Abstract
Fibrosing cholestatic hepatitis (FCH) is a rapidly progressive, sometimes fatal form of liver injury. Though originally reported in liver transplant recipients with recurrent hepatitis B, it has now been recognized frequently in chronic hepatitis B or C patients who are under immunosuppression. The histopathologic hallmarks in the liver include marked hepatocytic injury, severe cholestasis, and periportal and pericellular fibrosis. The pathogenesis is largely unknown. The aim of this review is to describe the spectrum of clinical conditions in which FCH occurs, common histopathologic findings, features unique to certain underlying diseases, factors to be considered in differential diagnosis, and our current understanding of pathogenesis of this disease.
Keywords: Viral hepatitis, hepatitis B virus, hepatitis C virus, liver transplantation, pathogenesis
Introduction
The term “fibrosing cholestatic hepatitis” (FCH) was first coined in 1991 to describe a severe, fulminant form of recurrent hepatitis B in liver transplant recipients [1, 2], although cases with similar clinicopathologic presentations had been recorded earlier [3, 4]. It has also been described under other names, such as “fibrosing cytolytic liver failure” [5] and “fibroviral hepatitis” [6]. Clinically, FCH is characterized by rapid and progressive deterioration in graft functions, evidenced by severe jaundice, coagulopathy, encephalo-pathy and death within 4–6 weeks of onset [2]. Laboratory tests usually show elevation of serum bilirubin level, prolonged prothrombin time, and mild to moderate increase in serum transaminases. The initially reported cases were all rapidly progressive and fatal, and were associated with high levels of viral antigen expression in the livers [2, 6, 7]. The histopathologic changes are characterized by marked hepatocyte ballooning (swelling), intracellular and canalicular cholestasis, and periportal and/or perisinusoidal collagen deposition.
With increasing awareness to clinicians and pathologists, the same condition has been subsequently reported with a broad spectrum of background diseases, including recurrent hepatitis C virus (HCV) infection following liver transplantation [8], kidney transplantation [9–11], kidney transplantation in hepatitis B virus (HBV)-infected recipients [12, 13], new HCV infection after kidney transplantation [14], and HCV/human immunodeficiency virus (HIV) coinfection [15–18]. Less frequently, FCH was seen in HBV patients undergone cardiac transplantation [19], patients with HBV/HIV coinfection [20], and HBV carriers during post-chemotherapy remission. Rarely, FCH was attributed to cytomegalovirus in kidney transplant recipients in the absence of HBV or HCV infection [21]. Despite the diverse background and complexity of clinicopathologic patterns of the disease, it has become evident that occurrence of FCH is underlined by the common thread of severe immunosuppression.
In one of the early series of analysis, of 29 HBV patients who underwent liver transplantation with a long-term follow up for reinfection, 6 patients developed histologically confirmed FCH and 4 additional patients had possible FCH, giving rise to an overall estimated rate of 34% for FCH as a form of recurrent HBV infection [2]. More careful analysis showed that a higher risk of developing severe progressive FCH is associated with older age of the recipients, more active inflammation and HBV core antigen (HBcAg) expression in the native livers [22]. Interestingly, coinfection with hepatitis D virus (HDV) appears to have a beneficial effect [2, 22]. Overall, in liver transplant recipients who have recurrent HBV infection, FCH accounts for approximately 33–42% of the cases [2, 6, 22].
Following liver transplantation for end-stage liver disease secondary to HCV infection, reinfection is almost universal. However, only rare cases of recurrent disease present in the form of FCH. The risk of developing FCH is increased if HCV patients are coinfected with HIV. It has been reported that FCH is responsible for 38% of the death in orthotopic liver transplant recipients who suffer from HCV/HIV coinfection [16]. Although FCH usually does not occur until 2–3 months after transplantation, it can occur much earlier in re-transplanted patients [3, 4, 22].
