Abstract
Interaction of curcumin with lipid bilayers is not well understood. A recent experiment showed that curcumin significantly affected the single-channel lifetime of gramicidin in a 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) bilayer without affecting its single-channel conductance. We performed two experiments to understand this result. By isothermal titration calorimetry, we measured the partition coefficient of curcumin binding to DOPC bilayers. By x-ray lamellar diffraction, we measured the thickness change of DOPC bilayers as a function of the curcumin/lipid ratio. A nonlinear membrane-thinning effect by curcumin was discovered. The gramicidin data were qualitatively interpreted by the combination of isothermal titration calorimetry and x-ray results. We show that not only does curcumin thin the lipid bilayer, it might also weaken its elasticity moduli. The result implies that curcumin may affect the function of membrane proteins by modifying the properties of the host membrane.
Cucumin (1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-di-one) is a natural compound extracted from rhizomes of turmeric (Curcuma longa), commonly used for centuries as a yellow spice. Curcumin has long been reported to be biologically active, most often as having antiinflammatory, antiangiogenic, antioxidant, wound-healing, and anticancer effects (1,2). However its efficacy has been a subject of controversy (3), and its mechanism of action remains obscure. In particular, curcumin modulates the function and expression of a wide range of structurally and functionally unrelated membrane proteins (see Table 1 of Ingolfsson (4)). There is also accumulated evidence of curcumin interacting with cell membranes (5,6). This suggested a hypothesis that curcumin could alter membrane protein function by modulating the properties of the host lipid bilayer (4). In a recent experiment, Ingolfsson et al. (4) found that curcumin significantly altered the lifetime of the gramicidin channel without affecting its single-channel conductance. Since the lifetime of the gramicidin channel is known to vary with the membrane thickness (7), the gramicidin result seems to be clear evidence that supports the hypothesis, if curcumin modifies the bilayer thickness. However the capacitance measurement by Ingolfsson et al. showed no thickness change (4). This puzzling result prompted us to investigate curcumin interaction with lipid bilayers. We measured the partition coefficient of curcumin to lipid bilayers by isothermal titration calorimetry (ITC) and its effect on the thickness of lipid bilayers by x-ray diffraction. We discovered an interesting nonlinear thinning effect on lipid bilayers by curcumin. Based on this result, we discuss the effect of curcumin on the lifetime of gramicidin channel.
Gramicidin is ideal for testing whether curcumin, or any other amphipathic drug, affects the functions of membrane proteins via its interaction with lipid bilayers. The major component of gramicidins produced by Bacillus brevis is a 15-amino acid peptide, gramicidin A or gA(15), which is known to form a well-defined dimeric channel in lipid bilayers (8). The backbone of the channel is in a right-handed β6.3-helix configuration due to its L-D alternating sequence, with largely hydrophobic side chains covering the exterior surface. Two monomers are joined by six hydrogen bonds formyl end/formyl end to form the dimeric channel. In lipid bilayers, gramicidin undergoes association-dissociation (dimmer-monomers) reactions. As a result, a channel has a well-defined lifetime (its survival probability exponentially decreases in time) that can be measured accurately by single-channel conductance experiments (9,10). It has been shown that the gramicidin lifetime is sensitive to the thickness of the lipid bilayer (7). This membrane thickness effect was explained as a consequence of hydrophobic matching between the gramicidin channel and the lipid bilayer (11). When there is a hydrophobic mismatch between the channel and the unperturbed state of the lipid bilayer, the effect of hydrophobic matching would create a local deformation in the lipid bilayer according to the elasticity theory (12). This membrane deformation energy and its effect on the gramicidin channel lifetime have been calculated, and the result successfully explained the membrane thickness effect (13). Thus it is reasonable to expect that membrane-active molecules, such as curcumin, are capable of changing the gramicidin channel lifetime if their binding to the lipid bilayer changes its thickness or elastic property.
Such a test was undertaken recently by Ingolfsson et al. (4). The team led by Andersen and Koeppe have, over the years, synthesized a large variety of gramicidin analogs and used them to investigate lipid-protein interactions (14,15). For the curcumin experiment (4), they measured the lifetime of two analogs [Ala1]gA (AgA(15)) and des-[Val1-Gly2]gA− (gA−(13)) in a black-lipid membrane made of 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) and the effect of adding curcumin to the electrolyte solution. They found that curcumin did not alter the single-channel conductance of either channel but systematically increased the lifetimes of both channels (as much as 10–15 times) as a function of curcumin concentration. No change of single-channel conductance is a strong indication that there is no direct curcumin-gramicidin interaction. Therefore the effect on the lifetime is most likely due to a change of the mechanical property of the bilayer induced by curcumin binding. Indeed Ingolfsson et al. measured and obtained a large partition coefficient for curcumin binding to DOPC. But their membrane capacitance measurement indicated no change in the bilayer thickness. Thus they concluded that the change of lifetime was due to curcumin's effect on the elasticity moduli of the lipid bilayer.
One possible reason for some of the controversies involving curcumin studies is that curcumin is difficult to handle. The molecule has low solubility in low pH, but in high pH it is susceptible to hydrolytic degradation (16). It is also sensitive to light and tends to adsorb to all surfaces. As a result, its actual concentration in solution is very difficult to determine. Therefore before the experiment, we first measured the solubility and stability of curcumin by its optical spectrum. We also measured the loss of curcumin to the walls of the containers. We used ITC to determine the partition coefficient of curcumin to lipid bilayers. For its effect on lipid bilayers, we used x-ray lamellar diffraction to measure the thickness as a function of curcumin/lipid ratio. We found that curcumin binding caused a nonlinear membrane thinning as a function of concentration. The combination of the ITC and x-ray results will be used to discuss how to explain the change of gramicidin channel lifetime by curcumin.
MATERIALS AND METHODS
Sample preparation
Curcumin (product number 28260), HEPES (product number H3375), dimethylsulfoxide (DMSO) (product number D5879), and sodium hydroxide (product number S8045) were purchased from Sigma-Aldrich (St. Louis, MO). DOPC was purchased from Avanti Polar Lipids (Alabaster, AL). All materials were used as delivered.
