA was found in clear cell and papillary RCCs with G/E features (13/39, 33% and 19/55, 35%, respectively). Chromophobe RCC and RCC without G/E features had essentially no EABA. With biotin blocker, benign renal tissue and clear cell RCC were negative for EABA; but a significant number of renal oncocytoma (29/60, 48%) and a few papillary RCC with G/E features (5/52, 10%) remained positive for EABA. In conclusion, high EABA may be used to differentiate oncocytoma from chromophobe RCC, and the staining results must be interpreted with caution when avidin-biotin detection system is used in diagnosing renal neoplasms.
Keywords: Oncocytoma, endogenous avidin binding activity (EABA), chromophobe renal cell carcinoma
Introduction
Biotin is a member of the vitamin B family (B7) that is involved in the biosynthesis of fatty acids, gluconeogenesis, energy production, and the metabolism of the branched-chain amino acids and the de novo synthesis of purine nucleotides. Biotin is the coenzyme for four carboxylases and several synthetases: pyruvic acid carboxylase and propionyl CoA carboxylase, methyl-crotonyl carboxylase, and acetyl CoA carboxylase; carbamoyl phosphate synthetase I, carbamoyl phosphate synthetase II. The first four are localized in mitochondria, and the last two are in cytosol [1, 2]. Large amounts of endogenous biotin (EB) are present in the liver, kidney, pancreas, mammary gland, skeletal muscle, salivary gland and adipose tissue [3, 4].
Avidin-biotin complex (ABC) detection system is commonly used in immunohistochemical laboratories to increase target antigen detecting sensitivity. It has been well documented that high level of EB in some tissues results in a high level of EABA in formalin fixed paraffin-embedded tissue sections [5–8].
Recently, while performing a study on a group of renal tumors, we noticed a significantly higher level of EABA in renal oncocytoma compared to other renal cell neoplasms. In this study, we extended our observation by comparing the staining quality of EABA in renal cell neoplasms using 7 different antigen retrieval methods and investigated whether high EABA is unique to oncocytoma among renal cell neoplasms and whether high EABA will pose a problem for immunohistochemical diagnosis of renal cell neoplasms.
Materials and Methods
A renal cell neoplasm tissue microarray (TMA) was constructed using formalin fixed, paraffin embedded tissue blocks from the pathology archive at University of Wisconsin-Madison. The TMA included oncocytoma (n=30), chromophobe renal cell carcinoma (chrRCC, n=18), clear cell RCC (clRCC, n=45), clRCC with granular/eosinophilic(G/E) features (clRCC G/E, n=19), papillary RCC (pRCC, n=21), pRCC with G/E features (pRCC G/E, n=29) and benign renal tissues (n=31). The pathological diagnoses were made according to the Heidelberg classification for renal tumors [9], and verified by a genitourinary pathologist (WH). Duplicate cores were obtained from each tumor/tissue. The core size was 0.6 mm in diameter. The cores were arranged 0.2 mm apart vertically and horizontally.
Three TMA slides were studied for EABA in renal neoplasms. BioCare Bull's Eye buffer (BioCare Medical, Concord, CA) with pressure cooking for 3 minutes was used for antigen retrieval. No target-specific primary antibody was applied to any of these three TMA slides. The three TMA slides were stained as follows: Slide #1 was stained according to the standard protocol for negative controls on Ventana Autostainer Benchmark XT (Ventana Medical, Inc, Tucson, AZ) with application of biotin blocker (endogenous biotin blocking kit, Ventana). Slides #2 and #3 were stained manually with BioCare avidin-biotin detection kit. Endogenous peroxidase (EP) blocker was applied to slides # 2 and #3 at room temperature for 10 minutes. Then BioCare background terminator was applied to the slides for 15 minutes at room temperature. No biotinylated secondary antibody was applied to slide #2 before adding avidin-horseradish peroxidase (HRP). No avidin-HRP was applied to slide #3. Immunohistochemical reactions were developed with diaminobenzidine as the chromogenic substrate. Hematoxylin was used as the counterstain. ACIS® (Clarient, Inc, Alisa Viego, CA), was used to evaluate the staining results [10, 11]. The core staining intensities (INT) varied from 0 to 255; core staining areas (S %) ranged from 0 to 100%. A composite score (CS = INT × S %) for each core is obtained and analyzed. A positive threshold was defined as CS = 25% of maximal INT × 15% (staining area) = 10.