Histopathologic Features and Differential Diagnosis
The histopathologic features of FCH were first described in recurrent HBV hepatitis following liver transplantation. Most commonly, the liver shows periportal or pericellular/sinusoidal fibrosis, ballooning degeneration of the hepatocytes, prominent cholestasis, and minimal infiltration by inflammatory cells [1, 6, 22]. These are usually accompanied by distinctive viral cytopathic changes such as ground-glass transformation of hepatocytes owing to cytoplasmic accumulation of HBV surface antigen (HBsAg). The ground-glass hepatocytes are sometimes recognized by their cytoplasmic inclusions with a partial clear halo [6]. The ballooned cells, as demonstrated by electron microscopy (EM), have markedly dilated endoplasmic reticulum containing flocculent material [6]. Cholestasis is both intracellular and canalicular, sometimes accompanied by periportal ductular reaction. It has been noted that cholestasis is usually predominantly canalicular and centrilobular at the early stage of the disease, but pancellular at the late stage where the entire cytoplasm is swollen and bile-stained. This late pancellular cholestasis has been considered an ominous sign [22]. Fibrosis can range from subtle, thin periportal and perisinusoidal collagen fibers, to a diffuse periportal and panlobular pericellular distribution [22].
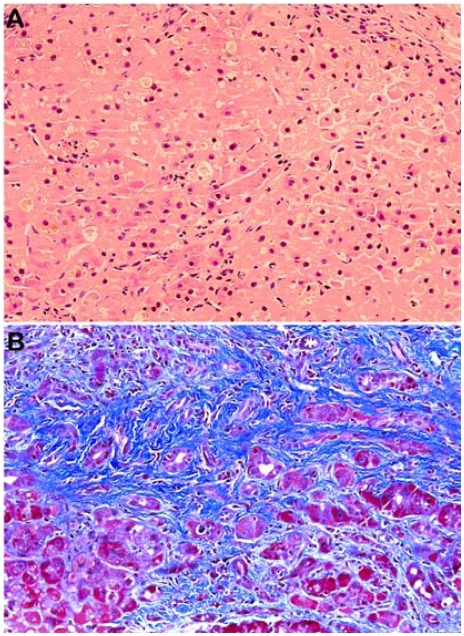
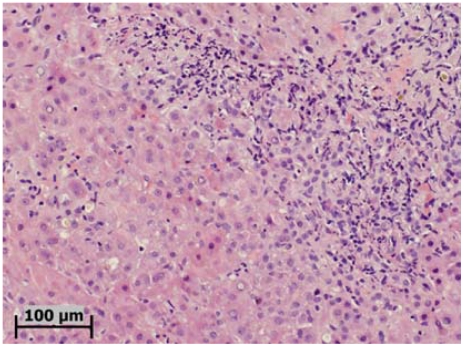
Similar to HBV-related cases, HCV-related FCH typically exhibits hepatocyte ballooning, intracellular and canalicular cholestasis, bile ductular reaction, bridging fibrosis and extensive pericellular/sinusoidal fibrosis (Figure 1). Many cases show marked parenchymal collapse, with confluent necrosis and rapid progression to fibrosis, sometimes with nodular regeneration. In fact, it is not uncommon that these livers also develop early or full-blown cirrhosis [8]. A syncytial giant cell form has also been described [17]. Somewhat different from HBV-related cases, inflammation in HCV-associated FCH may be more severe and the periportal and periseptal inflammatory infiltrate may consist predominantly of neutrophils (Figure 2). On EM examination, neither mitochondrial damage nor cytoplasmic or nuclear viral inclusion can be identified [15].
Figure 1.
A liver biopsy from a HCV-HIV coinfected patient. A. Note the prominent hepatocytic degeneration, intracellular and canalicular cholestasis (Hematoxylin & eosin stain). B. Extensive periportal and pericellular fibrosis (Masson's trichrome stain).
Figure 2.
A liver biopsy from another patient with HCV-HIV coinfection, showing in addition to the hepatocytic degeneration and cholestasis, a component of neutrophilic infiltration in the parenchyma-portal interface (Hematoxylin & eosin stain).