Since the main purpose of our investigation is to understand the result of the gramicidin experiment (4), we used the same curcumin solution reported in Ingolfsson and colleagues (4), i.e., curcumin in the buffer solution of HEPES at pH 7. However, we did not include NaCl because we intended to compare the results here with a future experiment with giant unilamellar vesicles that we believe will shed further light on curcumin-lipid interactions. It is well known that giant unilamellar vesicle experiments have difficulty with electrolytes.
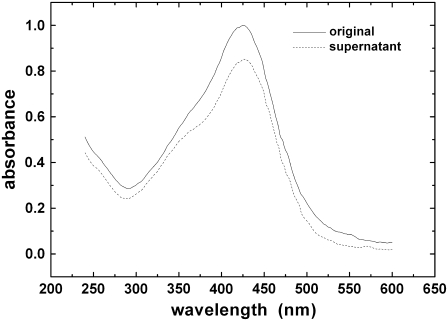
To determine the curcumin concentration, it is important to note 1), The solubility of curcumin is pH dependent. The solubility decreases rapidly as pH decreases below 7. 2), Curcumin degrades rapidly at pH above 7 (16). 3), Curcumin strongly adsorbs to the walls of containers. After trials and errors, we found that at pH 7 the highest achievable concentration for curcumin is ∼25 μM. At this concentration the solution appeared to be transparent to the eyes. However if the solution was subject to centrifugation (14,000 rpm at room temperature for 30 min), there was a small amount of yellow sedimentation. The absorbance of the supernate at the peak of the curcumin spectrum (426 nm) is 97% of the original solution (after a correction for the loss to the wall of the centrifuge tube; Fig. 1). The optical absorption spectroscopy was also employed to determine the percentage of curcumin loss to the container's wall. The solutions were kept in the dark as much as possible.
FIGURE 1.
Absorption spectrum of curcumin in the original 25 μM solution (solid line) and in the supernate after centrifugation (dotted line). The background absorption by the buffer has been removed. Taking into account the curcumin loss to the wall of the centrifuge tube (∼10%), the spectral amplitude of the supernate at 426 nm was estimated to be 97% of the original solution before centrifugation.
Large unilamellar vesicles for ITC
DOPC was dissolved in a solvent of methanol/chloroform (v/v, 1:1). An appropriate amount of the solution was dried under a nitrogen flow, then vacuumed overnight to remove any residue of solvent. An appropriate amount of buffer was added to hydrate the dried film. The dispersion was subjected to vortex mixing and then went through several freeze-thaw cycles between liquid nitrogen and a 40°C water bath. The multilamellar vesicles thus obtained were extruded at room temperature in a miniextruder (Avanti) through two polycarbonate membrane filters of 100 nm pore size more than 10 times. The resultant vesicle size was measured by dynamical light scattering at 90° angle (calibrated by 100-nm-diameter polystyrene beads). The measured diameter of the large unilamellar vesicles (LUVs) was 90–100 nm. The lipid concentration of LUVs was measured by phosphate assay (17). LUVs of 20 mM lipid concentration were prepared as a stock solution and used for experiments within 3 days.
Isothermal titration calorimetry
Before the ITC experiment, the curcumin solution was put in a vacuum degas system (provided by MicroCal, Northampton, MA; 140 mbar at 25°C for 8 min) to remove the possible air bubbles. The heat flow for curcumin binding to the vesicles was measured by a high-sensitivity ITC instrument (MicroCal) with a reaction cell volume of 1.4144 ml. The titration calorimetry experiment was performed as follows. A curcumin solution of 25 μM was placed in the reaction cell, and the LUVs of 5 mM were injected via an injection syringe in a series of aliquots of volume10 μL. The data of heat flow were acquired by computer software developed by MicroCal. The solution in the reaction cell was continually stirred during the measurement. The reaction heat for each injection was determined by integration of the heat flow tracing. In a control experiment, the corresponding LUVs were injected into a buffer solution in reaction cell. The resultant heat (usually called dilution heat) for each injection was subtracted from the corresponding reaction heat of LUVs injected into the curcumin solution. The reaction heat per injection thus obtained was that for curcumin binding to lipid vesicles. This titration calorimetry experiment provides the measurement of binding enthalpy and partition coefficient (18,19). The data reduction is briefly described below. We denote the reaction heat per injection 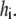 The cumulative heat up to the kth injection is defined as
The cumulative heat up to the kth injection is defined as
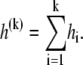 |
(1) |
The cumulative heat will be saturated once all the curcumin molecules in the reaction cell are bound to lipid vesicles. We denote the saturated cumulative heat 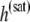 and the molar concentration of curcumin in reaction cell
and the molar concentration of curcumin in reaction cell  then the molar concentration of vesicle-bound curcumin up to the kth injection can be calculated thus
then the molar concentration of vesicle-bound curcumin up to the kth injection can be calculated thus
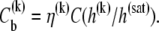 |
(2) |
The original volume of solution in the reaction cell is 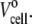 Every injection of the LUV suspension increased the volume by δv. The volume of the solution in the reaction cell up to the kth injection is
Every injection of the LUV suspension increased the volume by δv. The volume of the solution in the reaction cell up to the kth injection is 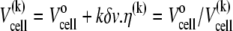 is the dilution factor. The corresponding free curcumin concentration left in the cell up to the kth injection is
is the dilution factor. The corresponding free curcumin concentration left in the cell up to the kth injection is
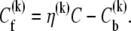 |
(3) |
The lipid concentration in the reaction cell up to the kth injection is
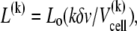 |
(4) |
where Lo is the molar concentration of lipid injected into the cell. The binding enthalpy per mole of curcumin can be measured by
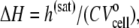 |
(5) |
and the partition coefficient K (or Kp) of curcumin to lipid can be deduced from the binding isotherm
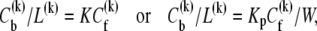 |
(6) |
where W = 55.5 M is the molarity of water.