Seven different antigen retrieval conditions were also tested on TMA slides to compare their effect on EABA in renal neoplasms: BioCare Bull's Eye (pressure cooking, 2 minutes), BioCare Borg (pressure cooking, 2 minutes), BioCare Diva (pressure cooking, 2 minutes), BioCare EDTA (pressure cooking, 2 minutes), Ventana (VMS) Cell Conditioning Solution 1 (CC1, online 37 C, 60 minutes), VMS Cell Conditioning Solution 2 (CC2, online 37°C, 60 minutes), and VMS Protease 1 (online 37°C, 4 minutes). After antigen retrieval, the TMA slides were manually stained with BioCare streptavidin-biotin detection kit, without using biotin block. Briefly, EP blocker was applied to all slides for 10 minutes at room temperature. Then BioCare background terminator was applied to all slides for 15 minutes at room temperature, and then streptavidin-HRP was applied to all slides for 30 minutes at room temperature. Hematoxylin was used as counterstain. Cores with poor quality were excluded from analysis. In this study, less than 5% of the cores in each group were disqualified.
Results
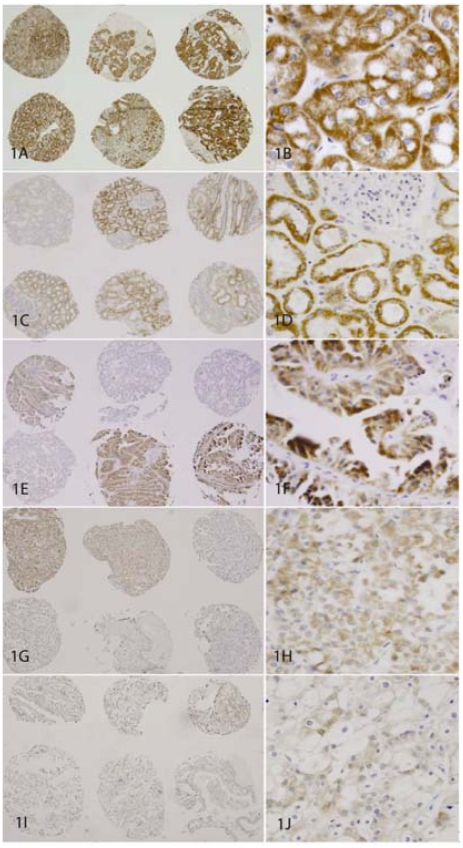
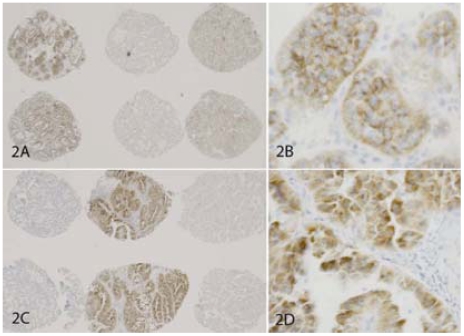
The results are summarized in Table 1. Without EB blocker (slide #2), a high positive rate of EABA was found in oncocytoma (56/60, 93%, Figures 1A and 1B) and normal renal tubules (46/60, 77%, Figures 1C and 1D). A moderate positive rate of EABA was found in clear cell and papillary RCCs with G/E features (pRCC G/E, 19/55, 35%, Figures 1E and 1F; clRCC G/E, 13/39, 33%; Figures 1G and 1H). Two cores of chromophobe RCC showed weak positive EABA (2/36, 6%, Figures 1I and 1J); and RCCs without G/E features had essentially no EABA. With EB blocker (slide #1), the EABA was removed almost completely from benign renal tissue, chrRCC and clear cell RCC; but a significant amount of EABA remained in renal oncocytoma (29/60, 48%, Figures 2A and 2B), and in a few pRCC with G/E features (pRCC G/E, 5/52,10%, Figures 2C and 2D).
Table 1.
EABA in renal neoplasms
| EABA Staining Positivity (%) (CS>=10) | EABA AQUATMScore (mean CS of positive cores) | ||||
|---|---|---|---|---|---|
| Diagnosis | N | w/o EB blocker | w/ EB blocker | w/o EB blocker | w/ EB blocker |
| Benign | 31 | 77 (46/60)* | 0 (0/62) | 37.4 | |
| Onco | 30 | 93 (56/60) | 48 (29/60)* | 64.9 | 26.2 |
| chrRCC | 18 | 6 (2/36) | 0 (0/34) | 25.8 | |
| clRCC | 45 | 0 (0/87) | 0 (0/88) | ||
| pRCC | 21 | 0 (0/39) | 0 (0/39) | ||
| clRCC G/E | 19 | 33 (11/39) | 0 (0/38) | 55.6 | |
| pRCC G/E | 29 | 35 (19/55) | 10 (5/52) | 65.3 | 37.6 |
EABA, endogenous avidin binding activity; EB, endogenous biotin; RCC, renal cell carcinoma; Onco, oncocytoma; chr, chromophobe; cl, clear cell; p, papillary; G/E, granular/eosinophilic; AQUA™, automated quantitative analysis; N, number of cases; CS, composite score;
numbers in parentheses = positive cores over total cores evaluated in each group; w/, with; w/o, without.