The list of histologic differential diagnosis can be long, but only the few which occur in a related clinical context are discussed here. Prominent hepatocyte ballooning and extensive pericellular fibrosis are the main features of alcoholic steatohepatitis (ASH) or severe forms of nonalcoholic steatohepatitis (NASH). Accurate clinical history is important in reaching the correct diagnosis. Histologic clues that favor the diagnosis of steatohepatitis include Mallory hyalines, which are usually subtle (“ill-formed”) in NASH but more typical in ASH, macrovesicular steatosis, and sometimes lobular neutrophilic infiltration. Although cholestasis can be seen in alcoholic liver disease, marked intracellular and canalicular cholestasis favors FCH.
In patients with liver transplantation, the differential diagnoses also include ischemic injury due to delayed hepatic artery thrombosis, extrahepatic biliary obstruction, and adverse drug reactions. All these conditions can show ballooning or necrosis of centrilobular hepatocytes, cholestasis, and Kupffer cell clustering. However, significant portal edema, ductular reaction, neutrophilic infiltration primarily surrounding the bile ducts and ductules, and sometimes periductal fibrosis, favor large bile duct obstruction, and are not features of FCH. It should be noted that in FCH, periductular infiltration by neutrophils can be seen at the interface between portal tracts and lobules, which should be differentiated from true neutrophilic cholangitis (ascending cholangitis) where neutrophils are seen within the lumens of the bile ducts. In all these differentials, particularly adverse drug reactions, clinical information remains critical and its importance cannot be overemphasized.
As severe cholestasis can sometimes occur in chronic rejection, it should also be considered in the differential diagnosis. Histologic features of chronic graft rejection include foamy cell change of the hepatic arterials, and paucity of bile ducts. Arterial foamy change may not always be present in a needle core biopsy, but it is usually not associated with prominent hepatocyte ballooning that is almost always seen in FCH. In addition, prominent pericellular fibrosis should help reach the diagnosis of FCH.
Pathogenesis
Because of the high level of viral replication in FCH cases associated with HBV, the less impressive inflammatory reactions and the immunosuppressive status in most patients, it has been thought that un-inhibited viral replication in hepatocytes is critical in the initiation and progression of FCH. High-level expression of viral antigens (HBsAg and HBcAg) has been directly visualized by immuno-histochemical staining in involved livers and measured by quantitative analysis such as radioimmunoassay of tissue homogenates [7]. The potential sources of reinfection by HBV include peripheral blood mononuclear cells, pancreas, spleen and bone marrow, as viral DNA has been demonstrated in these tissues [23, 24]. It has been postulated that the accumulation of abundant HBV surface antigen within the endoplasmic reticulum (ER) and Golgi complex leads to stress of these organelles, resulting in hepatocytic death either by apoptosis or necrosis, depending on the severity of the “over-loading.” However, this theory is challenged by the fact that a large number of ground-glass hepatocytes (GGH) are commonly seen in livers without FCH.
The presence of viral mutants with dysregulated viral replication has also been proposed to be important in the pathogenesis of FCH. Several HBV mutants have been reported in FCH patients, including the precore mutant [12, 25–27] and the preS mutation that has been suggested to be responsible for intracellular viral retention [28]. The genome of an HBV mutant isolated from a patient with FCH resulted in overexpression of the surface antigen upon transfection into cultured cells.
Factors implicated in the pathogenesis of HCV-associated FCH are even more scarce. Although genotype 1b has been suggested to be associated with more severe liver disease, such a correlation has not been observed in cases of FCH [8]. In one study of HCV quasispecies before and after liver transplantation, it was found that even though the number of variants did not change over time, a qualitative change in variants (divergence) occurred in immunocompromised patients, which was associated with and proceeded development of FCH [29]. While some cases showed an increased level of HCV viremia, other FCH patients secondary to HCV/HIV coinfection did not show a marked elevation of HCV RNA [15]. This may be partially due to the fact that HCV remains concentrated within the secretory apparatus of the affected hepatocytes, and thus the intrahepatic viral replication may not be accurately reflected by the viremia level.
