Abstract
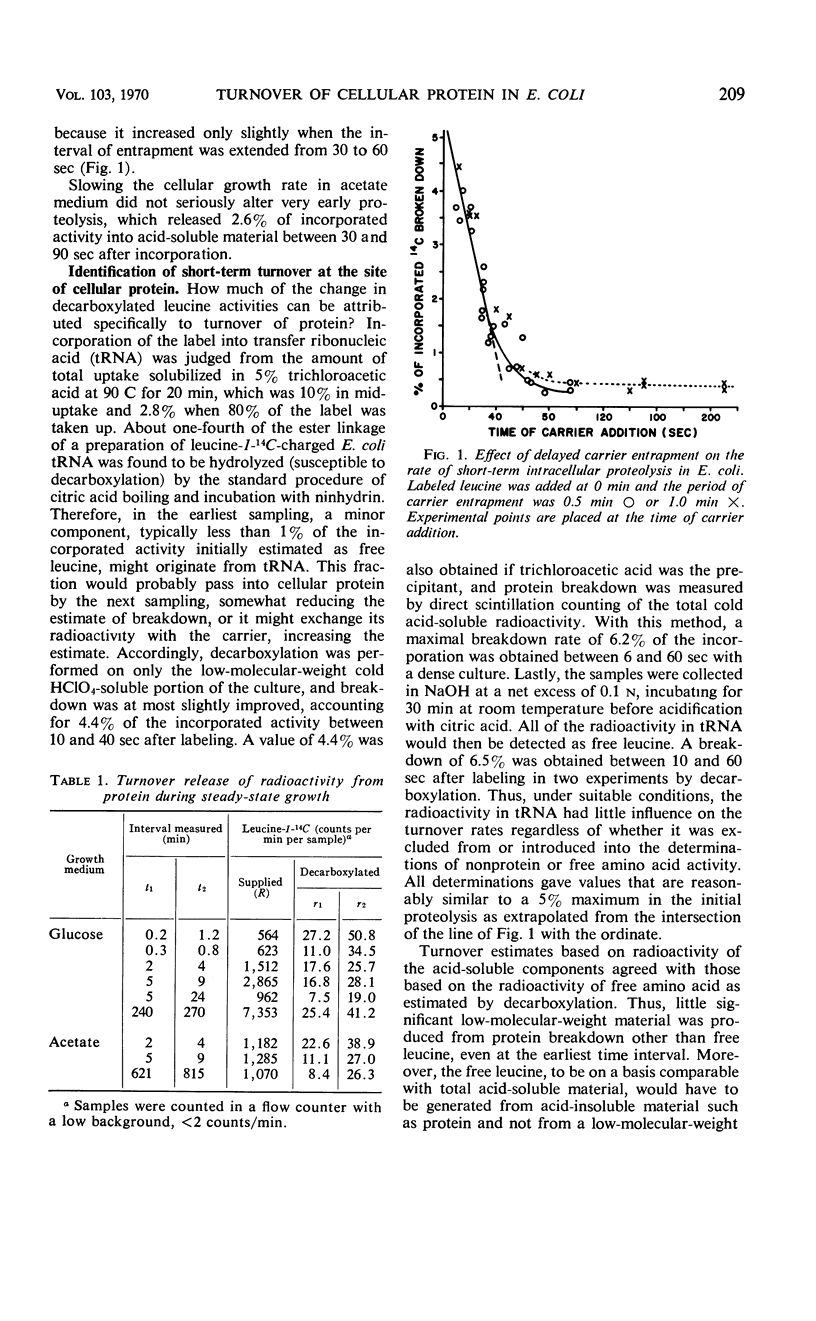
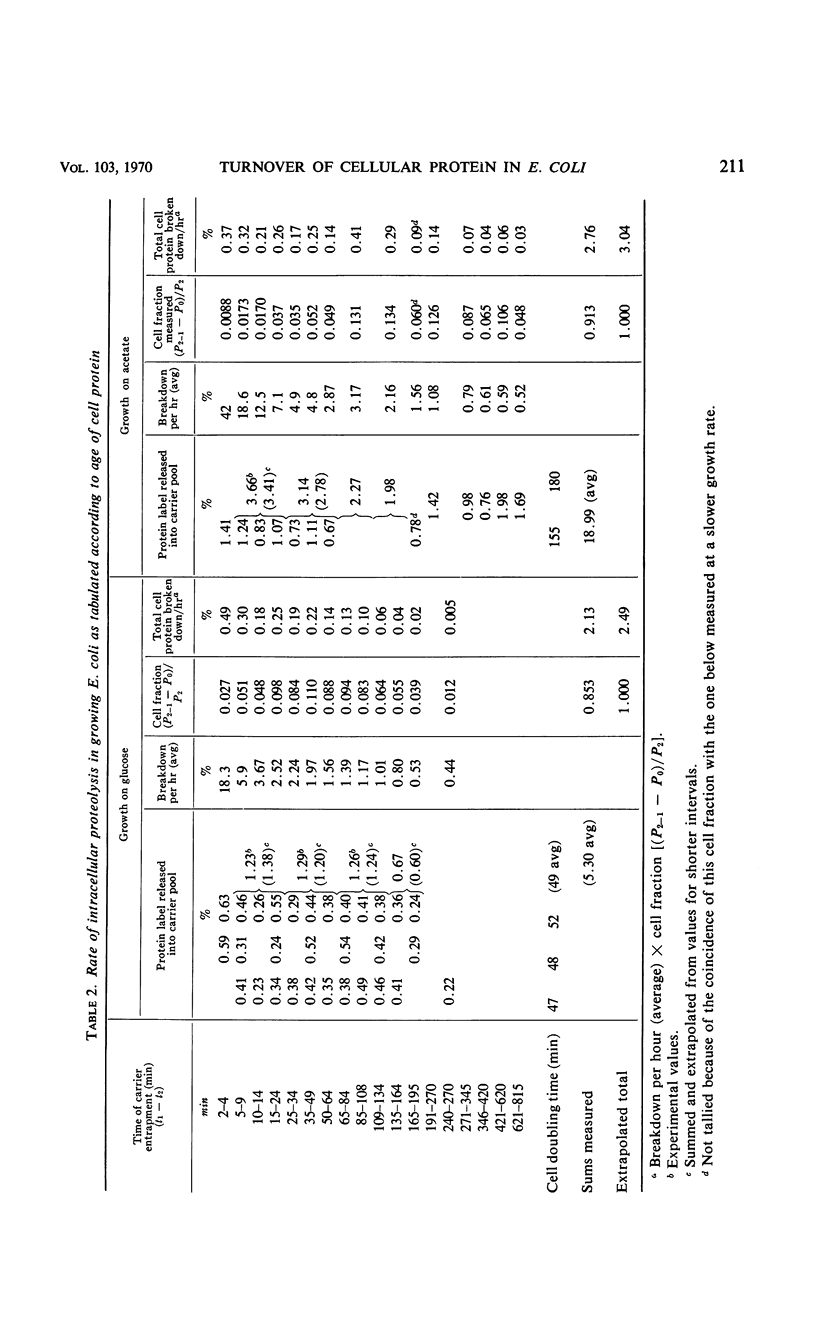
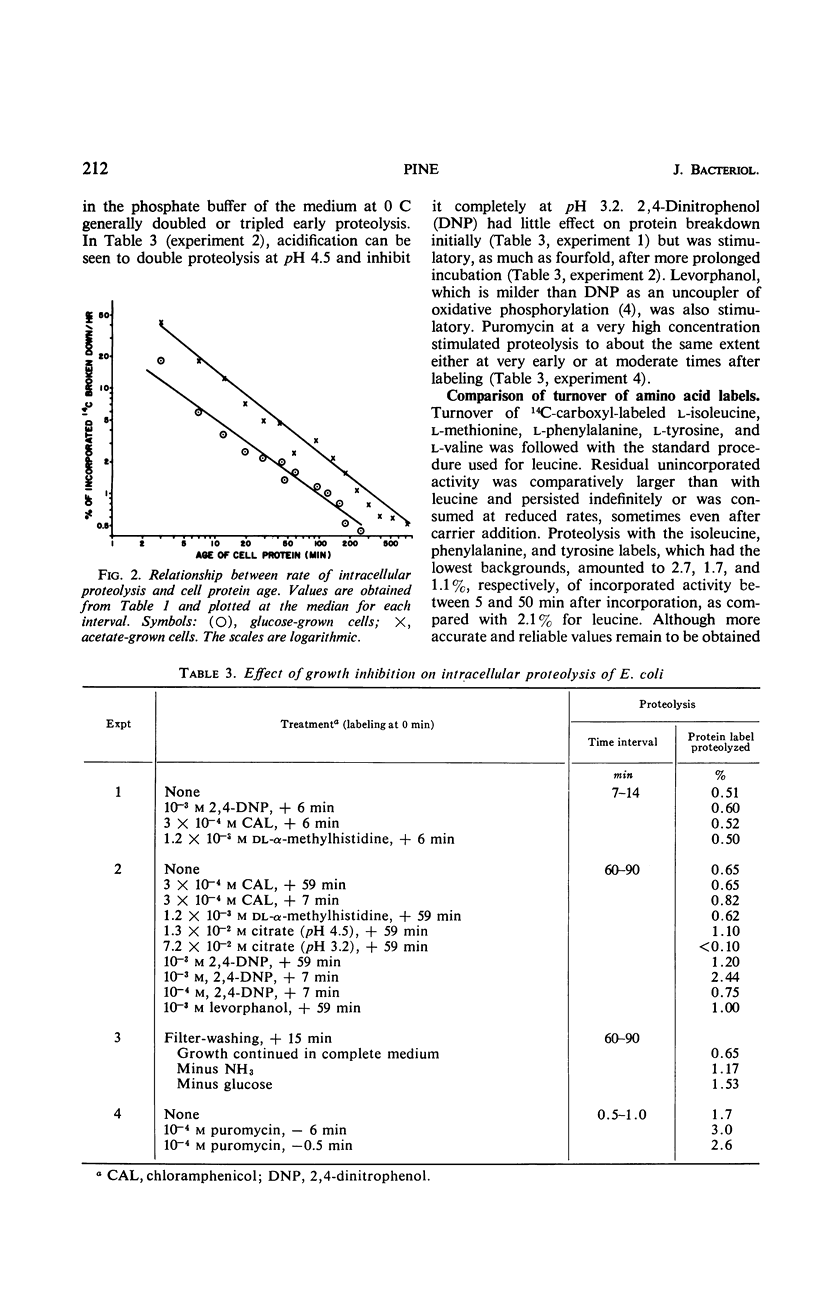
Turnover of cellular protein has been estimated in Escherichia coli during continuous exponential growth and in the absence of extensive experimental manipulation. Estimation is based upon the cumulative release into carrier pools of free leucine-1-14C over a number of time intervals after its pulsed incorporation into protein. Breakdown rates obtained with other labeled amino acids are similar to those obtained with leucine. Two kinetically separate processes have been shown. First, a very rapid turnover of 5% of the amino acid label occurs within 45 sec after its incorporation, most likely indicating maturative cleavages within the proteins after their assembly. A slower heterogeneous rate of true protein turnover follows, falling by 39% in the remaining proteins for each doubling of turnover time. At 36 C, the total breakdown rate of cellular protein is 2.5 and 3.0% per hr over a threefold range of growth rate in glucose and acetate medium, respectively. This relatively constant breakdown rate is maintained during slower growth by more extensive protein replacement, one fifth of the protein synthesized at any time in the acetate medium being replaced after 4.6 doubling times. Intracellular proteolysis thus appears to be a normal and integral reaction of the growing cell. The total rate equals minimal estimates obtained by others for arrested or decelerated growth but is kinetically more heterogeneous. Quantitatively