Abstract
The pathologic distinction of atypical fibroxanthomas (AFXs) from cutaneous spindle cell/sarcomatoid squamous cell carcinomas (SCSCCs) may occasionally pose a significant diagnostic challenge, given the substantial clinicopathologic overlap between these lesions. Recent studies indicate that p63 and CD10 are expressed in significant proportions of SCSCC and AFX, respectively. The purpose of this study is to investigate the utility of CD10 and p63 in distinguishing cutaneous SCSCCs and AFXs. The immunohistochemical expression of p63, CD10, cytokeratin AE-1/3, cytokeratin 5/6 and a cytokeratin cocktail (Kermix) was evaluated in an archived group of 23 AFXs and 10 SCSCCs. CD10 was positive in 18/23 AFXs (78%), with most demonstrating strong and/or diffuse staining. Three of 23 AFXs (13%), all negative for cytokeratins, showed focal and weak nuclear staining for p63. Two of 23 AFXs (9%) demonstrated very focal or weak staining for only one cytokeratin; in both cases, p63 and CD10 were negative. One AFX was negative with all immunostains. CD10 was positive in 6/10 SCSCCs (60%), with half demonstrating strong and/or diffuse staining. P63 was positive in 9/10 SCSCCs (90%), with most demonstrating strong and diffuse staining. One SCSCC was negative for p63, but positive with two cytokeratin immunostains. In conclusion, the expression of any of the cytokeratins evaluated herein significantly distinguished AFX from SCSCC. CD10 used in isolation, however, was not useful in making this distinction (positive in 18/23 AFXs versus 6/10 SCSCCs, p=0.4). The addition of CD10 to a panel that includes p63 did not provide any additional information to that obtained from the latter alone. Overall, the most effective combination to distinguish AFX from SCSCC was p63 and cytokeratin AE-1/3. Positivity for both p63 and cytokeratin AE-1/3 was seen in 9/10 SCSCCs (90%) and was not observed in any of the 23 AFXs (p<0.0001). The usefulness of CD10 in this differential diagnosis is limited.
Keywords: Atypical fibroxanthoma, p63, CD10, skin, sarcomatoid/spindle cell squamous cell carcinoma
Introduction
Atypical fibroxanthomas (AFXs) and cutaneous spindle cell/sarcomatoid squamous cell carcinomas (SCSCCs) can be morphologically indistinguishable on routine hematoxylin and eosin (H&E) stained sections. In addition to spindle cell melanomas, they represent two of the top diagnostic considerations for spindle cell lesions presenting in sun-damaged skin, particularly in the head and neck region of the elderly [1–14]. AFX was first described by Helwig in 1961 [15]. It is a pleomorphic lesion of uncertain histogenesis; however, most investigators now suggest AFX is of mesenchymal origin with variable histiocytic, fibroblastic, and/or myofibroblastic differentiation [1–3, 7, 11, 16, 17]. SCSCCs are well-documented albeit uncommon variants of poorly differentiated squamous cell carcinoma which on occasion lack expression of various epithelial markers, such as cytokeratin and epithelial membrane antigen [4, 5, 9, 11, 13, 18–23]. SCSCCs may also express markers of mesenchymal differentiation, such as vimentin [14].
In routine practice, AFX remains a diagnosis of exclusion, as they display no morphologic or immunohistochemical evidence of epithelial, melanocytic, and/or other specific line of differentiation. Recently, CD10 has been shown to be a useful positive marker for AFX [1, 24, 25]. Conversely, p63 has been shown to be a useful marker for cutaneous SCSCCs [26]; although, due to their relative infrequency, the number of SCSCCs studied to date has been small. We sought to further investigate the utility of CD10 and p63 in distinguishing cutaneous SCSCC and AFX.