In contrast to immune-mediated injuries of the original diseases (chronic hepatitis B, chronic hepatitis C), it is likely that in FCH high level of viruses directly induce cellular degeneration or death, with acute and uniform functional “shut down” in a relatively short period of time, thus causing progressive and rapid liver failure. The rapid functional shutdown is reflected by only modest elevation in serum transaminases and severe conjugated bilirubinemia in the absence of bile duct obstruction. The sudden widespread degeneration of the hepatocytes is mostly reflected by diffuse ballooning. In addition, the massive damage to the hepatocytes is a relatively prolonged (subacute) process, allowing the liver to express fibrogenesis that leads to prominent perisinusoidal fibrosis in most cases. This virus-induced direct liver cell damage in certain aspects resembles that caused by other “direct-hit” agents, such as alcohol and iron overload, both of which induce ballooning of hepatocytes and perisinusoidal fibrosis.
The mechanisms of cholestasis during acute or subacute disease process are much speculative. It is most likely resulted from functional compromise of the bile acid transporter proteins located on the canalicular membrane. This leads to the most frequently observed intracellular cholestasis and subsequent feathery degeneration of the hepatocytes (cholate stasis). This is understandable as the generation of bile flow is dependant on the adenosine triphosphate (ATP) and any acute severe “blow” to the hepatocytes would cause the “sudden” shut down of the transporting mechanisms. In turn, intracellular bile salts are strong toxins to the hepatocytes. In addition to the blockage of bile acid transport at the cell level, functional “shut down” at the downstream of bile flow also appears to occur as canalicular bile plugs are sometimes noted.
Treatment
The treatment of FCH depends on the underlying diseases. However, the outcome is usually dismal due to the severity of the disease. Even with intensive treatment, the prognosis remains poor, and rapid progression to hepatic failure and death are common.
For transplant patients, the current standard therapy is reduction or complete stop of immunosuppressive therapies, followed by aggressive antiviral treatment. For HBV-associated cases, lamivudine has shown benefit in some [30, 31] but without effect in others. Alternative agents have been used for those with lamivudine resistance, such as adevovir dipivoxil [32]. For HCV-related cases, interferon-α (IFN-α) [10] or peg-INF with ribavirin has shown to be effective in reversing the severe clinical course. In addition, double-filtration plasmapheresis (DFPP) combined with IFN and ribavirin has been used in a HCV liver transplant patient who later recovered from FCH [33]. It has been shown that among HCV liver transplantation recipients who developed end-stage liver disease again, retransplantation was associated with a higher risk of immediate peri-operative mortality, or death secondary to rapid recurrence of the disease including FCH [34].
For renal transplant patients, reduction of immunosuppressants has beneficial effect [35], as for liver transplant patients. However, the timing and regimen selection for anti-HCV therapy are more complex in these patients [36]. This is because the therapy sometimes carries adverse effect on renal allografts [37], thus making treatment decision rather tricky. For instance, ribavirin is contraindicated in these patients because of reduced renal clearance which may increase the risk of severe hemolysis. Therefore, for HCV patients with end-stage renal diseases who expect to receive renal transplantation, pre-transplant anti-viral therapy has been recommended, even with relatively mild histologic hepatitis and low HCV viremia.
It should be emphasized that in cases of liver transplantation, early and accurate diagnosis of FCH is critical. It is particularly important to distinguish FCH from acute rejection because this erroneous diagnosis can lead to stronger immunosuppressive regimen, which will precipitate further progression of FCH. It has been observed that expression of HBsAg and HBcAg precedes histologic evidence of lobular necroinflammation in recurrent hepatitis B. This leaves opportunity for early recognition and potential early treatment before more severe hepatitis occurs [6].
Synopsis
HBV infects ∼5% of the world's population and is responsible for more than 1 million deaths each year. It has a 2.1 per 100,000 incidence of acute infection in the US after the implementation of a rigorous vaccination campaign [38]. HCV is a major cause of chronic liver disease and affects 130 million people worldwide [39], including over 3 million in the US, causing ∼10,000 deaths each year in this country. It is responsible for about 27% of cirrhosis and 25% of hepatocellular carcinoma. Together, patients affected by these viruses constitute the majority of liver transplantation recipients.
Recurrence of HCV infection after liver transplantation is almost universal [40], and FCH remains a significant threat to graft and patient survival. Better awareness of its clinicopathologic features and better understanding of its pathogenesis will facilitate its early recognition so that more effective therapies may be implemented.