X-ray lamellar diffraction
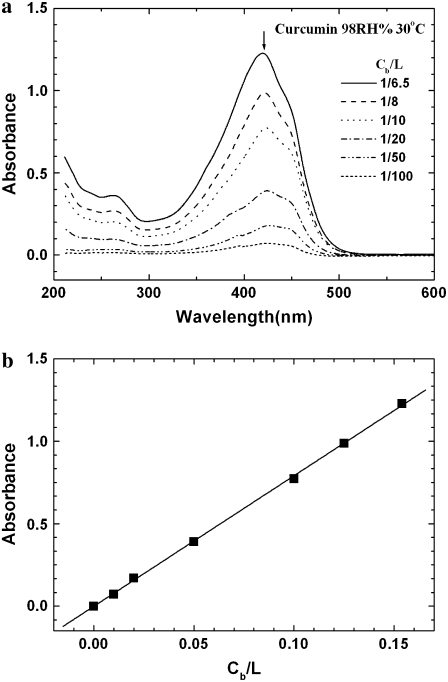
Curcumin was dissolved in a solvent of 1:1 (v/v) methanol and chloroform (1 mg in 4 ml). An appropriate amount was added to 10 mg of DOPC in chloroform solution, according to the desired curcumin/lipid molar ratio Cb/L. An appropriate amount of solution was spread onto a cleaned quartz surface (3 mg of lipid on an  mm2 area). After the solvent evaporated, the sample was placed under vacuum to remove the remaining solvent residues and then slowly hydrated with saturated water vapor until it appeared transparent. To ensure the Cb/L ratios for the x-ray measurement, each sample was measured for its absorption amplitude (Fig. 2 a). Fig. 2 b shows that the amounts of curcumin in the samples were at least in proportion to their Cb/Ls.
mm2 area). After the solvent evaporated, the sample was placed under vacuum to remove the remaining solvent residues and then slowly hydrated with saturated water vapor until it appeared transparent. To ensure the Cb/L ratios for the x-ray measurement, each sample was measured for its absorption amplitude (Fig. 2 a). Fig. 2 b shows that the amounts of curcumin in the samples were at least in proportion to their Cb/Ls.
FIGURE 2.
(a) Absorption spectra of curcumin in the prepared x-ray samples, each containing 3 mg of DOPC and an amount of curcumin according to the molar ratio Cb/L. The background absorption by the lipid and quartz substrate has been removed. (b) The measurement showed that the content was indeed proportional to Cb/L, as proven by the amplitudes of the spectral peak at 426 nm. This check was necessary because of the difficulty for handling curcumin.
For x-ray lamellar diffraction measurement, the sample was kept in a thermally insulated chamber (±0.1°C) that was equipped with Mylar windows for x-ray passage. The chamber also enclosed a polyethylene glycol (PEG) solution for the humidity control (20). The relative humidity (RH) corresponding to a PEG solution was measured by a hygrometer (Rotronic Instrument, Huntington, NY) in a calibration chamber provided by the manufacturer; e.g., 1.0 g of PEG400 dissolved in 4.0 g of water gave a vapor pressure equivalent to 98% RH at 30°C.
The diffractometer consisted of a two-circle goniometer and a Cu Kα radiation source filtered by Ni and operated at 40 kV/30 mA. The two-circle goniometer was designed for vertical θ-2θ scan, so that the sample substrate was kept nearly horizontal during the entire measurement. This allowed us to measure the lipid samples at high hydration levels without the problem of sample running that would otherwise occur if the substrate were oriented vertically as in a horizontal θ-2θ scan experiment. Both the incident and the diffracted x-rays were collimated by two sets of x-y slits. An attenuator was used to prevent the first-order Bragg peak from saturating the detector. Each θ-2θ scan was measured from θ = 0.5° to 10.5° with a step size of Δθ = 0.01° at 1 s per step. Each sample was measured at several different hydration levels from 90% RH to 98% RH to use the swelling method to determine the phases of diffraction amplitudes. The equilibrium of the sample at each humidity setting was ensured by an agreement of at least three consecutive diffraction patterns whose average was subsequently analyzed. Each curcumin-lipid mixture was measured with at least two separately prepared samples. Each sample was measured twice to check its reproducibility. This procedure also ensured that the samples were not damaged by radiation. In previous experiments we observed diffraction pattern changes when a sample was overexposed; such samples also produced extra spots in the thin layer chromatogram (20).
The procedure for data reduction was described in many of our previous works (20–22). Briefly, the procedure started with the background removal and corrections for absorption and diffraction volume. Then the integrated peak intensities were corrected for the polarization and the Lorentz factors. The magnitude of the diffraction amplitude was the square root of the integrated intensity. The phases were determined by the swelling method (23). With their phases determined, the diffraction amplitudes were Fourier transformed to obtain the transbilayer electron density profiles. The profiles were not normalized to the absolute scale, but they gave the correct phosphate peak to phosphate peak distances (PtP), since this distance is independent of normalization (21).
RESULTS
Binding enthalpy and partition coefficient
Curcumin is visibly soluble up to 25 μM in the buffer solution of HEPES at pH 7. Precipitation occurs in higher concentrations. However even at 25 μM, we detected sedimentation if the solution was subject to centrifugation. The spectral amplitude of the supernate was 97% of the original solution before centrifugation (Fig. 1). We wanted to know whether the insoluble part, presumably in a form of suspension of small particles, binds to membranes. Thus we compared the binding isotherm of the original solution with the supernate.
