Abstract
A variety of experiments suggest that membrane proteins are important targets of anesthetic molecules, and that ion channels interact differently with anesthetics in their open and closed conformations. The availability of an open and a closed structural model for the KirBac1.1 potassium channel has made it possible to perform a comparative analysis of the interactions of anesthetics with the same channel in its open and closed states. To this end, all-atom molecular dynamics simulations supplemented by normal mode analysis have been employed to probe the interactions of the inhalational anesthetic halothane with both an open and closed conformer of KirBac1.1 embedded in a lipid bilayer. Normal mode analysis on the closed and open channel, in the presence and absence of halothane, reveals that the anesthetic modulates the global as well as the local dynamics of both conformations differently. In the case of the open channel, the observed reduction of flexibility of residues in the inner helices suggests a functional modification action of anesthetics on ion channels. In this context, preferential quenching of the aromatic residue motion and modulation of global dynamics by halothane may be seen as steps toward potentiating or favoring open state conformations. These molecular dynamics simulations provide the first insights into possible specific interactions between anesthetic molecules and ion channels in different conformations.
INTRODUCTION
Despite the widespread clinical use of anesthetics since the 19th century, a clear understanding of the mechanism of anesthetic action has yet to emerge. The main contenders regarding the site of action for anesthetics are lipids and membrane proteins underlying the nonspecific and specific theories, respectively. Specific interactions of anesthetic molecules with the membrane proteins have been studied since 1952 (1) and experiments have shown that neurotransmitters like GABAA and glycine receptors are sensitive to clinically relevant concentrations of inhaled anesthetics (2). Photo-labeling experiments have revealed possible sites of halothane binding to nicotinic acetylcholine receptors (3). The demonstration of specific action of anesthetics on ion channels through several experiments is a departure from the traditional view of a nonspecific mechanism of anesthetic action through their interactions with lipid bilayers. The nature and mechanism of the specific action of anesthetics on ion channels has been discussed extensively in several recent reviews (4–7).
On the computational side, molecular dynamics (MD) simulations have been used to understand the interactions of inhalational anesthetics molecules such as halothane with both model lipid bilayers and ion channels. Simulations of model lipid bilayers demonstrated the preferential distribution of halothane molecules close to the lipid-water interface in agreement with experiments and effects on hydrophobic chain ordering of the lipids (8–10). Other MD simulations of interactions of anesthetic molecules with ion channels include studies of binding of an inhalational anesthetic, halothane, to soluble native and synthetic four-helix bundles (11,12), to gramicidin in a DMPC membrane (13), to ketosteroid isomerase (14) and to subunits of nicotinic acetylcholine receptors (15).
Ion channels exist in different conformations, i.e., open, closed, resting, or inactive. A natural question is the following: do anesthetic molecules interact with these distinct conformations of the same ion channel differently? Addressing the effects of anesthetics on different conformations is difficult experimentally because ion channels remain in an open state for a much shorter time than in the closed state. Unfortunately, limited availability of high-resolution ion channel structures, which have been implicated in anesthetic mechanism, has impeded potentially insightful MD simulation studies.
A family of K+ channels, tandem pore domains, has been shown to be activated by volatile anesthetics (16) and studies on G-protein-gated-inwardly rectifying K channels have shown that these inward rectifiers contain an intrapore binding site for local anesthetics (17). It has been predicted that anesthetics may prolong open-state components. Also, inhalational anesthetics were shown to potentiate open states in ion channels like the nicotinic acetylcholine receptor (18). Potassium (K+) channels are found in a wide range of cells and tissues and play an important role in controlling the resting potential of the membrane through channel opening and closing by selectively transporting K+ ions across membranes. The KirBac1.1 channel belongs to the inward rectifier family of K+ channels. The three-dimensional crystal structure of this channel in a closed state at a resolution of 3.65 Å was first reported in 2003 (19). KirBac1.1 has a pore-forming tetrameric transmembrane domain (four monomers are named U1, U2, U3, and U4, respectively) similar to that of the prototypical K+ channel, KcsA. Recently, an open-state structure of KirBac1.1 was modeled starting from the closed state and with further refinement using projection maps obtained from electron microscopy experiments (20). The resulting open-state structural model was validated through extensive MD simulations (21). We performed additional MD simulations on both the open and closed states of the KirBac1.1 channel in a DOPC lipid membrane environment and showed that the aromatic residues through localization play an important role in directing and stabilizing structural changes within the transmembrane region of this integral membrane protein (22).