The histopathologic changes manifested by FCH are considered to represent a unique reactive pattern associated with a well defined spectrum of hepatic injuries. It can be viewed as a special form of liver disease that occurs at certain stages of several hepatitic diseases complicated by immunosuppression. In a simplified fashion, this disease is most likely caused by a “rapid” built-up of the attacking agent, usually a virus, in the setting of reduced host immune response. These injuries quickly induce intracellular change (degeneration), and at the same time elicit marked fibrogenic response, leading to pericellular (perisinusoidal) fibrosis. Shutdown of the bile secretory mechanisms (not the conjugation process) at the local hepatocyte level and/or canaliculi leads to intrahepatic bile stasis (conjugated hyperbilirubinemia).
It is interesting to note that similar, but somewhat different, patterns of liver injuries have been observed in other diseases including: (1) yellow fever, in which the host immune status is normal, but the infecting viruses are highly hepatotrophic and virulent. The infection leads to rapid and severe hepatocytic apoptosis and necrosis, often resulting in fulminant hepatic damage [41, 42]. The process is either quickly fatal, or self-limiting with the liver recovering without the development of fibrosis; (2) alcoholic liver disease, in which the host is also immunocompetent, and the attacking agent (alcohol) induces severe hepatocytic injuries, such as ballooning, Mallory hyalines, steatosis and marked pericellular fibrosis, by mechanisms likely similar to those for FCH. In a small number of cases, severe cholestasis may develop, leading to a microscopic picture difficult to be distinguished from FCH. However, as aforementioned, the presence of prominent Mallory hyalines and sometimes prominent neutrophilic infiltration helps in the differential diagnosis. From a practical stand of view, when treating patients with chronic viral hepatitis who are immunocompromised (such as HIV coinfection) or therapeutically immunosuppressed (such as organ transplantation, chemotherapy, and steroid use), caution needs to be exercised to avoid the negligence of possible occurrence of FCH.
References
- 1.Davies SE, Portmann BC, O'Grady JG, Aldis PM, Chaggar K, Alexander GJ, Williams R. Hepatic histological findings after transplantation for chronic hepatitis B virus infection, including a unique pattern of fibrosing cholestatic hepatitis. Hepatology. 1991;13:150–157. [PubMed] [Google Scholar]
- 2.O'Grady JG, Smith HM, Davies SE, Daniels HM, Donaldson PT, Tan KC, Portmann B, Alexander GJ, Williams R. Hepatitis B virus reinfection after orthotopic liver transplantation. Serological and clinical implications. J Hepatol. 1992;14:104–111. doi: 10.1016/0168-8278(92)90138-f. [DOI] [PubMed] [Google Scholar]
- 3.Demetris AJ, Tod S, Van Thiel DH, Fung JJ, Iwaki Y, Sysyn G, Ming W, Trager J, Starzl TE. Evolution of hepatitis B virus liver disease after hepatic replacement. Practical and theoretical considerations. Am J Pathol. 1990;137:667–676. [PMC free article] [PubMed] [Google Scholar]
- 4.Todo S, Demetris AJ, Van Thiel D, Teperman L, Fung JJ, Starzl TE. Orthotopic liver transplantation for patients with hepatitis B virus-related liver disease [see comment] Hepatology. 1991;13:619–626. [PMC free article] [PubMed] [Google Scholar]