proteolysis is not directly affected by growth arrestment per se as caused by α-methylhistidine, chloramphenicol, or uncouplers of oxidative phosphorylation, but qualitatively it can gradually become more homogeneous kinetically as a secondary event of starvation. Under more extreme conditions as with extensive washing, prolonged phosphorylative uncoupling, or acidification of the growth medium, the proteolytic rate can increase severalfold.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Capecchi M. R. Initiation of E. coli proteins. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1517–1524. doi: 10.1073/pnas.55.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlin G., Broda P. Physiology and genetics of the "ribonucleic acid control" locus in escherichia coli. Bacteriol Rev. 1968 Sep;32(3):206–226. doi: 10.1128/br.32.3.206-226.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein I., Grossowicz N. Intracellular protein breakdown in a thermophile. J Bacteriol. 1969 Aug;99(2):418–421. doi: 10.1128/jb.99.2.418-421.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene R., Magasanik B. The mode of action of levallorphan as an inhibitor of cell growth. Mol Pharmacol. 1967 Sep;3(5):453–472. [PubMed] [Google Scholar]
- Holland J. J., Kiehn E. D. Specific cleavage of viral proteins as steps in the synthesis and maturation of enteroviruses. Proc Natl Acad Sci U S A. 1968 Jul;60(3):1015–1022. doi: 10.1073/pnas.60.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Morphogenesis of poliovirus. I. Association of the viral RNA with coat protein. J Mol Biol. 1968 Apr 28;33(2):369–378. doi: 10.1016/0022-2836(68)90195-2. [DOI] [PubMed] [Google Scholar]