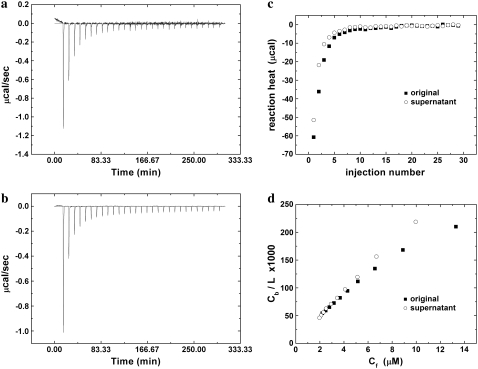
The heat trace of curcumin binding to lipid vesicles is shown in Fig. 3, a and b, for the original 25 μM solution and for the corresponding supernate, respectively. After 10 injections, the heat trace was indistinguishable from the corresponding heat of LUVs injecting into the buffer. We thus took the first 10 injections (max k = 10) to build the binding isotherm. The reaction heat per injection is shown in Fig. 3 c for the original solution and for the supernate, where the corresponding heat of LUVs injecting into the buffer has been removed. The binding enthalpy was estimated by Eq. 5 and the partition coefficient by Eq. 6. For the value of the bound curcumin/lipid ratio Cb/L, we assumed that all lipid molecules (not just the outer monolayers of the vesicles) participated in curcumin binding.
FIGURE 3.
The heat trace of curcumin binding to DOPC vesicles from the original 25 μM curcumin solution (a) and from the corresponding supernatant (b). (c) The reaction heat per injection (the corresponding heat of LUVs injecting into the buffer has been removed). (d) The bound curcumin/lipid ratio as a function of the free curcumin concentration in solution.
In calculating the binding enthalpy and partition coefficient, we corrected for all the losses to the walls of containers in the entire process of measurement. Eq. 6 is plotted in Fig. 3 d for the binding from the original 25 μM solution and from the supernate. Only the latter is a straight line. We conclude that the original 25 μM solution contained 3% undissolved curcumin particles that could be precipitated out by centrifugation and that these particles did not bind to the lipid vesicles.
From the results of the supernate, we found the enthalpy of binding −4.7 kcal/mol and the partition coefficient K = 2.4 × 104 M−1 (or Kp = 1.3 × 106). In comparison, Ingolfsson et al. (4) obtained K = 1.1 × 104 M−1 (or Kp = 6.3 × 105) by a method using the second derivative of the optical spectra (24). We cannot exclude the possibility that the difference is due to the presence of 1 M NaCl in the latter measurement.
To electrically neutral membranes, curcumin has a very large partition coefficient for binding. In comparison, the membrane-active antimicrobial peptide magainin has a partition coefficient of 2 × 103 M−1 for binding to POPC, measured by the same ITC method (25).
X-ray experiment
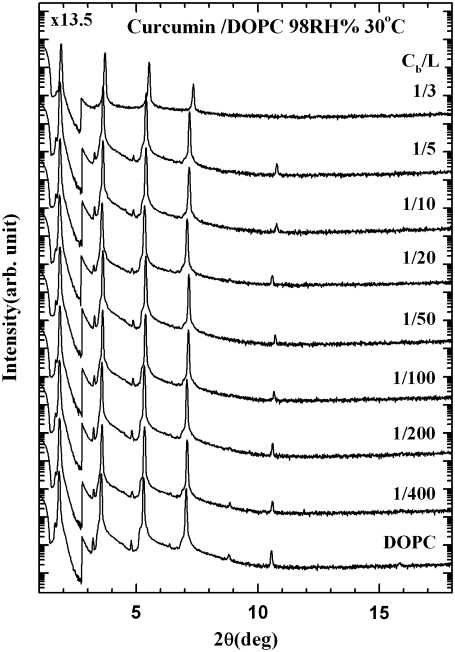
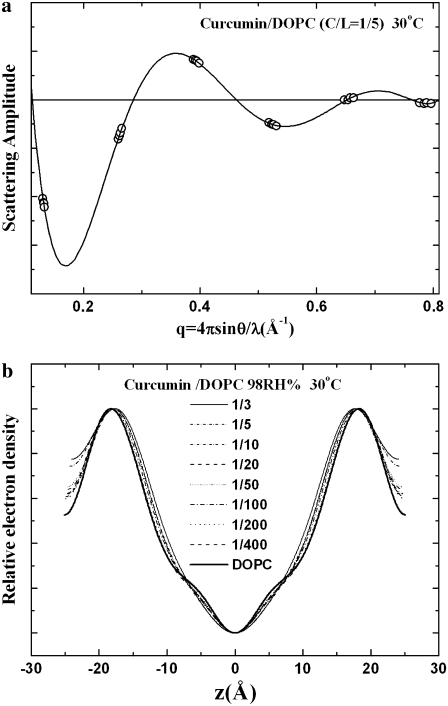
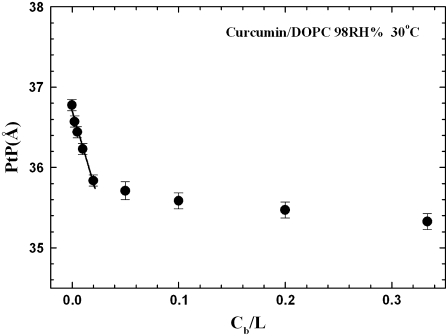
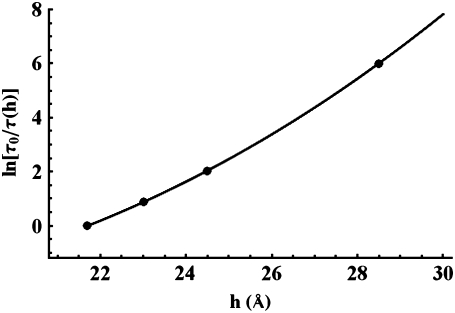
Fig. 4 shows the diffraction patterns of curcumin-DOPC mixtures for a series of curcumin/lipid molar ratios Cb/L = 0 (pure DOPC), 1:400, 1:200, 1:100, 1:50, 1:20, 1:10, 1:5, and 1:3, all at 30°C and 98% RH. Diffraction patterns deteriorated at higher RH values due to undulation fluctuations of membranes (22). Fig. 5 a shows a representative phasing diagram that determined the phases of the diffraction amplitudes. Fig. 5 b shows the electron density profiles across the bilayers for the series of Cb/L. Clearly the PtP decreases with increasing Cb/L. The PtP versus Cb/L is plotted in Fig. 6.
FIGURE 4.