The limited timescales of MD simulations (typically a few tens of nanoseconds) may not capture the global conformational motions of proteins, which play an important role in the function of the protein. Ligands, such as anesthetics, upon binding to ion channels or proteins can affect the function by altering their global motions. Structure-based coarse-grained approaches such as normal mode analysis (NMA) have been used to understand effects of ligands on protein dynamics (23) and functional motions responsible for catalytic reactions (24–26), and to fit high resolution crystal structures into low-resolution electron density maps (27). In the coarse-grained elastic network models used in NMA, the residues are represented as nodes in the network with complex force-field interactions between them replaced by harmonic potentials with a cutoff (28–30). The collective modes representing global (delocalized) changes have been shown to be adequately represented by few low frequency modes (31), which are well captured by such coarse-grain models. In the current work, an elastic network model based on Gaussian-distributed fluctuations (referred to as the Gaussian network model or GNM) is used to understand the effects of halothane molecules on the functional motions of both closed and open conformations of the KirBac1.1 ion channel. For comparative analysis, we have performed the NMA on crystal structure models of closed and open conformations as well as equilibrated MD structures of liganded and unliganded ion channels as inputs. KirBac1.1 provides us with a unique opportunity to compare the effects of inhaled anesthetics on two different conformations within the same family. In this study, we focus on how inhaled anesthetics interact with certain key aromatic residues, as well as influence both local and global protein dynamics in the closed and open conformations of this channel.
METHODS
Description of the systems
The starting configurations before addition of halothane molecules were taken from our previous MD simulations (20 ns for each conformation) and details of setting up closed and open systems can be found in the Supplementary Material of that work (22). Since in this study we are interested in understanding the effects of halothane molecules on the transmembrane domain of the KirBac channel, we retained the slide helices and the transmembrane portions and did not include the intracellular domain. In these MD simulations, 80 halothane molecules were added to the water phase. This concentration of the halothane molecules, which is much higher than clinical concentration, is needed to improve statistics concerning the behavior of the anesthetics. The halothane molecules were initially inserted near the headgroups of both the inner and outer membrane leaflets. This was done because spontaneous translocation of the anesthetic molecules across the lipid membrane is not likely within the MD simulation time (∼15 ns). Each system was then subjected to additional energy minimization runs using the conjugate gradient method, followed by MD equilibration runs.
Molecular dynamics simulations
MD simulations were performed using the NAMD2 (32) software package. The Nosé-Hoover method with Langevin dynamics and Langevin piston were applied to maintain a pressure of 1 bar and temperature of 305 K. A multiple time step (33,34) was used and all the hydrogen atoms were constrained using the RATTLE algorithm to allow a time step of 1.5 fs. The CHARMM 22 (35) and CHARMM 27 (36) force fields were used for the peptide bundles and lipids, respectively and water was modeled by TIP3P (37). Parameters developed by Scharf and Laasonen (38) were employed for halothane molecules. A cutoff distance of 12 Å and a pair-list distance of 15 Å were used to compute all nonbonded interactions and periodic boundary conditions were imposed. The full electrostatics interactions were computed with the particle-mesh Ewald method with a tolerance of 10−6 and updated every two time steps.
Normal mode analysis (NMA)
The elastic network models in NMA are based on random polymer network models (39). As described in the Introduction, in this approach, protein residues are coarse-grained and each residue (represented by Cα) becomes a node in this three-dimensional network. The underlying assumption in elastic network models is that the protein under consideration represents the native state and the global motions can be derived from superposition of various normal modes assuming the harmonic approximation. In the GNM, used in this study, the nodes (hence the residues) are subjected to Gaussian fluctuations (40). In detail, a connectivity matrix (Kirchoff) matrix (Γ) is constructed based on a cutoff distance (rC) as
 |
By diagonalization of the Kirchoff matrix, a set of eigenvalues and eigenmodes are obtained. The root mean-squared fluctuations (RMSF) of the residues of the protein are obtained from the inverse of the Kirchoff matrix as
 |
Here, γ is parameter of the Hookean potential and serves as a scaling factor for the comparison of fluctuations from the simulation and experiments (if B-factors are available) (40), kB is the Boltzmann constant, and T is the temperature in the elastic network. The RMSF values of residues are indicative of the mobility of those residues, and the terms “RMSF” and “mobility” will be used interchangeably in the text. The above equation indicates that the connectivity of a node (or a residue) is inversely proportional to the flexibility of the node. In this regard, the RMSF values depend on the value of rC. A cutoff value <10 Å yielded >0 eigenvalues for the systems with halothane, rendering the results unreliable. Convergence studies on the optimal value of rC yielded 15 Å for all the systems studied in this work. It is to be noted that the eigenmodes from the Kirchoff matrix do not have information about the direction of motion. A Hessian matrix, whose elements are second derivatives of the spring potential used to connect the nodes of the Gaussian network, needs to be constructed for the directional fluctuations of the individual residues, which is implemented in anisotropic network model (ANM) (41). (Both GNM and ANM have online implementations and can be found at http://www.ignm.ccbb.pitt.edu.) Halothane molecules were included in the NMA computations as described in the recent work of Szarecka et al. (23). More details on the inclusion of halothane molecules in the GNM calculations are described in the next section.