- 5.Benner KG, Lee RG, Keeffe EB, Lopez RR, Sasaki AW, Pinson CW. Fibrosing cytolytic liver failure secondary to recurrent hepatitis B after liver transplantation [see comment] Gastroenterology. 1992;103:1307–1312. doi: 10.1016/0016-5085(92)91521-5. [DOI] [PubMed] [Google Scholar]
- 6.Phillips MJ, Cameron R, Flowers MA, Blendis LM, Greig PD, Wanless I, Sherman M, Superina R, Langer B, Levy GA. Post-transplant recurrent hepatitis B viral liver disease. Viral-burden, steatoviral, and fibroviral hepatitis B. Am J Pathol. 1992;140:1295–1308. [PMC free article] [PubMed] [Google Scholar]
- 7.Lau JY, Bain VG, Davies SE, O'Grady JG, Alberti A, Alexander GJ, Williams R. High-level expression of hepatitis B viral antigens in fibrosing cholestatic hepatitis. Gastroenterology. 1992;102:956–962. doi: 10.1016/0016-5085(92)90182-x. [DOI] [PubMed] [Google Scholar]
- 8.Schluger LK, Sheiner PA, Thung SN, Lau JY, Min A, Wolf DC, Fiel I, Zhang D, Gerber MA, Miller CM, Bodenheimer HC., Jr Severe recurrent cholestatic hepatitis C following orthotopic liver transplantation. Hepatology. 1996;23:971–976. doi: 10.1002/hep.510230505. [DOI] [PubMed] [Google Scholar]
- 9.Zylberberg H, Carnot F, Mamzer MF, Blancho G, Legendre C, Pol S. Hepatitis C virus-related fibrosing cholestatic hepatitis after renal transplantation. Transplantation. 1997;63:158–160. doi: 10.1097/00007890-199701150-00029. [DOI] [PubMed] [Google Scholar]
- 10.Toth CM, Pascual M, Chung RT, Graeme-Cook F, Dienstag JL, Bhan AK, Cosimi AB. Hepatitis C virus-associated fibrosing cholestatic hepatitis after renal transplantation: response to interferon-alpha therapy. Transplantation. 1998;66:1254–1258. doi: 10.1097/00007890-199811150-00023. [DOI] [PubMed] [Google Scholar]
- 11.Munoz De Bustillo E, Ibarrola C, Colina F, Castellano G, Fuertes A, Andres A, Aguado JM, Rodicio JL, Morales JM. Fibrosing cholestatic hepatitis in hepatitis C virus-infected renal transplant recipients. J Am Soc Nephrol. 1998;9:1109–1113. doi: 10.1681/ASN.V961109. [DOI] [PubMed] [Google Scholar]
- 12.Chen CH, Chen PJ, Chu JS, Yeh KH, Lai MY, Chen DS. Fibrosing cholestatic hepatitis in a hepatitis B surface antigen carrier after renal transplantation. Gastroenterology. 1994;107:1514–1518. doi: 10.1016/0016-5085(94)90557-6. [DOI] [PubMed] [Google Scholar]
- 13.Lam PW, Wachs ME, Somberg KA, Vincenti F, Lake JR, Ferrell LD. Fibrosing cholestatic hepatitis in renal transplant recipients. Transplantation. 1996;61:378–381. doi: 10.1097/00007890-199602150-00008. [DOI] [PubMed] [Google Scholar]
- 14.Delladetsima I, Psichogiou M, Sypsa V, Psimenou E, Kostakis A, Hatzakis A, J.N. Boletis JN. The course of hepatitis C virus infection in pretransplantation anti-hepatitis C virus-negative renal transplant recipients: a retrospective follow-up study. Am J Kidney Dis. 2006;47:309–316. doi: 10.1053/j.ajkd.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Rosenberg PM, Farrell JJ, Abraczinskas DR, Graeme-Cook FM, Dienstag JL, Chung RT. Rapidly progressive fibrosing cholestatic hepatitis–hepatitis C virus in HIV coinfection. Am J Gastroenterol. 2002;97:478–483. doi: 10.1111/j.1572-0241.2002.05459.x. [DOI] [PubMed] [Google Scholar]
- 16.de Vera ME, Dvorchik I, Tom K, Eghtesad B, Thai N, Shakil O, Marcos A, Demetris A, Jain A, Fung JJ, Ragni MV. Survival of liver transplant patients coinfected with HIV and HCV is adversely impacted by recurrent hepatitis C. Am J Transplant. 2006;6:2983–2993. doi: 10.1111/j.1600-6143.2006.01546.x. [DOI] [PubMed] [Google Scholar]