Figure 1.
Endogenous avidin binding activity (EABA) in renal neoplasms. A pair of panels (objective magnification 4× and 40×) for each group are shown. EABA was detected in 93% of oncocytomas (1A and 1B); 77% of normal kidney tubules (1C and 1D); in 35% of papillary RCCs with G/E features (1E and 1F); in 33% of clear cell RCCs with G/E features (1G and 1H). Weakly positive EABA was detected in two cores of chromophobe RCC (1I and 1J).
Figure 2.
Endogenous biotin blocker (endogenous biotin blocking kit, Ventana Medical, Inc) has limited power in blocking EABA in renal neoplasms with high EB content. A pair of panels (objective magnification 4× and 40×) for each group are shown. With EB blocker, EABA was detected in 48% of oncocytomas (2A and 2B) and in 10% of papillary RCCs with G/E features (2C and 2D).
Similar staining pattern was also observed in all 7 TMA slides with the aforementioned different antigen retrieval conditions. We found that the EABA staining quality varied when different antigen retrieval conditions were applied. Antigen retrieval with BioCare Borg, BioCare EDTA, VMS CC1, and VMS CC2 showed stronger signal and higher background staining. Strong staining signal yet less background was observed in slides pretreated with BioCare Bull's eye, BioCare Diva and VMS Protease 1.
Discussion
Our data confirmed previous reports [3, 4, 6] that kidney tissue has high level endogenous biotin. We found that the Ventana endogenous biotin blocking kit is efficient in removing endogenous biotin from normal renal tissue. However, it has limited blocking effect on some renal neoplasms that have high EB, such as oncocytoma and renal cell carcinoma with granular/eosinophilic features. This finding indicates that a false positive result may be produced using biotin-avidin complex detection system in diagnosing renal neoplasms, which could potentially create a problem for pathologists, especially when only a small amount of tissue, such as needle core biopsy, is available for diagnosis.
Not surprisingly, high level EABA was found in renal oncocytoma which has abundant cytoplasmic mitochondria [12–14], and very low level of EABA was found in chromophobe RCC which is rich in cytoplasmic microvesicles [13]. As we know that chromophobe RCC sometimes presents as an imitator of oncocytoma, especially when there is only limited tissue to evaluate. Differentiating chromophobe RCC from oncocytoma can be a daunting task for pathologists especially when a kidney sparing surgery is necessary. Hale's colloidal iron remains the most commonly used stain so far in differentiating chromophobe RCC from oncocytoma, yet it is often a technical and interpretive challenge [13, 15]. Therefore, markers capable of distinguishing chromophobe RCC from oncocytoma have been explored [10, 16–18]. Based on our findings, we propose that EABA may be useful as a marker in differentiating chromophobe RCC from oncocytoma. We believe EABA as a marker is advantageous, as the detection reagents are readily available, the test of EABA is easier to perform, and cost-effective, over other proposed antibody-based markers such as S100A1 [19], ankyrin-repeated protein with a proline-rich region (ARPP) [20], caveolin-1 [17], CD63 [21], and claudin-7 [22]. However, since EABA is also present in some clear cell and papillary RCCs with granular/eosinophilic features, additional markers that are distinctive for clear cell or papillary RCCs such as RCCma, CD10, CK7 and vimentin should be also included to make a differential diagnosis [23, 24].
ACIS® is a robust system and is precise and high throughput in quantifying core staining intensities of a TMA. However, it lacks accuracy sometimes, as it does not discriminate real staining signals from other similar pigments, such as hemosiderin. We found that high composite scores were produced from a few tissue cores of papillary RCC with abundant hemosiderin yet with minimal or without EABA. Therefore, validation of the results by correlating staining morphology with composite score is necessary to avoid robotic bias.
In conclusion, high level of EABA was present in oncocytoma whereas little EABA was found in chromophobe RCC. We believe EABA could be used as a marker in differentiating oncocytoma from chromophobe RCC. When biotin-avidin detection system is used in diagnosing renal cell neoplasms, false positive staining may result from high EB content in some tumors such as oncocytoma, clear cell and papillary RCC with granular/eosinophilic features. Antigen retrieval with BioCare Bull's eye, BioCare Diva and VMS Protease 1 produced less background staining when EABA was used in differentiating oncocytoma from chromophobe RCC.