Materials and Methods
We retrieved a total of 36 archived cases diagnosed as or favored to be AFXs, 9 cutaneous SCSCCs, and one case in which the diagnosis of AFX or SCSCC could not be conclusively made (indeterminate). All cases were originally evaluated in the Departments of Pathology at Brooke Army Medical Center (BAMC), Fort Sam Houston, Texas or Wilford Hall Medical Center (WHMC), Lackland air force base, Texas. BAMC and WHMC are both tertiary care medical treatment facilities with robust dermatopathology sections. All cases were biopsies or small excisions. The original H&E stained sections and immunohistochemical studies were reviewed for each case to confirm the original diagnoses. One additional H&E stained section was prepared and examined for each case. Only 26/36 AFX cases (72%) and 6/9 SCSCC cases (67%) had residual formalin-fixed, paraffin-embedded tissue available for study.
For immunohistochemistry, 5 μm-thick sections were cut and mounted on a glass slide, deparaffinized and rehydrated. Appropriate negative and positive controls were assayed in parallel. All assays were performed in an Axiom 36 autostainer (LabVision Corporation, Fremont, CA). The following primary monoclonal antibodies were utilized: p63, CD10, a cytokeratin cocktail (Kermix), cytokeratin 5/6, and cytokeratin AE1/3. Assay specifications for each antibody are outlined in Table 1. All assays entailed heat-induced epitope retrieval. For p63, only unequivocal nuclear staining in lesional cells was considered as immunopositivity, whereas cytoplasmic staining was the standard used for all of the other antibodies. For each case, the extent of staining was graded as: 0 (negative), 1+ (<5% cells staining), 2+ (5–25% cells staining), 3+ (26–75% cells staining), and 4+ (>75% cells staining). The intensity of staining was graded as: 0 (negative), 1+ (weakly positive), and 2+ (strongly positive). For statistical comparisons, Fisher's Exact test was used, with a 2-tailed p-value of less than 0.05 considered as significant.
Table 1.
Immunohistochemistry specifications
| Marker | Clone | Dilution | Vendor |
|---|---|---|---|
| p63 | 4A4+Y4A3 | Prediluted | LabVision, Fremont, CA |
| CD10 | 56C6 | Prediluted | Biocare, Concord, CA |
| Cytokeratin cocktail (Kermix) | AE1/AE3 + LP34 | 1:200 | Signet, England |
| Cytokeratin 5/6 | D5/16 B4 | Prediluted | LabVision, Fremont, CA |
| Cytokeratin AE-1/3 | AE-1/AE-3 | 1:100 | Signet, England |
Results and Discussion
The patient demographics and distribution of lesions are presented in Table 2. The immunohistochemical results for AFXs and SCSCCs are presented in Table 3 and Table 4, respectively.
Table 2.
Distribution of lesions by anatomic site and patient demographic data
| AFX(n=23) | SCSCC(n=10) | |
|---|---|---|
| Location of lesions | 7 | 3 |
| Scalp | 10 | 3 |
| Face | 1 | 2 |
| Ear | 1 | 0 |
| Neck | 2 | 1 |
| Trunk | 2 | 0 |
| Upper Limb | 0 | 1 |
| Lower Limb | 7 | 3 |
| Age | ||
| Range (years) | 37–85 | 53–90 |
| Median (years) | 70 | 75 |
| Gender | ||
| Male | 22 | 9 |
| Female | 1 | 1 |
AFX, atypical fibroxanthomas; SCSCC, spindle cell/sarcomatoid squamous cell carcinomas
Table 3.