X-ray diffraction patterns for a series of curcumin-DOPC mixtures of ratios Cb/L in aligned multiple bilayers. An attenuator was used to prevent the first-order Bragg peak from saturating the detector. The patterns are displaced for clarity.
FIGURE 5.
(a) An example of a phasing diagram for DOPC bilayers containing curcumin at Cb/L = 1:5 by the swelling method (23). Several sets of data points were obtained from diffraction measured at different humidity levels to change the lamellar spacing. One set of data was used to construct the Shannon curve (solid line). The phases (i.e., the signs of the amplitudes) were chosen such that the curve would go through all the data points. (b) With the phases determined, the diffraction amplitudes were used to construct the electron density profiles for DOPC bilayers containing curcumin at different Cb/L, all at 30°C and 98% RH.
FIGURE 6.
The PtP measured from Fig. 5 b plotted as a function of Cb/L. The error bars represent the ranges of variation of at least three independent measurements.
The PtP is a good measure of the membrane thickness. To a good approximation, the thickness of the hydrocarbon region is 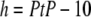 Å or PtP minus twice the length of the glycerol region (from the phosphate to the first methylene of the hydrocarbon chains) (26–28). In using this formula, we note that the electron density profiles (Fig. 5 b) indicate that there is no significant change in the headgroup-glycerol configuration as the C/L increases; the main changes occur in the central chain region. The vertical distance from the phosphate to the first methylene is ∼5 Å as long as the majority of the glycerol backbones are more or less vertical, which should be the case because C/L is ≪1:10 in most cases. Note that the majority of thinning occurred at C/L ≤ 1:50. Also in numerous measurements with antimicrobial peptides binding to lipid bilayers, the PtP initially thins (by an amount ranging from 0.5 to 2 Å; all lipids are PCs) then levels off (constant) with increasing concentrations of peptides (for a review, see Huang (29)), indicating no significant effect on the glycerol configuration by higher concentrations of peptide binding.
Å or PtP minus twice the length of the glycerol region (from the phosphate to the first methylene of the hydrocarbon chains) (26–28). In using this formula, we note that the electron density profiles (Fig. 5 b) indicate that there is no significant change in the headgroup-glycerol configuration as the C/L increases; the main changes occur in the central chain region. The vertical distance from the phosphate to the first methylene is ∼5 Å as long as the majority of the glycerol backbones are more or less vertical, which should be the case because C/L is ≪1:10 in most cases. Note that the majority of thinning occurred at C/L ≤ 1:50. Also in numerous measurements with antimicrobial peptides binding to lipid bilayers, the PtP initially thins (by an amount ranging from 0.5 to 2 Å; all lipids are PCs) then levels off (constant) with increasing concentrations of peptides (for a review, see Huang (29)), indicating no significant effect on the glycerol configuration by higher concentrations of peptide binding.
If we assume that the thinning is caused by the binding of curcumin at the interface of the bilayer that stretches the membrane area, the increase of the interfacial area 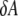 is related to the thickness decrease of the hydrocarbon region
is related to the thickness decrease of the hydrocarbon region 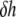 by
by 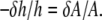 This is due to the fact that the volume compressibility of hydrocarbon chains is very small (30). Assuming that each curcumin molecule binding to the interface increases the interfacial area by an amount AS,
This is due to the fact that the volume compressibility of hydrocarbon chains is very small (30). Assuming that each curcumin molecule binding to the interface increases the interfacial area by an amount AS, 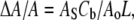 where Ao is the cross sectional area of pure DOPC per lipid. Therefore we have
where Ao is the cross sectional area of pure DOPC per lipid. Therefore we have
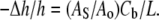 |
(7) |
Ao = 73.4 Å2 was calculated from the PtP of pure DOPC (36.8 Å) and its chain volume per lipid (984 Å 3) (31). From the average initial slope of Fig. 6 (from Cb/L = 0 to 1:50), we obtain AS ≈ 130 Å2. This compares with the cross sectional area along the long axis of curcumin, which we estimated to be ∼115 Å2. This indicates that curcumin initially binds to the bilayer interface with its long axis parallel to the plane of the interface. The change of the thinning slope upon further binding indicates a change of the curcumin binding state. We will investigate this problem by a future experiment with giant unilamellar vesicles.
DISCUSSION
The maximum solubility of curcumin in the pH 7 buffer solution of HEPES is 25 μM. In higher pH, curcumin degrades rapidly (on the timescale of minutes) and its solubility decreases rapidly with decreasing pH. Since the method of ITC requires introducing a vesicle solution into a curcumin solution, the binding curve is limited to below ∼10 μM of free curcumin. Within this range the binding is well described by a single partition coefficient 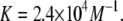 This is 10 times higher than that of the membrane-active antimicrobial peptide magainin; so there is no question that curcumin has a high affinity for binding to lipid bilayers. If DOPC lipid bilayers are exposed to a 25 μM curcumin solution, the bound curcumin/lipid molar ratio would be 3:5 in equilibrium, if the binding curve is extrapolated linearly to this high concentration.
This is 10 times higher than that of the membrane-active antimicrobial peptide magainin; so there is no question that curcumin has a high affinity for binding to lipid bilayers. If DOPC lipid bilayers are exposed to a 25 μM curcumin solution, the bound curcumin/lipid molar ratio would be 3:5 in equilibrium, if the binding curve is extrapolated linearly to this high concentration.
There is also no question that bound curcumin causes membrane thinning. Interestingly the initial binding of curcumin, up to Cb/L ≈ 1:50, thins the bilayer with a high thinning rate per molecule. This initial thinning decreases the thickness by ∼1 Å at Cb/L ≈ 1:50. Further binding of curcumin thins the bilayer at a rate only 1/30 of the initial rate.