RESULTS AND DISCUSSION
Characterization of membrane-protein system
Within ∼15 ns, most of the halothane molecules initially placed in the solution phase spontaneously partitioned into the lipid-protein system. In Fig. 1, the partitioning of halothane molecules for both the closed and open channel systems is shown at t = 0 and t = 15 ns, respectively. The partitioning of halothane molecules is asymmetric with respect to the membrane normal (z axis) in both systems. However, we observed a higher preference for the halothane molecules to be concentrated near the entry of the channel pore at the intracellular region of the open conformation, which is not the case in the closed conformation. Approximately 75% of halothane molecules (60 out of 80) are close to the headgroup region in the inner lipid leaflet in the open conformation, as opposed to somewhat equal distribution of the halothane molecules in both side lipid leaflets in the closed conformation.
FIGURE 1.
Snapshots taken from MD simulations of the closed (upper) and open (lower) conformations of the KirBac1.1 ion channel embedded in a lipid bilayer at t = 0 (left) and t = 15 ns (right). The protein backbones are rendered as ribbons with aromatic residues drawn in white and the halothane molecules in stick representation. The lipid headgroups are also shown and indicate the membrane-water interface. The lipid hydrocarbon chains and water are not shown for visual clarity.
In previous bilayer-halothane computational studies, the most favored locations of halothane molecules is just below the polar headgroups in a complex interfacial region of hydrophilic headgroups and hydrophobic tail groups reflecting the amphiphilic nature of the halothane molecules (8,9,15,42,43). The spontaneous partitioning of halothane molecules into bilayers in times accessible to computer simulations was shown, using steered molecular dynamics calculations, to be due to the absence of significantly high energy barriers against entry into the bilayer (15). In the current simulations also, as described in the previous section, halothane molecules preferentially target the locations below the headgroup region. The presence of anesthetic molecules near the interface of the membrane protein and lipid bilayer, thus disrupting the protein-lipid contacts, has been observed before (13). In the current simulations, anesthetics molecules also partitioned such that they are located near the protein-lipid interface. The number of halothane molecules near the protein-headgroup interface in the simulations of the closed conformation was higher than those of the open conformation.
We have also investigated the distribution of halothane molecules around the protein and in both conformations; halothane molecules are mostly concentrated close to aromatic residues. Density profiles of halothane molecules projected along the bilayer normal (z direction) and averaged over the last 6 ns of the simulation are shown in Fig. 2. The distribution of aromatic residues significantly changes in the presence of halothane molecules in both the open and closed conformations. Importantly, the width of the TRP residue distribution peak (near the inner leaflet) is reduced and is sharper in the presence of halothane molecules, suggesting increase in the localization of these residues.
FIGURE 2.
The density profiles along the bilayer normal, z, averaged over last 6 ns of the MD trajectory for closed and open conformations of KirBac1.1 (z = 0 represents the center of the bilayer). The distribution of halothane molecules is magnified five times for clarity. Distributions of different aromatic residues, charged residues, and lipid molecules are also shown.
Fluorescence experiments (44) have suggested that halothane molecules quench the motion of Trp residues, reducing the fluctuations in the motion of the indole ring. Similar modulation of protein motion in the presence of halothane molecules was observed in previous MD simulations (11,13). The halothane molecule distribution (magnified by five times for clarity) is also shown in Fig. 2. From the distribution we can see that, near the entry of the channel pore at the intracellular side of the closed conformation, the anesthetic molecules are occluded (∼−20 Å), whereas in the open conformation, the distribution peaks near the intracellular pore entry.
Previous atomistic MD simulations showed the distribution of halothane molecules at low anesthetic concentration to be essentially bimodal in nature (8,9,43), with halothane molecules preferentially occupying sites close to the interfacial headgroup water region. However, in recently performed coarse-grain simulations (10), it was seen that this bimodal distribution of halothane molecules is not valid if the concentration of the anesthetic molecules is high. In the latter study, it was observed that as the concentration of the coarse-grain halothane molecules increased, there was an appreciable presence of halothane molecules at the center of the hydrophobic hydrocarbon region. In these simulations also, we observe that halothane molecules penetrate the lipid bilayer and have an appreciable presence at the bilayer center, as can be seen from Fig. 2.
In the open conformation, the halothane molecules were observed to be concentrated at the entry of the channel near the intracellular domain, which is not the case for the closed conformation. Fig. 3 shows average representations of the open and closed conformations of the pore region both with and without halothane molecules. The entry of the intracellular pore in the closed conformation remains occluded to anesthetic molecules throughout the 15-ns MD simulation time, and on an average, six halothane molecules are found near the pore region. The pore of the closed conformation has four Phe residues occluding the pore and more aromatic residues (including Trp and Tyr) line the spacing between the inner and outer loops. On the other hand, in the case of the open conformer, on average 25 halothane molecules are found near and in the pore region as the inner loops open up. One of the effects of the presence of the anesthetic molecules on the open conformer is to reduce the flexibility of the inner helices near the intracellular pore entry.
FIGURE 3.