Immunohistochemical features of AFX
| Case | Extent (Intensity) | ||||
|---|---|---|---|---|---|
| P63 | CD10 | Kermix | CK5/6 | AE1/3 | |
| 1 | 0 | 4+(2+) | 0 | 0 | 0 |
| 2 | 0 | 4+(2+) | 0 | 0 | 0 |
| 3 | 0 | 4+(2+) | 0 | 0 | 0 |
| 4 | 1(1+) | 0 | 0 | 0 | 0 |
| 5 | 0 | 0 | 0 | 1+(2+) | 0 |
| 6 | 0 | 4+(1+) | 0 | 0 | 0 |
| 7 | 2(1+) | 4+(2+) | 0 | 0 | 0 |
| 8 | 0 | 4+(1+) | 0 | 0 | 0 |
| 9 | 1+(1+) | 4+(2+) | 0 | 0 | 0 |
| 10 | 0 | 4+(2+) | 0 | 0 | 0 |
| 11 | 0 | 4+(2+) | 0 | 0 | 0 |
| 12 | 0 | 4+(2+) | 0 | 0 | 0 |
| 13 | 0 | 0 | 4+(1+) | 0 | 0 |
| 14 | 0 | 4+(2+) | 0 | 0 | 0 |
| 15 | 0 | 0 | 0 | 0 | 0 |
| 16 | 0 | 3+(1+) | 0 | 0 | 0 |
| 17 | 0 | 4+(2+) | 0 | 0 | 0 |
| 18 | 0 | 2+(1+) | 0 | 0 | 0 |
| 19 | 0 | 3+(2+) | 0 | 0 | 0 |
| 20 | 0 | 0 | 0 | 0 | 0 |
| 21 | 0 | 4+(2+) | 0 | 0 | 0 |
| 22 | 0 | 4+(2+) | 0 | 0 | 0 |
| 23 | 0 | 3+(1+) | 0 | 0 | 0 |
Extent of staining: 0, negative; 1+, <5% cells; 2+, 5–25%; 3+, 26–75%; 4+, >75%. Intensity of staining: 0, strength; 1+, weakly positive; 2+, strongly positive. AFX: atypical fibroxanthoma
Table 4.
Immunohistochemical features of SCSCC
| Case | Extent (Intensity) | ||||
|---|---|---|---|---|---|
| P63 | CD10 | Kermix | CK5/6 | AE1/3 | |
| 1 | 4+(2+) | 0 | 3+(2+) | 4+(2+) | 4+(2+) |
| 2 | 4+(2+) | 1+(1+) | 4+(2+) | 4+(2+) | 4+(2+) |
| 3 | 4+(2+) | 2+(1+) | 4+(2+) | 4+(2+) | 4+(2+) |
| 4 | 4+(2+) | 0 | 4+(2+) | 4+(2+) | 4+(2+) |
| 5 | 4+(2+) | 1+(1+) | 4+(2+) | 4+(2+) | 4+(2+) |
| 6 | 3+(2+) | 3+(1+) | 4+(2+) | 4+(2+) | 4+(2+) |
| 7 | 3+(2+) | 4+(2+) | 4+(2+) | 4+(2+) | 4+(2+) |
| 8 | 0 | 0 | 1+(1+) | 0 | 3+(1+) |
| 9 | 1+(2+) | 3+(2+) | 3+(2+) | 3+(2+) | 2+(2+) |
| 10 | 4+(2+) | 0 | 2+(2+) | NP | 2+(2+) |
Extent of staining: 0, negative; 1+, <5% cells; 2+, 5–25%; 3+, 26–75%; 4+, >75%; Intensity of staining: 0, strength; 1+, weakly positive; 2+, strongly positive; NP, not performed. SCSCC: spindle cell/sarcomatoid squamous cell carcinoma
Since there is no absolutely objective external validator of the rendered diagnoses, we selected the expression of cytokeratins as the most likely diagnostic end point for the purpose of this study. Essentially, in the differential between AFX and SCSCC, we considered the diffuse expression of cytokeratins as evidence of the latter. 3 of the 26 cases originally classified as AFXs demonstrated strong and/or diffuse staining for multiple cytokeratins and were accordingly reclassified as SCSCCs. In addition, the indeterminate case was also found to demonstrate staining for multiple cytokeratins. After reclassification of these 4 cases, we observed the immunohistochemical staining for all antibodies using a final total of 23 AFXs and 10 SCSCCs.
CD10 was positive in 18/23 AFXs (78%), with most demonstrating strong and/or diffuse staining (Figure 1a-d). 3 AFXs (13%), negative for cytokeratins, showed focal and weak nuclear staining for p63. 2 AFXs (9%) demonstrated very focal or very weak staining for only one of the cytokeratin immunostains; in these two cases, both p63 and CD10 were negative. There was one AFX that was negative for all immunostains.