Effect of curcumin on the gramicidin channel lifetime
Ingolfsson et al. (4) performed gramicidin single-channel experiments by using a bilayer-forming solution of DOPC in n-decane. It was known that such bilayers would contain an unknown amount of solvent (n-decane), unlike solvent-free bilayers prepared from squalene (7). This makes quantitative interpretation of the gramicidin data from Ingolfsson et al. (4) very difficult. Theoretical expressions (13) derived from Helfrich's bilayer elasticity (12) are applicable only to solvent-free bilayers, which were used in the experiment by Elliott et al. (7). Nevertheless the results by Ingolfsson et al. (4) are informative. Although the elastic responses of a solvent-containing bilayer and a solvent-free bilayer are different, they are qualitatively similar. Therefore we expect the effect of curcumin on gramicidin channels embedded in solvent-free bilayers to be qualitatively similar to what was observed by Ingolfsson et al. (4). In the following text we demonstrate how the curcumin-induced membrane thinning can be used to interpret a change in the gramicidin channel lifetime. We will use the data from Ingolfsson et al. (4) as if they were performed on solvent-free DOPC bilayers for the purpose of reaching a qualitative conclusion.
Ingolfsson et al. (4) measured the effect of curcumin on two gramicidin analogs, AgA(15) and gA−(13), and found that curcumin binding increased the lifetimes of both channels. The effect on gA−(13) is greater than that on AgA(15). To understand these results, let's review the membrane thickness effect on the gramicidin channel measured by Elliott et al. (7). Single channels of gA(15) were measured in lipid bilayers of thickness from below 21.7 Å up to 28.5 Å. They found that the lifetime remained the same for thicknesses below 21.7 Å, but above 21.7 Å the lifetime systematically decreased with increasing thickness. This implies that the hydrophobic thickness of the gA(15) channel is 21.7 Å. In a thicker membrane, the local deformation of the bilayer creates a pulling force that facilitates dissociation of the dimeric channel, hence shortening the channel lifetime (13). These data and the theoretical fit by one of us (13) were reproduced in the form of 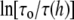 vs. h in Fig. 7 for the following discussion, where
vs. h in Fig. 7 for the following discussion, where 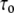 is the lifetime of the gA(15) channel in the bilayer of thickness 21.7 Å; this is the natural lifetime of the gA(15) channel without subjecting it to membrane stress.
is the lifetime of the gA(15) channel in the bilayer of thickness 21.7 Å; this is the natural lifetime of the gA(15) channel without subjecting it to membrane stress.
FIGURE 7.
The lifetime τ of gramicidin A single channel in membranes of (hydrocarbon) thickness h, reproduced from Elliott et al. (7) and Huang (13). For numerical analyses, the fitting curve can be reproduced by the expression (1.25864 − 0.639155h + 0.0220003h2 + 0.000220317h3).
Pure DOPC bilayer has a (hydrocarbon) thickness of 26.8 Å that has a 5.1 Å mismatch with the gA(15) channel. Let 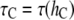 and
and 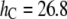 Å, i.e., τC is the lifetime of the gA(15) channel in the pure DOPC bilayer. Curcumin binding reduces the bilayer thickness by Δh (
Å, i.e., τC is the lifetime of the gA(15) channel in the pure DOPC bilayer. Curcumin binding reduces the bilayer thickness by Δh (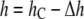 ) and hence reduces the mismatch with the channel. Therefore, the channel lifetime in the presence of curcumin should be longer than τC.
) and hence reduces the mismatch with the channel. Therefore, the channel lifetime in the presence of curcumin should be longer than τC. 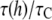 should increase with the curcumin concentration. This is exactly what Ingolfsson et al. (4) found.
should increase with the curcumin concentration. This is exactly what Ingolfsson et al. (4) found.
The case of gA(13) should be qualitatively similar. However, since gA(13) is shorter than gA(15) by 3 Å (32), the data in Fig. 7 are not directly applicable. If we use Fig. 7 qualitatively for gA(13), we have to increase the mismatch by 3 Å, then the DOPC thickness would be at 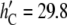 Å. The data for gA(13) would be
Å. The data for gA(13) would be 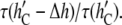 Since
Since 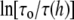 vs. h (Fig. 7) has a greater slope at higher values of h, the ratio
vs. h (Fig. 7) has a greater slope at higher values of h, the ratio 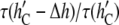 for gA(13) is
for gA(13) is 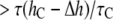 for gA(15). This was found by Ingolfsson et al. (4).
for gA(15). This was found by Ingolfsson et al. (4).
For quantitative analyses, we compare the data of AgA(15) presented in Fig. 5 of Ingolfsson et al. (4) with the data in Fig. 7, by using the equality
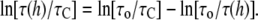 |
(8) |
Consider the data at the highest curcumin concentration of the gramicidin experiment, 6 μM, where 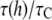 was found to be ∼10 or
was found to be ∼10 or 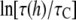 ≈ 2.3. Ingolfsson et al. estimated that under this condition, the Cb/L of the black lipid membrane (BLM) is ∼0.01. The bilayer thinning would be
≈ 2.3. Ingolfsson et al. estimated that under this condition, the Cb/L of the black lipid membrane (BLM) is ∼0.01. The bilayer thinning would be 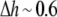 Å or
Å or 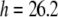 Å from Fig. 6. However this value of Cb/L must be the minimum possible value. It was calculated by assuming the same Cb/L throughout the BLM and its lipid reservoir, which was in an unknown configuration. The authors also mentioned a possible local accumulation of curcumin adjacent to the gramicidin channel. In another extreme, if we assume that curcumin merely adsorbed to the surface of the lipid reservoir so that the free curcumin concentration in the electrolyte was ∼6 μM, then according to the partition coefficient
Å from Fig. 6. However this value of Cb/L must be the minimum possible value. It was calculated by assuming the same Cb/L throughout the BLM and its lipid reservoir, which was in an unknown configuration. The authors also mentioned a possible local accumulation of curcumin adjacent to the gramicidin channel. In another extreme, if we assume that curcumin merely adsorbed to the surface of the lipid reservoir so that the free curcumin concentration in the electrolyte was ∼6 μM, then according to the partition coefficient 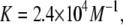 the curcumin concentration in the BLM would be Cb/L = 0.144. This is the maximum possible value of Cb/L. From Fig. 6, at Cb/L = 0.144 the membrane-thinning
the curcumin concentration in the BLM would be Cb/L = 0.144. This is the maximum possible value of Cb/L. From Fig. 6, at Cb/L = 0.144 the membrane-thinning 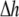 is 1.3 Å, or
is 1.3 Å, or 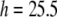 Å. At the minimum value of
Å. At the minimum value of 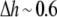 Å, we found by using Fig. 7,
Å, we found by using Fig. 7, 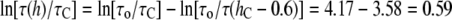 or
or 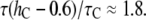 On the other hand, at the maximum value of
On the other hand, at the maximum value of 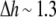 Å, we found
Å, we found 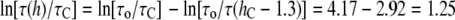 or
or 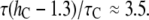 Compared with the measured
Compared with the measured 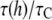 ≈ 10, the thinning effect alone is a factor of 5 to 3 too small. This discrepancy can be understood as follows.