Shown in the upper-left and -right panels are the average closed and open conformations, respectively, of KirBac taken from the MD simulations and viewed looking down the selectivity filter from extracellular side. The protein backbone α-helices are rendered as ribbons. Only the key aromatic residues lining the pore are shown. Halothane molecules that are found near the pore are shown in the bottom two panels. In the case of the closed conformation (bottom, left), the pore restricts entry of halothane molecules, but in the case of the open conformer (bottom, right), on an average, 25 halothane molecules are present.
To understand the effects of halothane on the local dynamics of the open and closed conformations, root mean-square fluctuation (RMSF) calculations were performed. In Fig. 4, the RMSF calculations of open and closed conformations without the slide helices are shown. The overall shapes of the RMSF profiles in the case of both open and closed conformations in the presence and absence of halothane molecules is somewhat similar. In both cases, an increase in the flexibility of the loops, which are close to the lipid-protein interface, has been observed. Halothane molecules were found close to these loops, and as suggested in previous simulations (13,14), the increase in flexibility can be related to perturbation of lipid-protein interactions due to presence of anesthetic molecules. We also looked at the effect of halothane on the lipid-protein interaction and it was seen that in the presence of halothane molecules, there is an appreciable decrease in the contacts in case of Trp and Tyr aromatic residues in both open and closed conformations, whereas there is essentially no difference in the contacts in the case of Phe residues (data not shown). It has also been observed that the presence or absence of anesthetics has no appreciable effect on the mobility of the selectivity filter.
FIGURE 4.
Influence of anesthetics on the dynamical behavior of the KirBac ion channel. Shown is a comparison of the computed root mean-squared fluctuations (RMSF) of the backbone Cα atoms for the closed (upper) and open (lower) conformations in the presence and absence of halothane molecules, respectively. The subunits in both the conformations are named U1, U2, U3, and U4.
Anesthetic modulation of global dynamics
The total length of the MD simulation for each of the conformations is ∼15 ns. Although this timescale may capture atomistic details of anesthetic interactions with different conformations of the ion channel, this time is likely to be too short to capture the effect on global dynamics, if any, of the anesthetic molecules. We calculated the low frequency global modes for the crystal structure as well as the MD equilibrated structure of the closed form of the KirBac1.1 channel. The average of three slowest modes will be henceforth referred to as the “global mode”. Our results, shown in Fig. 5, agree very well with those obtained in the work of Shrivastava et al. (45) on pore opening mechanisms of different potassium channels; this is perhaps not surprising, since the same Gaussian model was used (especially the results for the closed conformation). The residue mobilities in the low frequency global mode of the MD equilibrated structure overlap very well with those of the crystal structure, even though the fourfold degeneracy as exhibited by the highly symmetric crystal structure is somewhat lost in the MD. The selectivity filter and the Gly residues (equivalent to Gly134 in the crystal structure (19)) of inner helices are the minima of the global modes, as observed previously (45,46). The global minimum at Gly residues suggests that the residue may serve as hinge site for the protein, which was seen in a previous simulation (46) looking at the conformational flexibility of the inner helices of KirBac1.1. We have also plotted the residue mobilities in the global mode for the open model and MD equilibrated structures. The global minimum near the selectivity filter is still preserved in the open conformation. The MD equilibrated structure in the open configuration exhibits more mobility in the N-terminal residues as compared to the model. In a previous work (46), it has been shown that the inner helices (M2 helices) of KirBac channels exhibit dimer-of-dimers type of motion in both octane and a POPC membrane. In this simulation, the KirBac channels in closed-form exhibit dimer-of-dimers motion both in the absence and presence of halothane molecules (see Fig. S1 in Supplementary Material, Data S1).
FIGURE 5.
Comparisons of mean-square fluctuations of residues in the global mode (average of three slowest modes) for closed (top) and open (bottom) conformations between the crystal/model and equilibrated MD structures in the absence of halothane molecules from IGNM calculations are shown. The arrows represent the minimum at the selectivity filter (solid) and at the Gly kink in M2 helix (shaded).
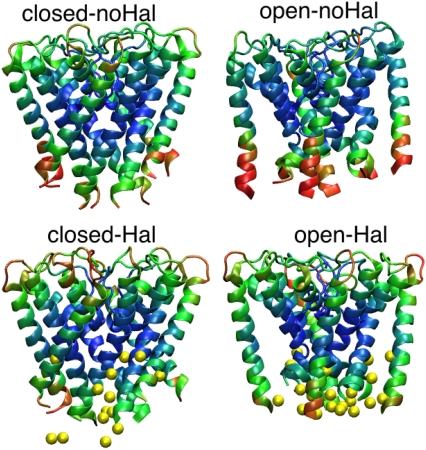
As described in Methods, the halothane molecules are also included in the NMA calculations as single nodes to understand the effect of anesthetics on functional modes and dynamics of KirBac1.1 channels. The starting configurations for the GNM are taken from structures averaged over the last 1 ns of the MD trajectory. In the GNM calculations only halothane molecules near the entry of intracellular pore are included in both conformations. The mobilities of the closed- and open-conformations in the presence and absence of halothane molecules have been mapped to the structure and are shown in Fig. 6. The color coding used in the figure ranges from red (most mobile) to green (intermediate) and to blue (least mobile). The halothane molecules included in the calculation are also included in the figure. From the figure, it is very clear that the presence of halothane molecules near the intracellular pore entry in open conformation has a significant effect on quenching the motion of residues as compared to the closed conformation.