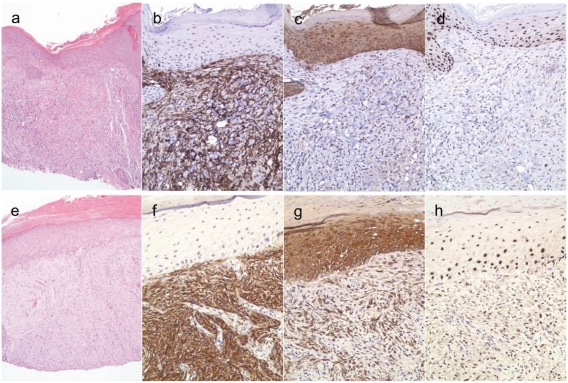
Figure 1.
Examples of H&E, CD10, cytokeratin AE1/3 and p63 staining patterns in atypical fibroxanthoma (AFX) (a-d) and spindle cell/sarcomatoid squamous cell carcinoma (SCSCC) (e-h). H&E at 100X and immunohistochemical stains at 200X. AFX (a) showing diffuse and strong staining for CD10 (b) and negative staining for cytokeratin AE1/3 (c) and p63 (d). SCSCC (e) showing diffuse and strong staining for CD10 (f), cytokeratin AE1/3 (g), and nuclear staining for p63 (h).
CD10 was positive in 6/10 SCSCCs (60%), with half of these demonstrating strong and/or diffuse staining (Figure 1e-h). P63 was positive in 9/10 SCSCCs (90%), with most demonstrating strong and diffuse staining, similar to the results obtained with our cytokeratin panel. One SCSCC was negative for p63, but positive with two cytokeratin immunostains. Among the three AFXs reclassified as SCSCCs, two were found to be positive for p63.
Table 5 summarizes the data on the proportions of each lesion that displayed any immunoreactivity for each of the markers, irrespective of extent. As expected, the expression of any of the cytokeratins evaluated herein significantly distinguished AFX from SCSCC. Notably, CD10 used in isolation, is not useful in making this distinction (18/23 versus 6/10 respectively, p = 0.4). The addition of CD10 to a panel that includes p63 does not provide any additional information to that obtained with the latter alone. Overall, the best combination to distinguish AFX from SCSCC appears to be p63 and cytokeratin AE-1/3. Positivity for both p63 and cytokeratin AE-1/3 was seen in 9/10 SCSCCs (90%) and was not observed in any of the 23 AFXs (p <0.0001). As previously noted, the solitary case of p63-negative SCSCC was positive for 2 cytokeratins.
Table 5.
Comparison of the immunohistochemical features of AFX and SCSCC
| Marker | Proportion displaying any extent of staining | p value | |
|---|---|---|---|
| AFX | SCSCC | ||
| p63 | 3/23 (13%) | 9/10 (90%) | <0.0001 |
| CD10 | 18/23 (78%) | 6/10 (60%) | 0.4 |
| Kermix | 1/23 (4%) | 10/10 (100%) | <0.0001 |
| Cytokeratin 5/6 | 1/23 (4%) | 8/9 (89%) | <0.0001 |
| Cytokeratin AE-1/3 | 0/23 (0%) | 10/10 (100%) | <0.0001 |
| p63 and AE-1/3 | 0/23 (0%) | 9/10 (90%) | <0.0001 |
AFX, atypical fibroxanthoma; SCSCC, spindle cell/sarcomatoid squamous cell carcinoma
Our findings are consistent with those of Dotto et al [26], in which p63 was positive in 13/13 SCSCCs (100%) and focally positive in 2/10 AFXs (20%). Similar to Mirza et al [1] and Weedon et al [24], we also found CD10 to be positive in the majority of AFXs. Our results are comparable to those reported by Hultgren et al [25], in which 15/16 AFXs (94%) showed strong and diffuse CD10 staining compared to 5/10 poorly-differentiated SCCs (50%), with 3/5 poorly-differentiated SCCs (60%) showing only weak CD10 expression. Overall, our findings suggest that CD10 is positive in a significant number of SCSCCs and that the distribution and intensity of CD10 expression can be similar to that seen in AFXs. Although, the number of cases studied is small, it appears that CD10 is less helpful in this differential diagnosis.