- Kepes A., Beguin S. Peptide chain initiation and growth in the induced synthesis of beta-galactosidase. Biochim Biophys Acta. 1966 Sep;123(3):546–560. doi: 10.1016/0005-2787(66)90222-x. [DOI] [PubMed] [Google Scholar]
- Lacroute F., Stent G. S. Peptide chain growth of -galactosidase in Escherichia coli. J Mol Biol. 1968 Jul 14;35(1):165–173. doi: 10.1016/s0022-2836(68)80044-0. [DOI] [PubMed] [Google Scholar]
- Pine M. J. Heterogeneity of protein turnover in Escherichia coli. Biochim Biophys Acta. 1965 Jul 8;104(2):439–456. doi: 10.1016/0304-4165(65)90349-1. [DOI] [PubMed] [Google Scholar]
- Pine M. J. Metabolic control of intracellular proteolysis in growing and resting cells of Escherichia coli. J Bacteriol. 1966 Oct;92(4):847–850. doi: 10.1128/jb.92.4.847-850.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine M. J. Response of intracellular proteolysis to alteration of bacterial protein and the implications in metabolic regulation. J Bacteriol. 1967 May;93(5):1527–1533. doi: 10.1128/jb.93.5.1527-1533.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer P. Sporulation and the production of antibiotics, exoenzymes, and exotonins. Bacteriol Rev. 1969 Mar;33(1):48–71. doi: 10.1128/br.33.1.48-71.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger D., Ben-Hamida F. Turnover of protein in Escherichia coli starving for nitrogen. Biochim Biophys Acta. 1966 Apr 18;119(1):171–182. doi: 10.1016/0005-2787(66)90048-7. [DOI] [PubMed] [Google Scholar]