FIGURE 6.
Influence of anesthetics on the dynamical behavior of the KirBac 1.1 ion channel. The mobilities of the residues in the global mode are mapped onto the ribbon representation of the closed and open conformations. The color-coding is from red (most mobile) to blue (least mobile). The halothane molecules represented as single nodes in the GNM calculations are also shown in yellow.
The mobilities of the closed conformation in the presence and absence of halothane molecules are not significantly different, as can be seen in Fig. 6. Due to the constriction at the entry of the pore near the intracellular side of the closed channel, the number of halothane molecules entering and hence affecting the inner helices (where the gate is located) is much smaller. In contrast, the mobilities of inner helices are significantly affected by the presence of halothane molecules in the case of the open conformation, as shown in Fig. 6. Due to the favorable pore space available in the open conformation, a significant number of halothane molecules are found inside and near the intracellular pore entry. From the crystal structure calculations (19), the KirBac1.1 channel in closed conformation is understood to prevent K+ ion conduction by occluding the pore entry from the intracellular side using the phenylalanine residues that are the blocking residues near the gate of the channel. The flexibility of the open channel, especially that of the Phe residues on the inner helices, has been implicated in playing a crucial role in channel gating (22). The present normal mode analysis does reveal that the anesthetic molecules affect the open conformation of the KirBac1.1 channel by reducing the mobility of the inner helices. The decrease in flexibility in the region close to the entry of the channel pore may play a role in potentiating the open conformation in the presence of halothane molecules. The root mean-square fluctuations of the protein residues in the global mode in the presence and absence of halothane molecules is shown in Fig. 7. The global minima near the selectivity filter (indicated by arrows) also remain unaltered in both conformations in the presence of halothane molecules.
FIGURE 7.
The mean-square fluctuations of the residues in the global mode in the presence and absence of halothane molecules are shown. The arrows represent the minimum at the selectivity filter (solid) and at the Gly kink in M2 helix (shaded). Only halothane molecules near the entry of intracellular pore are included in the GNM calculations.
The ANM calculations reveal the direction of motions involved in the slowest normal modes. As in the GNM calculations, only halothane molecules near the intracellular pore of the proteins were included in the ANM calculations. Halothane molecules do not significantly affect the direction of motion of the first three global modes of the closed conformation. However, in the open conformation, halothane molecules significantly alter the direction of motion of the third slowest global mode. This mode contains a hingelike symmetric movement of outer and inner helices involving dimer-of-dimer motions (U1–U3 and U2–U4). These symmetric motions may play an important role in gating of the KirBac1.1 channel (19), required for transition between open and closed conformations. Addition of halothane molecules to the system changes these motions from symmetric to antisymmetric. This change of direction may hamper the gating transition, and along with the reduction of flexibility as described earlier, may prolong the open state. In the open conformation of the KirBac1.1 ion channel, the cavity near the intracellular pore entry flanked by aromatic residues (hydrophobic environment) and polar headgroups and water (polar environment) seem an appropriate location for binding of anesthetics as observed in the current MD simulation. Once the anesthetics occupy this cavity, they affect the functional modes of the open conformation as described in this section.
Aromatic localization and anesthetics
In our previous simulations (22) we have observed that the aromatic residues, especially Phe, close to the lipid-protein interface may play a role in controlling channel gating through localization. In our current MD simulations we explore the dynamic nature of the aromatic residues in the closed and open conformations with and without halothane molecules. An order parameter, characterizing the motion of the aromatic residues, SN is defined as the angle between the normal to the bilayer and a vector drawn perpendicular to the aromatic ring. We evaluated the histogram distribution of the order parameter over the last eight nanoseconds for both closed and open conformers in the presence and absence of halothane. From the comparative analysis, we find that in the case of the closed conformer, the effect of the presence of halothane molecules on the order parameter, SN, is negligible. In the case of the open conformation, however, for the Phe residues near the pore of the channel, where halothane molecules accumulate in the MD simulation, there is an appreciable quenching of the motion of the aromatic rings. Order parameter results for three such residues are shown in Fig. 8.
FIGURE 8.
Interaction of aromatic residues with halothane molecules for the open structure of KirBac. The aromatic residues in question are Phe residues close to the pore of the open conformation, where accumulation of halothane molecules was observed in the MD simulations (as shown in Fig. 3). The term SN is the angle between the normal to the bilayer and a vector drawn perpendicular to the aromatic ring of Phe.