Although both AFXs and SCSCCs are associated with a favorable prognosis, the true biological potential of AFX remains uncertain. In regards to SCSCCs, recurrence is infrequent and cases of metastasis have been rare [2, 4, 18]. Similarly, recurrent AFX has been shown to be an uncommon event [1, 2, 7, 10–12, 17]. While metastasis of AFX is rare, recent case reports have suggested that it may be underestimated [27–29]. As our ability to distinguish these two entities improves, their true biological behavior can be better delineated. In the current study, SCSCC case #7 was originally diagnosed as an AFX with immunohistochemical studies showing the lesional cells to be negative for cytokeratin AE1/3, S-100, MART-1, and positive for CD68 (KP-1). Eight months later, the patient presented with recurrent tumor, which was again negative for cytokeratin AE1/3 and S-100, and subsequently diagnosed as recurrent AFX. We found the original and the recurrent tumors to be positive for both p63 and multiple cytokeratins, consistent with a SCSCC with recurrence. We also found two additional cases of SCSCC (case #8 and #9), both originally favored to be AFXs, which we found to demonstrate positivity for more than one cytokeratin immunostain. Case #9 was also positive for p63. The indeterminate case (SCSCC case #6) was also found to be positive with multiple cytokeratins and p63. These findings illustrate the potential difficulty in distinguishing AFXs and SCSCCs and support the suggestion that some cases reported in the past as AFX, with further study, may actually prove to be SCSCCs.
Lastly, we found 2/23 AFXs (9%) exhibited focal or weak staining for one cytokeratin immunostain. AFX case #5 showed strong cytokeratin 5/6 expression in only few tumor cells, while AFX case #13 showed diffuse, but very weak staining with Kermix. In both cases, all other cytokeratins, p63, and CD10 were negative. Bansal et al [30] recently reported two cases of AFX with weak cytokeratin positivity and offered possible explanations including aberrant expression of epithelial antigens, phagocytosis of cytokeratins by tumor cells, or in some cases AFXs may actually represent de-differentiated squamous cell carcinomas with loss of epithelial antigens. Whether or not any or all of these theories is true remains to be determined. However, as with AFXs with weak cytokeratin positivity, the significance of p63 expression in AFXs in the absence of cytokeratin expression is also uncertain.
Conclusions
To our knowledge, this study is only the second to investigate CD10 and p63 expression in cutaneous SCSCCs. Our results show that p63 is a useful adjunct to the immunohistochemical evaluation of cutaneous spindle cell lesions, and in particular, the combination of p63 with a cytokeratin will distinguish SCSCCs from AFXs in the vast majority of cases. We also found that although CD10 is positive in the majority AFXs, it is not uncommonly positive in SCSCCs and can show a similar pattern of CD10 expression. Therefore the usefulness of CD10 in this differential diagnosis is limited.
Acknowledgments
This study was funded in part by a grant from the Clinical Research Squadron, Wilford Hall Medical Center, Lackland AFB, TX, USA.
Footnotes
The views expressed in this article are those of the authors and do not reflect the official policy of the Department of Defense or other Departments of the United States Government.