Acknowledgments
This work was supported by Department of Pathology and Laboratory Medicine TRIP laboratory at the University of Wisconsin-Madison.
References
- 1.McCormick DB. Biotin. Nutr Rev. 1975;33:97–102. doi: 10.1111/j.1753-4887.1975.tb07426.x. [DOI] [PubMed] [Google Scholar]
- 2.Depeint F, Bruce WR, Shangari N, Mehta R, O'Brien PJ. Mitochondrial function and toxicity: role of the B vitamin family on mitochondrial energy metabolism. Chem Biol Interact. 2006;163:94–112. doi: 10.1016/j.cbi.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 3.Patard JJ, Leray E, Rioux-Leclercq N, Cindolo L, Ficarra V, Zisman A, De La Taille A, Tostain J, Artibani W, Abbou CC, Lobel B, Guille F, Chopin DK, Mulders PF, Wood CG, Swanson DA, Figlin RA, Belldegrun AS, Pantuck AJ. Prognostic value of histologic subtypes in renal cell carcinoma: a multicenter experience. J Clin Oncol. 2005;23:2763–2771. doi: 10.1200/JCO.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 4.Bussolati G, Gugliotta P, Volante M, Pace M, Papotti M. Retrieved endogenous biotin: a novel marker and a potential pitfall in diagnostic immunohistochemistry. Histopathology. 1997;31:400–407. doi: 10.1046/j.1365-2559.1997.3020895.x. [DOI] [PubMed] [Google Scholar]
- 5.Parkin RK, Boeckh MJ, Erard V, Huang ML, Myerson D. Specific delineation of BK polyomavirus in kidney tissue with a digoxigenin-labeled DNA probe. Mol Cell Probes. 2005;19:87–92. doi: 10.1016/j.mcp.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 6.Zhou X, Wang P, Lu M, Liu L, Zhang Y, Zhang S, Chen G, Zhang C, Huang S. [The influence of heat-induced epitope retrieval on endogenous avidin-binding activity (EABA) and blocking of EABA in immunohistochemistry] Zhonghua Bing Li Xue Za Zhi. 2002;31:491–496. [PubMed] [Google Scholar]
- 7.Cauli A, Yanni G, Panayi GS. Endogenous avidin-binding activity in epithelial cells of the ducts of human salivary glands. Clin Exp Rheumatol. 1994;12:45–47. [PubMed] [Google Scholar]
- 8.Banerjee D, Pettit S. Endogenous avidin-binding activity in human lymphoid tissue. J Clin Pathol. 1984;37:223–225. doi: 10.1136/jcp.37.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kovacs G, Akhtar M, Beckwith BJ, Bugert P, Cooper CS, Delahunt B, Eble JN, Fleming S, Ljungberg B, Medeiros LJ, Moch H, Reuter VE, Ritz E, Roos G, Schmidt D, Srigley JR, Storkel S, van den Berg E, Zbar B. The Heidelberg classification of renal cell tumours. J Pathol. 1997;183:131–133. doi: 10.1002/(SICI)1096-9896(199710)183:2<131::AID-PATH931>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 10.van Gijssel HE, Divi RL, Olivero OA, Roth MJ, Wang GQ, Dawsey SM, Albert PS, Qiao YL, Taylor PR, Dong ZW, Schrager JA, Kleiner DE, Poirier MC. Semiquantitation of polycyclic aromatic hydrocarbon-DNA adducts in human esophagus by immunohistochemistry and the automated cellular imaging system. Cancer Epidemiol Biomarkers Prev. 2002;11:1622–1629. [PubMed] [Google Scholar]
- 11.Jiang Z, Wu CL, Woda BA, Iczkowski KA, Chu PG, Tretiakova MS, Young RH, Weiss LM, Blute RD, Jr, Brendler CB, Krausz T, Xu JC, Rock KL, Amin MB, Yang XJ. Alpha-methylacyl-CoA racemase: a multi-institutional study of a new prostate cancer marker. Histopathology. 2004;45:218–225. doi: 10.1111/j.1365-2559.2004.01930.x. [DOI] [PubMed] [Google Scholar]
- 12.van der Walt JD, Reid HA, Risdon RA, Shaw JH. Renal oncocytoma. A review of the literature and report of an unusual multicentric case. Virchows Arch A Pathol Anat Histopathol. 1983;398:291–304. doi: 10.1007/BF00583586. [DOI] [PubMed] [Google Scholar]