The implication of such quenching is important for potentiating the open state. In this particular case of the KirBac channel, we have shown earlier that the orientation of the Phe residues close to the lipid-protein interface is very dynamic and changes drastically over the MD simulation time (∼10 ns) as compared to the closed conformation (22). The dynamic nature of the Phe orientation is thought to play a role in preventing the channel from being stabilized in the open state. It was suggested earlier (47) that one of the fundamental effects of volatile anesthetics on protein action may be through attenuation of local side-chain dynamics and stabilization of certain folded protein conformations.
CONCLUDING REMARKS
An intimate relationship exists between protein function and flexibility. Fluorescence anisotropy measurements, among other experimental techniques, are able to provide insights into the physical effects of anesthetics in proteins. It has been shown that anesthetics may alter protein function by decreasing protein flexibility and stabilizing certain protein conformations (47–49). Simulations of halothane with gramicidin and luciferase (13,23) have suggested that anesthetic molecules modulate the protein global dynamics. Simulations of halothane interactions with subunits of nicotinic acetylcholine receptor have shown that the anesthetic molecules modulate the dynamics of loops connecting the M2 and M3 subunits, a loop that is implicated in gating mechanism (15,50) by allosterically transmitting the effects to channel gate. Likewise, in the current MD simulations we see that halothane molecules influence both the local and global dynamics of different conformations, but clearly affect the open conformer more. Halothane molecules target the inner helices of the open conformer and decrease the flexibility significantly, as revealed by first three slowest global normal modes. A gate in the KirBac1.1 channel is located at the intracellular pore entry and the four phenylalanine residues form the channel gate. The KirBac1.1 is a unique channel in that it has an abundance of Phe residues. The presence of aromatic residues close to the lipid headgroups have been implicated in stable insertion of the channel using favorable (cation-π) interactions between polar headgroups and aromatic rings. As these aromatic residues are conserved throughout the Kir family, we proposed that aromatic localization could contribute to the forces driving conformational changes (22). From the analysis of order parameters measuring the flexibility of the aromatic residues, it was observed that the Phe residues near the interface experience much higher mobility compared to the closed conformation in the absence of any anesthetic molecules. From that study, the entropy associated with the increased flexibility of the aromatic residues in the open conformer was implicated in preventing the stabilization of the open conformer. With the addition of halothane molecules, we see that the mobility of the inner helices and Phe residues near the gate is significantly decreased. A unitary explanation of anesthetic action on proteins by Tang et al. (13) suggests that functionally significant changes caused by anesthetics are a result of protein's intrinsic susceptibility to the presence of such modulators, as is also seen here.
Cavities inside proteins, especially those embedded in membranes, with appropriate mixture of hydrophobic and polar environment have been implicated as possible locations for anesthetic binding (49). Binding of hydrophobic molecules in cavities of proteins has been shown to reduce the protein motion (51). The binding of anesthetics in the cavities of a protein is expected to play a role in preferentially stabilizing those conformers, which are otherwise poorly populated (49). Membrane proteins spend more time in closed conformations than in open ones. The open conformations, on the other hand, possess cavities large enough to bind small hydrophobic molecules such as anesthetics. This scenario is very close to the conformational ensemble concept described above. The current study suggests that the anesthetic molecules, by preferentially occupying the available (and favorable) cavity in the open conformation, reduce the protein motion in that vicinity and thereby affect the residues implicated in the gating of the channel. This in turn may eventually potentiate the open conformer, consistent with the attractive cavity hypothesis (49). Simulations with nonimmobilizers and other anesthetic molecules on different conformations of membrane proteins may shed further light on the underlying principles of anesthetic action.
SUPPLEMENTARY MATERIAL
To view all of the supplemental files associated with this article, visit www.biophysj.org.
Supplementary Material
Acknowledgments
We thank our collaborators Rod Eckenhoff, Jonas Johansson, Werner Treptow, and Grace Brannigan for useful discussions. Computer time on “Vindhya” at The Institute of Mathematical Sciences is greatly appreciated.
The research described herein was supported by National Institutes of Health grant No. P01-GM055876-09. C.D. is supported by the Royal Society of London.
Editor: Klaus Schulten.