- 17.Moreno A, Moreno A, Perez-Elias MJ, Quereda C, Fernandez-Munoz R, Antela A, Moreno L, Barcena R, Lopez-San Roman A, Celma ML, Garcia-Martos M, Moreno S. Syncytial giant cell hepatitis in human immunodeficiency virus-infected patients with chronic hepatitis C: 2 cases and review of the literature. Hum Pathol. 2006;37:1344–1349. doi: 10.1016/j.humpath.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Vallet-Pichard A, Pol S. Natural history and predictors of severity of chronic hepatitis C virus (HCV) and human immunodeficiency virus (HIV) co-infection. J Hepatol. 2006;44:S28–34. doi: 10.1016/j.jhep.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Delgado J, Munoz de Bustillo E, Ibarrola C, Colina F, Morales JM, Rodriguez E, Aguado JM, Fuertes A, Gomez MA. Hepatitis C virus-related fibrosing cholestatic hepatitis after cardiac transplantation: is azathioprine a contributory factor? J Heart Lung Transplant. 1999;18:607–610. doi: 10.1016/s1053-2498(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 20.Fang JW, Wright TL, Lau JY. Fibrosing cholestatic hepatitis in patient with HIV and hepatitis B. Lancet. 1993;342:1175. doi: 10.1016/0140-6736(93)92160-u. [DOI] [PubMed] [Google Scholar]
- 21.Agarwal SK, Kalra V, Dinda A, Gupta S, Dash SC, Bhowmik D, Tiwari SC. Fibrosing cholestatic hepatitis in renal transplant recipient with CMV infection: a case report. Int Urol Nephrol. 2004;36:433–435. doi: 10.1007/s11255-004-6196-9. [DOI] [PubMed] [Google Scholar]
- 22.Lucey MR, Graham DM, Martin P, Di Bisceglie A, Rosenthal S, Waggoner JG, Merion RM, Campbell DA, Nostrant TT, Appelman HD. Recurrence of hepatitis B and delta hepatitis after orthotopic liver transplantation. Gut. 1992;33:1390–1396. doi: 10.1136/gut.33.10.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pontisso P, Poon MC, Tiollais P, Brechot C. Detection of hepatitis B virus DNA in mononuclear blood cells. BMJ Clin Res. 1984;288:1563–1566. doi: 10.1136/bmj.288.6430.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davison F, Alexander GJ, Trowbridge R, Fagan EA, Williams R. Detection of hepatitis B virus DNA in spermatozoa, urine, saliva and leucocytes, of chronic HBsAg carriers. A lack of relationship with serum markers of replication. J Hepatol. 1987;4:37–44. doi: 10.1016/s0168-8278(87)80007-7. [DOI] [PubMed] [Google Scholar]
- 25.Angus PW, Locarnini SA, McCaughan GW, Jones RM, McMillan JS, D.S. Bowden DS. Hepatitis B virus precore mutant infection is associated with severe recurrent disease after liver transplantation. Hepatology. 1995;21:14–18. [PubMed] [Google Scholar]
- 26.Waguri N, Ichida T, Fujimaki R, Ishikawa T, Nomoto M, Asakura H, Nakamar T, Saitoh A, Arakawa M, Saitoh K, Takahashi K. Fibrosing cholestatic hepatitis after living related-donor renal transplantation. J Gastroenterol Hepatol. 1998;13:1133–1137. doi: 10.1111/j.1440-1746.1998.tb00589.x. [DOI] [PubMed] [Google Scholar]
- 27.Brind AM, Bennett MK, Bassendine MF. Nucleoside analogue therapy in fibrosing cholestatic hepatitis–a case report in an HBsAg positive renal transplant recipient [see comment] Liver. 1998;18:134–139. doi: 10.1111/j.1600-0676.1998.tb00139.x. [DOI] [PubMed] [Google Scholar]
- 28.Bock CT, Tillmann HL, Maschek HJ, Manns MP, Trautwein C. A preS mutation isolated from a patient with chronic hepatitis B infection leads to virus retention and misassembly. Gastroenterology. 1997;113:1976–1982. doi: 10.1016/s0016-5085(97)70018-0. [DOI] [PubMed] [Google Scholar]
- 29.Pessoa MG, Bzowej N, Berenguer M, Phung Y, Kim M, Ferrell L, Hassoba H, Wright TL. Evolution of hepatitis C virus quasispecies in patients with severe cholestatic hepatitis after liver transplantation [see comment] Hepatology. 1999;30:1513–1520. doi: 10.1002/hep.510300610. [DOI] [PubMed] [Google Scholar]