References
- 1.Mirza B, Weedon D. Atypical fibro-xanthoma: a clinicopathological study of 89 cases. Australas J Dermatol. 2005;46:235–238. doi: 10.1111/j.1440-0960.2005.00190.x. [DOI] [PubMed] [Google Scholar]
- 2.Calonje E, Wadden C, Wilson-Jones E, Fletcher CDM. Spindle-cell non-pleomorphic atypical fibroxanthoma: analysis of a series and delineation of a distinctive variant. Histopathology. 1993;22:247–254. doi: 10.1111/j.1365-2559.1993.tb00114.x. [DOI] [PubMed] [Google Scholar]
- 3.Heintz PW, White CR. Diagnosis: atypical fibroxanthoma or not? Evaluating spindle cell malignancies on sun damaged skin: a practical approach. Semin Cutan Med Surg. 1999;18:78–83. doi: 10.1016/s1085-5629(99)80012-1. [DOI] [PubMed] [Google Scholar]
- 4.Cassarino DS, DeRienzo DP, Barr RJ. Cutaneous squamous cell carcinoma: a comprehensive clinicopathologic classification. J Cutan Pathol. 2006;33:191–206. doi: 10.1111/j.0303-6987.2006.00516_1.x. [DOI] [PubMed] [Google Scholar]
- 5.Lewis JS, Ritter JH, El-Mofiy S. Alternative epithelial markers in sarcomatoid carcinomas of the head and neck, lung, and bladder-p63, MOC-31, and TTF-1. Mod Pathol. 2005;18:1471–1481. doi: 10.1038/modpathol.3800451. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein SC, Lim KK, Brodland DG, Heidelberg KA. The many faces of squamous cell carcinoma. Dermatol Surg. 1996;22:243–254. doi: 10.1111/j.1524-4725.1996.tb00315.x. [DOI] [PubMed] [Google Scholar]
- 7.Weiss SW, Goldblum JR. Enzinger and Weiss's Soft Tissue Tumors. 5th ed. St. Louis: Mosby; 2008. Malignant fibrous histiocytoma (pleomorphic undifferentiated sarcoma) pp. 403–426. [Google Scholar]
- 8.McKee PH, Calonje E, Granter SR. Pathology of the skin with clinical correlations. 3rd edition. Philadelphia: Elsevier Mosby; 2005. Connective tissue tumors; pp. 1684–1864. [Google Scholar]
- 9.Weedon D. Skin Pathology. 2nd ed. Philadelphia: Churchill Livingstone; 2002. Tumors of the Epidermis; pp. 753–802. [Google Scholar]
- 10.Granter SR, Folpe AL. Fibrous and fibrohistiocytic tumors. In: Barnhill RL, Crowson AN, editors. Textbook of dermatopathology. 2nd ed. New York: McGraw-Hill; 2004. pp. 785–820. [Google Scholar]
- 11.Heenan PJ. Tumors of the fibrous tissue involving the skin. In: Elder D, Elenitsas, Jaworsky C, Johnson B, editors. Lever's Histopathology of the Skin. 8th ed. Philadelphia: Lippincott Williams & Wilkins; 1997. pp. 847–887. [Google Scholar]
- 12.Fretzin DF, Helwig EB. Atypical fibroxanthoma of the skin. A clinicopathologic study of 140 cases. Cancer. 1973;31:1541–1552. doi: 10.1002/1097-0142(197306)31:6<1541::aid-cncr2820310635>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 13.Wick MR, Fitzgibbon J, Swanson PE. Cutaneous sarcomas and sarcomatoid neoplasms of the skin. Semin Diagn Pathol. 1993;10:148–158. [PubMed] [Google Scholar]
- 14.Smith KJ, Skelton HG, Morgan AM, Barrett TL, Lupton GP. Spindle cell neoplasms coexpressing cytokeratin and vimentin (metaplastic squamous cell carcinoma) J Cutan Pathol. 1992;19:286–293. doi: 10.1111/j.1600-0560.1992.tb01364.x. [DOI] [PubMed] [Google Scholar]
- 15.Helwig EB. Atypical fibroxanthoma. Tex State J Med. 1963;59:664. [PubMed] [Google Scholar]
- 16.Weedon D. Tumors and tumor-like proliferations of fibrous and related tissues. In: Weedon D, editor. Skin Pathology. 2nd ed. Philadelphia: Churchill Livingstone; 2002. pp. 917–954. [Google Scholar]