- 13.Skinnider BF, Jones EC. Renal oncocytoma and chromophobe renal cell carcinoma. A comparison of colloidal iron staining and electron microscopy. Am J Clin Pathol. 1999;111:796–803. doi: 10.1093/ajcp/111.6.796. [DOI] [PubMed] [Google Scholar]
- 14.Mazal PR, Stichenwirth M, Koller A, Blach S, Haitel A, Susani M. Expression of aquaporins and PAX-2 compared to CD10 and cytokeratin 7 in renal neoplasms: a tissue microarray study. Mod Pathol. 2005;18:535–540. doi: 10.1038/modpathol.3800320. [DOI] [PubMed] [Google Scholar]
- 15.Wu SL, Kothari P, Wheeler TM, Reese T, Connelly JH. Cytokeratins 7 and 20 immunoreactivity in chromophobe renal cell carcinomas and renal oncocytomas. Mod Pathol. 2003;15:712–717. doi: 10.1097/01.MP.0000017566.29755.8A. [DOI] [PubMed] [Google Scholar]
- 16.Huo L, Sugimura J, Tretiakova MS, Patton KT, Gupta R, Popov B, Laskin WB, Yeldandi A, Teh BT, Yang XJ. C-kit expression in renal oncocytomas and chromophobe renal cell carcinomas. Hum Pathol. 2005;36:262–268. doi: 10.1016/j.humpath.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Garcia E, Li M. Caveolin-1 immuno-histochemical analysis in differentiating chromophobe renal cell carcinoma from renal oncocytoma. Am J Clin Pathol. 2006;125:392–398. [PubMed] [Google Scholar]
- 18.Choi YD, Kim KS, Ryu S, Park Y, Cho NH, Rha SH, Jang JJ, Ro JY, Juhng SW, Choi C. Claudin-7 is highly expressed in chromophobe renal cell carcinoma and renal oncocytoma. J Korean Med Sci. 2007;22:305–310. doi: 10.3346/jkms.2007.22.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li G, Barthelemy A, Feng G, Gentil-Perret A, Peoc'h M, Genin C, Tostain J. S100A1: a powerful marker to differentiate chromophobe renal cell carcinoma from renal oncocytoma. Histopathology. 2007;50:642–647. doi: 10.1111/j.1365-2559.2007.02655.x. [DOI] [PubMed] [Google Scholar]
- 20.Shomori K, Nagashima Y, Kuroda N, Honjo A, Tsukamoto Y, Tokuyasu N, Maeta N, Matsuura K, Hijiya N, Yano S, Yokoyama S, Ito H, Moriyama M. ARPP protein is selectively expressed in renal oncocytoma, but rarely in renal cell carcinomas. Mod Pathol. 2007;20:199–207. doi: 10.1038/modpathol.3800730. [DOI] [PubMed] [Google Scholar]
- 21.Mete O, Kilicaslan I, Gulluoglu MG, Uysal V. Can renal oncocytoma be differentiated from its renal mimics? The utility of anti-mitochondrial, caveolin 1, CD63 and cytokeratin 14 antibodies in the differential diagnosis. Virchows Arch. 2005;447:938–946. doi: 10.1007/s00428-005-0048-6. [DOI] [PubMed] [Google Scholar]
- 22.Hornsby CD, Cohen C, Amin MB, Picken MM, Lawson D, Yin-Goen Q, Young AN. Claudin-7 immunohistochemistry in renal tumors: a candidate marker for chromophobe renal cell carcinoma identified by gene expression profiling. Arch Pathol Lab Med. 2007;131:1541–1546. doi: 10.5858/2007-131-1541-CIIRTA. [DOI] [PubMed] [Google Scholar]
- 23.Liu L, Qian J, Singh H, Meiers I, Zhou X, Bostwick DG. Immunohistochemical analysis of chromophobe renal cell carcinoma, renal oncocytoma, and clear cell carcinoma: an optimal and practical panel for differential diagnosis. Arch Pathol Lab Med. 2007;131:1290–1297. doi: 10.5858/2007-131-1290-IAOCRC. [DOI] [PubMed] [Google Scholar]
- 24.Pan CC, Chen PC, Ho DM. The diagnostic utility of MOC31, BerEP4, RCC marker and CD10 in the classification of renal cell carcinoma and renal oncocytoma: an immunohistochemical analysis of 328 cases. Histopathology. 2004;45:452–459. doi: 10.1111/j.1365-2559.2004.01962.x. [DOI] [PubMed] [Google Scholar]