- 30.Al Faraidy K, Yoshida EM, Davis JE, Vartanian RK, Anderson FH, Steinbrecher UP. Alteration of the dismal natural history of fibrosing cholestatic hepatitis secondary to hepatitis B virus with the use of lamivudine. Transplantation. 1997;64:926–928. doi: 10.1097/00007890-199709270-00024. [DOI] [PubMed] [Google Scholar]
- 31.Chan TM, Wu PC, Li FK, Lai CL, Cheng IK, Lai KN. Treatment of fibrosing cholestatic hepatitis with lamivudine. Gastroenterology. 1998;115:177–181. doi: 10.1016/s0016-5085(98)70380-4. [DOI] [PubMed] [Google Scholar]
- 32.Walsh KM, Woodall T, Lamy P, Wight DG, Bloor S, Alexander GJ. Successful treatment with adefovir dipivoxil in a patient with fibrosing cholestatic hepatitis and lamivudine resistant hepatitis B virus. Gut. 2001;49:436–440. doi: 10.1136/gut.49.3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taniguchi M, Furukawa H, Shimamura T, Suzuki T, Yamashita K, Ota M, Todo S. Impact of double-filtration plasmapheresis in combination with interferon and ribavirin in living donor liver transplant recipients with hepatitis C. Transplantation. 2006;81:1747–1749. doi: 10.1097/01.tp.0000226075.04938.43. [DOI] [PubMed] [Google Scholar]
- 34.Berenguer M, Prieto M, Palau A, Rayon JM, Carrasco D, Juan FS, Lopez-Labrador FX, Moreno R, Mir J, Berenguer J. Severe recurrent hepatitis C after liver retransplantation for hepatitis C virus-related graft cirrhosis. Liver Transplant Surgery. 2003;9:228–235. doi: 10.1053/jlts.2003.50029. [DOI] [PubMed] [Google Scholar]
- 35.Delladetsima JK, Boletis JN, Makris F, Psichogiou M, Kostakis A, Hatzakis A. Fibrosing cholestatic hepatitis in renal transplant recipients with hepatitis C virus infection. Liver Transplant Surgery. 1999;5:294–300. doi: 10.1002/lt.500050417. [DOI] [PubMed] [Google Scholar]
- 36.Berenguer M, Aguilera V, Prieto M, San Juan F, Rayon JM, Benlloch S, Berenguer J. Significant improvement in the outcome of HCV-infected transplant recipients by avoiding rapid steroid tapering and potent induction immunosuppression. J Hepatol. 2006;44:717–722. doi: 10.1016/j.jhep.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Gane E, Pilmore H. Management of chronic viral hepatitis before and after renal transplantation. Transplantation. 2002;74:427–437. doi: 10.1097/00007890-200208270-00001. [DOI] [PubMed] [Google Scholar]
- 38.Centers for Disease Control. Hepatitis B vaccination coverage among adults–United States 2004. MMWR. 2006;55:509–511. [PubMed] [Google Scholar]
- 39.Alter MJ. Epidemiology of hepatitis C virus infection. World J Gastroenterol. 2007;13:2436–2441. doi: 10.3748/wjg.v13.i17.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanchez-Fueyo A, Restrepo JC, Quinto L, Bruguera M, Grande L, Sanchez-Tapias JM, Rodes J, Rimola A. Impact of the recurrence of hepatitis C virus infection after liver transplantation on the long-term viability of the graft. Transplantation. 2002;73:56–63. doi: 10.1097/00007890-200201150-00010. [DOI] [PubMed] [Google Scholar]
- 41.Monath T. Yellow fever. In: Guerrant R, Walker D, Weller P, editors. Tropical Infectious Diseases. Philadelphia: Churchill Livingston; 1999. pp. 1253–1264. [Google Scholar]
- 42.Xiao SY, Zhang H, Guzman H, Tesh R. Experimental yellow fever virus infection in golden hamster (Mesocricetus auratus) II. Pathology. J Infect Dis. 2001;183:1437–1444. doi: 10.1086/320200. [DOI] [PubMed] [Google Scholar]