References
- 1.Larrabee, M. G., and J. M. Postenack. 1952. Selective action of anesthetics on synapses and axons in mammalian sympathetic ganglia. J. Neurophysiol. 15:91–114. [DOI] [PubMed] [Google Scholar]
- 2.Mihic, S. J., Q. Ye, M. J. Wick, V. V. Koltchine, M. A. Krasowski, S. E. Finn, M. P. Mascia, C. F. Valenzuela, K. K. Hanson, E. P. Greenblatt, R. A. Harris, and N. L. Harrison. 1997. Sites of alcohol and volatile anesthetic action on GABAA and glycine receptors. Nature. 389:385–389. [DOI] [PubMed] [Google Scholar]
- 3.Chiara, D. C., L. J. Dangott, R. G. Eckenhoff, and J. B. Cohen. 2003. Identification of nicotinic acetylcholine receptor amino acids photolabeled by the volatile anesthetic halothane. Biochemistry. 42:13457–13467. [DOI] [PubMed] [Google Scholar]
- 4.Yamakura, T., E. Bertaccini, J. R. Trudell, and R. A. Harris. 2001. Anesthetics and ion channels: molecular models and sites of action. Annu. Rev. Pharmacol. Toxicol. 41:23–51. [DOI] [PubMed] [Google Scholar]
- 5.Dilger, J. P. 2002. The effects of general anesthetics on ligand-gates ion channels. Br. J. Anaesth. 89:41–51. [DOI] [PubMed] [Google Scholar]
- 6.Campagna, J. A., K. W. Miller, and S. A. Forman. 2003. Mechanisms of actions of inhaled anesthetics. N. Engl. J. Med. 348:2110–2124. [DOI] [PubMed] [Google Scholar]
- 7.Miller, K. W. 2002. The nature of sites of general anesthetic action. Br. J. Anaesth. 89:17–31. [DOI] [PubMed] [Google Scholar]
- 8.Tu, K. C., M. Tarek, M. L. Klein, and D. Scharf. 1998. Effects of anesthetics on the structure of a phospholipid bilayer: molecular dynamics investigation of halothane in the hydrated liquid crystal phase of dipalmitoylphosphatidylcholine. Biophys. J. 75:2123–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koubi, L., M. Tarek, M. L. Klein, and D. Scharf. 2000. Distribution of halothane in a dipalmitoylphosphatidylcholine bilayer from molecular dynamics calculations. Biophys. J. 78:800–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pickholz, M., L. Saiz, and M. L. Klein. 2005. Concentration effects of volatile anesthetics on the properties of model membranes: a coarse-grain approach. Biophys. J. 88:1524–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies, L. A., Q. F. Zhong, M. L. Klein, and D. Scharf. 2000. Molecular dynamics simulation of four-α-helix bundles that bind the anesthetic halothane. FEBS Lett. 478:61–66. [DOI] [PubMed] [Google Scholar]
- 12.Davies, L. A., M. L. Klein, and D. Scharf. 1999. Molecular dynamics simulation of a synthetic four-α-helix bundle that binds the anesthetic halothane. FEBS Lett. 455:332–338. [DOI] [PubMed] [Google Scholar]
- 13.Tang, P., and Y. Xu. 2002. Large-scale molecular dynamics simulations of general anesthetic effects on the ion channel in the fully hydrated membrane: the implication of molecular mechanisms of general anesthesia. Proc. Natl. Acad. Sci. USA. 99:16035–16040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yonkunas, M. J., Y. Xu, and P. Tang. 2005. Anesthetic interaction with ketosteroid isomerase: insights from molecular dynamics simulations. Biophys. J. 89:2350–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vemparala, S., L. Saiz, M. F. Eckenhoff, and M. L. Klein. 2006. Partitioning of anesthetics into a lipid bilayer and their interaction with membrane-bound peptide bundles. Biophys. J. 91:2815–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yost, C. S. 2000. Tandem pore domain K channels: an Important site of volatile anesthetic action? Curr. Drug Targets. 1:207–217. [DOI] [PubMed] [Google Scholar]
- 17.Slesinger, P. A. 2001. Ion selectivity filter regulates local anesthetic inhibition of G-protein-gated inwardly rectifying K+ channels. Biophys. J. 80:701–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forman, S. A., K. W. Miller, and G. Yellen. 1995. A discrete site for general anesthetics on a polysynaptic receptor. Mol. Pharmacol. 48:574–581. [PubMed] [Google Scholar]
- 19.Kuo, A., J. M. Gulbis, J. F. Antcliff, T. Rahman, E. D. Lowe, J. Zimmer, J. Cuthbertson, F. M. Ashcroft, T. Ezaki, and D. A. Doyle. 2003. Crystal structure of the potassium channel KirBac1.1 in the closed state. Science. 300:1922–1926. [DOI] [PubMed] [Google Scholar]
- 20.Kuo, A., C. Domene, L. N. Johnson, D. A. Doyle, and C. Venien-Bryan. 2005. Two different conformational states of the KirBac3.1 potassium channel revealed by electron crystallography. Structure. 13:1463–1472. [DOI] [PubMed] [Google Scholar]