- 17.Longacre TA, Smoller BR, Rouse RV. Atypical fibroxanthoma. Multiple immunohistologic profiles. Am J Surg Pathol. 1993;17:1199–1209. doi: 10.1097/00000478-199312000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Brenn T, McKee PH. Tumors of the surface epithelium. In: McKee PH, Calonje E, Granter SR, editors. Pathology of the skin with clinical correlations. 3rd ed. Philadelphia: Elsevier Mosby; 2005. pp. 1153–1238. [Google Scholar]
- 19.Boyd AS. Tumors of the epidermis. In: Barnhill RL, Crowson AN, editors. Textbook of Dermatopathology. 2nd ed. New York: McGraw-Hill; 2004. pp. 575–633. [Google Scholar]
- 20.Sigel JE, Skacel M, Bergfeld WF, House NS, Rabkin MS, Goldblum JR. The utility of cytokeratin 5/6 in the recognition of cutaneous spindle cell squamous cell carcinoma. J Cutan Pathol. 2001;28:520–524. doi: 10.1034/j.1600-0560.2001.281005.x. [DOI] [PubMed] [Google Scholar]
- 21.Reis-Filho JS, Torio B, Albergaria A, Schmitt FC. p63 expression in normal skin and usual cutaneous carcinomas. J Cutan Pathol. 2002;29:517–523. doi: 10.1034/j.1600-0560.2002.290902.x. [DOI] [PubMed] [Google Scholar]
- 22.Eyden B, Banerjee SS. Spindle-cell squamous carcinoma exhibiting myofibro-blastic differentiation. A study of two cases showing fibronexus junctions. Virchows Arch. 2002;440:36–44. doi: 10.1007/s004280100482. [DOI] [PubMed] [Google Scholar]
- 23.Winfield HL, Rosenberg AS, Antonescu CR, Weil M, Wang AR. Monophasic sarcomatoid carcinoma of the scalp: a case mimicking inflammatory myofibroblastic tumor and a review of cutaneous spindle cell tumors with myofibroblastic differentiation. J Cutan Pathol. 2003;30:393–400. doi: 10.1034/j.1600-0560.2003.00079.x. [DOI] [PubMed] [Google Scholar]
- 24.Weedon D, Williamson R, Mirza B. CD10, a useful marker for atypical fibroxanthomas. Am J Dermatopathol. 2005;27:181. doi: 10.1097/01.dad.0000150766.74493.19. [DOI] [PubMed] [Google Scholar]
- 25.Hultgren TL, DiMaio DJ. Immunohistochemical staining of CD10 in atypical fibroxanthomas. J Cutan Pathol. 2007;34:415–419. doi: 10.1111/j.1600-0560.2006.00635.x. [DOI] [PubMed] [Google Scholar]
- 26.Dotto JE, Glusac EJ. p63 is a useful marker for cutaneous spindle cell squamous cell carcinoma. J Cutan Pathol. 2006;33:413–417. doi: 10.1111/j.0303-6987.2006.00477.x. [DOI] [PubMed] [Google Scholar]
- 27.Cooper JZ, Newman SR, Scott GA, Brown MD. Metastasizing atypical fibroxanthoma (cutaneous malignant histiocytoma): report of five cases. Dermatol Surg. 2005;31:221–225. doi: 10.1111/j.1524-4725.2005.31046. [DOI] [PubMed] [Google Scholar]
- 28.Helwig EB, May D. Atypical fibroxanthoma of the skin with metastasis. Cancer. 1986;57:368–376. doi: 10.1002/1097-0142(19860115)57:2<368::aid-cncr2820570230>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 29.Giuffrida TJ, Kligora CJ, Goldstein GD. Localized cutaneous metastases from an atypical fibroxanthoma. Dermatol Surg. 2004;30:1561–1564. doi: 10.1111/j.1524-4725.2004.30560.x. [DOI] [PubMed] [Google Scholar]
- 30.Bansal C, Sinkre P, Stewart D, Cockerell CJ. Two cases of cytokeratin positivity in atypical fibroxanthoma. J Clin Pathol. 2007;60:716–717. doi: 10.1136/jcp.2006.044206. [DOI] [PMC free article] [PubMed] [Google Scholar]