≈ 10, the thinning effect alone is a factor of 5 to 3 too small. This discrepancy can be understood as follows.
The membrane thickness effect on the gramicidin channel lifetime was previously expressed in a theoretical expression in terms of the thickness and elasticity moduli of the bilayer (13). The theoretical expression for 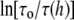 is proportional to a factor
is proportional to a factor 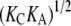 where KC is the bending rigidity and KA is the stretch coefficient of the bilayer (13). The effect of curcumin applies only to the second term on the right-hand side of Eq. 8. For example, if the elasticity moduli depend on curcumin concentration, the term for pure DOPC (i.e.,
where KC is the bending rigidity and KA is the stretch coefficient of the bilayer (13). The effect of curcumin applies only to the second term on the right-hand side of Eq. 8. For example, if the elasticity moduli depend on curcumin concentration, the term for pure DOPC (i.e., 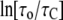 ) is not affected, but the term for
) is not affected, but the term for 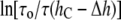 should be modified by the changes of the elasticity moduli. It is reasonable to expect that curcumin binding would alter the elasticity moduli. For example, recently Zhou and Raphael (33) measured the effect of salicylate binding on KC and KA and found that both values could be reduced by a factor of up to ∼2, depending on the salicylate concentration.
should be modified by the changes of the elasticity moduli. It is reasonable to expect that curcumin binding would alter the elasticity moduli. For example, recently Zhou and Raphael (33) measured the effect of salicylate binding on KC and KA and found that both values could be reduced by a factor of up to ∼2, depending on the salicylate concentration.
Since 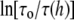 is proportional to
is proportional to 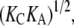 if both KC and KA were reduced by a factor of α, the value of
if both KC and KA were reduced by a factor of α, the value of 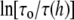 would also be reduced by the factor α. It is easy to find the factor α necessary to explain the data of Ingolfsson et al. For
would also be reduced by the factor α. It is easy to find the factor α necessary to explain the data of Ingolfsson et al. For 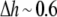 Å, it is
Å, it is 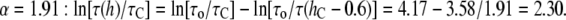 For
For 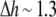 Å, it is
Å, it is 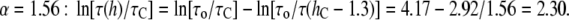 These examples show how to calculate the change of the gramicidin channel lifetime by the changes of bilayer thickness and bilayer elasticity moduli.
These examples show how to calculate the change of the gramicidin channel lifetime by the changes of bilayer thickness and bilayer elasticity moduli.
In conclusion, curcumin binding causes a nonlinear membrane-thinning effect. But the thinning alone probably could not explain the very large effect by curcumin on the lifetime of the gramicidin channel (an increase by a factor of 10–15). The most reasonable explanation is that curcumin binding also weakens the elasticity moduli of the bilayers, a conclusion also reached by Ingolfsson et al. (4). Binding to lipid bilayers is a general property of curcumin. This implies that curcumin could affect the functions of membrane proteins since it will modify the thickness and the elastic property of the host lipid bilayers.
Acknowledgments
This work was supported by National Science Council (NSC; Taiwan) Contract NSC 95-2112-M-145-001 (to W.-C.H.), NSC 95-2112-M-008-018-MY3 (to F.-Y.C.), NSC 96-2112-M-213-007-MY3 (to M.-T.L.) and by the National Institutes of Health (US) Grants GM55203 and the Robert A. Welch Foundation Grant C-0991 (to H.W.H.).
Editor: Lukas K. Tamm.
References
- 1.Joe, B., M. Vijaykumar, and B. R. Lokesh. 2004. Biological properties of curcumin-cellular and molecular mechanisms of action. Crit. Rev. Food Sci. Nutr. 44:97–111. [DOI] [PubMed] [Google Scholar]
- 2.Maheshwari, R. K., A. K. Singh, J. Gaddipati, and R. C. Srimal. 2005. Multiple biological activities of curcumin: a short review. Life Sci. 78:2081–2087. [DOI] [PubMed] [Google Scholar]
- 3.Mall, M., and K. Kunzelmann. 2005. Correction of the CF defect by curcumin: hypes and disappointments. Bioessays. 27:9–13. [DOI] [PubMed] [Google Scholar]
- 4.Ingolfsson, H. I., R. E. Koeppe 2nd, and O. S. Andersen. 2007. Curcumin is a modulator of bilayer material properties. Biochemistry. 46:10384–10391. [DOI] [PubMed] [Google Scholar]
- 5.Jaruga, E., A. Sokal, S. Chrul, and G. Bartosz. 1998. Apoptosis-independent alterations in membrane dynamics induced by curcumin. Exp. Cell Res. 245:303–312. [DOI] [PubMed] [Google Scholar]
- 6.Khajavi, M., K. Shiga, W. Wiszniewski, F. He, C. A. Shaw, J. Yan, T. G. Wensel, G. J. Snipes, and J. R. Lupski. 2007. Oral curcumin mitigates the clinical and neuropathologic phenotype of the Trembler-J mouse: a potential therapy for inherited neuropathy. Am. J. Hum. Genet. 81:438–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elliott, J. R., D. Needham, J. P. Dilger, and D. A. Hayden. 1983. The effects of bilayer thickness and tension on gramicidin single-channel lifetime. Biochim. Biophys. Acta. 735:95–103. [DOI] [PubMed] [Google Scholar]
- 8.Arseniev, A. S., I. L. Barsukov, V. F. Bystrov, A. L. Lomize, and A. Ovchinnikov Yu. 1985. 1H-NMR study of gramicidin A transmembrane ion channel. Head/head right-handed, single-stranded helices. FEBS Lett. 186:168–174. [DOI] [PubMed] [Google Scholar]
- 9.Hladky, S. B., and D. A. Haydon. 1970. Discreteness of conductance change in biomolecular lipid membranes in the presence of certain antibiotics. Nature. 225:451–453. [DOI] [PubMed] [Google Scholar]
- 10.Hladky, S. B., and D. A. Haydon. 1984. Ion Movements in Gramicidin Channels. In Current Topics in Membranes and Transport. Vol. 21. W. D. Stein, editor. Academic Press, New York. 327–372.
- 11.Harroun, T. A., W. T. Heller, T. M. Weiss, L. Yang, and H. W. Huang. 1999. Experimental evidence for hydrophobic matching and membrane-mediated interactions in lipid bilayers containing gramicidin. Biophys. J. 76:937–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helfrich, W. 1973. Elastic properties of lipid bilayers: theory and possible experiments. Z. Naturforsch. [C]. 28c:693–703. [DOI] [PubMed] [Google Scholar]
- 13.Huang, H. W. 1986. Deformation free energy of bilayer membrane and its effect on gramicidin channel lifetime. Biophys. J. 50:1061–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andersen, O. S., and R. E. Koeppe 2nd. 2007. Bilayer thickness and membrane protein function: an energetic perspective. Annu. Rev. Biophys. Biomol. Struct. 36:107–130. [DOI] [PubMed] [Google Scholar]
- 15.Greathouse, D. V., R. E. Koeppe 2nd, L. L. Providence, S. Shobana, and O. S. Andersen. 1999. Design and characterization of gramicidin channels. Methods Enzymol. 294:525–550. [DOI] [PubMed] [Google Scholar]
- 16.Oetari, S., M. Sudibyo, J. N. Commandeur, R. Samhoedi, and N. P. Vermeulen. 1996. Effects of curcumin on cytochrome P450 and glutathione S-transferase activities in rat liver. Biochem. Pharmacol. 51:39–45. [DOI] [PubMed] [Google Scholar]
- 17.Chen, P. S., T. Y. Toribara, and H. Warner. 1956. Micro-determination of phosphorus. Anal. Chem. 28:1756–1758. [Google Scholar]
- 18.Wieprecht, T., and J. Seelig. 2002. Isothermal titration calorimetry for studying interactions between peptides and lipid membranes. Current Topics in Membranes. 52:31–56. [Google Scholar]
- 19.Wiseman, T., S. Williston, J. F. Brandts, and L. N. Lin. 1989. Rapid measurement of binding constants and heats of binding using a new titration calorimeter. Anal. Biochem. 179:131–137. [DOI] [PubMed] [Google Scholar]
- 20.Chen, F. Y., M. T. Lee, and H. W. Huang. 2003. Evidence for membrane thinning effect as the mechanism for peptide-induced pore formation. Biophys. J. 84:3751–3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu, Y., K. He, S. J. Ludtke, and H. W. Huang. 1995. X-ray diffraction study of lipid bilayer membrane interacting with amphiphilic helical peptides: diphytanoyl phosphatidylcholine with alamethicin at low concentrations. Biophys. J. 68:2361–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen, F. Y., W. C. Hung, and H. W. Huang. 1997. Critical swelling of phospholipid bilayers. Phys. Rev. Lett. 79:4026–4029. [Google Scholar]
- 23.Blaurock, A. E. 1971. Structure of the nerve myelin membrane: proof of the low-resolution profile. J. Mol. Biol. 56:35–52. [DOI] [PubMed] [Google Scholar]
- 24.Kitamura, K., N. Imayoshi, T. Goto, H. Shiro, T. Mano, and Y. Nakai. 1995. Second derivative spectrophotometric determination of partition coefficients of chlorpromazine and promazine between lecithin bilayer vesicles and water. Anal. Chim. Acta. 304:101–106. [Google Scholar]
- 25.Wieprecht, T., M. Beyermann, and J. Seelig. 1999. Binding of antibacterial magainin peptides to electrically neutral membranes: thermodynamics and structure. Biochemistry. 38:10377–10387. [DOI] [PubMed] [Google Scholar]
- 26.Hung, W. C., F. Y. Chen, and H. W. Huang. 2000. Order-disorder transition in bilayers of diphytanoyl phosphatidylcholine. Biochim. Biophys. Acta. 1467:198–206. [DOI] [PubMed] [Google Scholar]
- 27.McIntosh, T. J., and S. A. Simon. 1986. Area per molecule and distribution of water in fully hydrated dilauroylphosphatidylethanolamine bilayers. Biochemistry. 25:4948–4952. [DOI] [PubMed] [Google Scholar]
- 28.Nagle, J. F., and S. Tristram-Nagle. 2000. Structure of lipid bilayers. Biochim. Biophys. Acta. 1469:159–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang, H. W. 2006. Molecular mechanism of antimicrobial peptides: the origin of cooperativity. Biochim. Biophys. Acta. 1758:1292–1302. [DOI] [PubMed] [Google Scholar]
- 30.Seemann, H., and R. Winter. 2003. Volumetric properties, compressibilities, and volume fluctuations in phospholipid-cholesterol bilayers. Z. Phys. Chem. 217:831–846. [Google Scholar]
- 31.Armen, R. S., O. D. Uitto, and S. E. Feller. 1998. Phospholipid component volumes: determination and application to bilayer structure calculations. Biophys. J. 75:734–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hwang, T. C., R. E. Koeppe 2nd, and O. S. Andersen. 2003. Genistein can modulate channel function by a phosphorylation-independent mechanism: importance of hydrophobic mismatch and bilayer mechanics. Biochemistry. 42:13646–13658. [DOI] [PubMed] [Google Scholar]
- 33.Zhou, Y., and R. M. Raphael. 2005. Effect of salicylate on the elasticity, bending stiffness, and strength of SOPC membranes. Biophys. J. 89:1789–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]