- 21.Domene, C., D. A. Doyle, and C. Venien-Bryan. 2005. Modeling of an ion channel in its open conformation. Biophys. J. 89:L01–L03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Domene, C., S. Vemparala, M. L. Klein, C. Venien-Bryan, and D. A. Doyle. 2006. Role of aromatic localization in the gating process of a potassium channel. Biophys. J. 90:L01–L03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szarecka, A., Y. Xu, and P. Tang. 2007. Dynamics of firefly Luciferase inhibition by general anesthetics: Gaussian and anisotropic network analysis. Biophys. J. 93:1895–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurt, N., W. R. P. Scott, C. A. Schiffer, and T. Haliloglu. 2003. Cooperative fluctuations of unliganded and substrate-bound HIV-1 protease: a structure-based analysis on a variety of conformations from crystallography and molecular dynamics simulations. Proteins Struct. Funct. Bioinf. 51:409–422. [DOI] [PubMed] [Google Scholar]
- 25.Delarue, M., and Y. H. Sanejouand. 2002. Simplified normal mode analysis of conformational transitions in DNA-dependent polymerases: the elastic network model. J. Mol. Biol. 320:1011–1024. [DOI] [PubMed] [Google Scholar]
- 26.Yang, L. W., and I. Bahar. 2005. Coupling between catalytic site and collective dynamics: a requirement for mechanochemical activity of enzymes. Structure. 13:893–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suhre, K., and Y. H. Sanejouand. 2004. On the potential of normal mode analysis for solving difficult molecular replacement problems. Acta Crystallogr. Sect. D Biol. Crystallogr. 60:796–799. [DOI] [PubMed] [Google Scholar]
- 28.Tirion, M. M. 1996. Large amplitude elastic motions in proteins from a single-parameter atomic analysis. Phys. Rev. Lett. 77:1905–1908. [DOI] [PubMed] [Google Scholar]
- 29.Bahar, I., A. R. Atilgan, and B. Erman. 1997. Direct evaluation of thermal fluctuations in proteins using a single-parameter harmonic potential. Fold. Des. 2:173–181. [DOI] [PubMed] [Google Scholar]
- 30.Tama, F., and Y. H. Sanejouand. 2002. Conformational change of proteins arising from normal mode calculations. Protein Eng. 14:1–6. [DOI] [PubMed] [Google Scholar]
- 31.Isin, B., P. Doruker, and I. Bahar. 2002. Functional motions of influenza virus hemagglutinin: a structure-based analytical approach. Biophys. J. 82:569–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kale, L., R. Skeel, M. Bhandarkar, R. Brunner, A. Gursoy, N. Krawetz, J. Philips, A. Shinozaki, K. Varadarajan, and K. Schulten. 1999. Molecular dynamics programs design—NAMD2: greater scalability for parallel molecular dynamics. J. Comput. Phys. 151:283. [Google Scholar]
- 33.Martyna, G. J., M. E. Tuckerman, D. J. Tobias, and M. L. Klein. 1996. Explicit reversible integrators for extended systems. Mol. Phys. 87:1117–1157. [Google Scholar]
- 34.Tuckerman, M. E., and G. J. Martyna. 2000. Understanding modern molecular dynamics: techniques and applications. J. Phys. Chem. B. 104:159–178. [Google Scholar]
- 35.MacKerell, A. D., D. Bashford, M. Bellott, R. L. Dunbrack, J. D. Evanseck, M. J. Field, S. Fischer, J. Gao, H. Guo, S. Ha, D. Joseph-McCarthy, L. Kuchnir, K. Kuczera, F. T. K. Lau, C. Mattos, S. Michnick, T. Ngo, D. T. Nguyen, B. Prodhom, W. E. Reiher, B. Roux, M. Schlenkrich, J. C. Smith, R. Stote, J. Straub, M. Watanabe, J. Wiorkiewicz-Kuczera, D. Yin, and M. Karplus. 1998. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B. 102:3586–3616. [DOI] [PubMed] [Google Scholar]
- 36.Feller, S. E., and A. D. MacKerell. 2000. An improved empirical potential energy function for molecular simulations of phospholipids. J. Phys. Chem. B. 104:7510–7515. [Google Scholar]
- 37.Jorgensen, W. L., J. Chandrasekhar, J. D. Madura, R. W. Impey, and M. L. Klein. 1983. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79:926–935. [Google Scholar]
- 38.Scharf, D., and K. Laasonen. 1996. Structure, effective pair potential and properties of halothane. Chem. Phys. Lett. 258:276–282. [Google Scholar]
- 39.Flory, P. J. 1976. Statistical thermodynamics of random networks. Proc. R. Soc. Lond. A. 351:351–380. [Google Scholar]
- 40.Haliloglu, T., I. Bahar, and B. Erman. 1997. Gaussian dynamics of folded proteins. Phys. Rev. Lett. 79:3090–3093. [Google Scholar]
- 41.Atilgan, A. R., S. R. Durell, R. L. Jernigan, M. C. Demirel, O. Keskin, and I. Bahar. 2001. Anisotropy of fluctuation dynamics of proteins with an elastic network model. Biophys. J. 80:505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang, P., B. Yan, and Y. Xu. 1997. Different distribution of fluorinated anesthetics and nonanesthetics in model membranes: a 19F NMR study. Biophys. J. 72:1676–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koubi, L., L. Saiz, M. Tarek, D. Scharf, and M. L. Klein. 2003. Influence of anesthetic and nonimmobilizer molecules on the physical properties of a polyunsaturated lipid bilayer. J. Phys. Chem. B. 107:14500–14508. [Google Scholar]
- 44.Tsao, D. H. H., J. R. Casa-Finet, A. H. Maki, and J. W. Chase. 1989. Triplet state properties of tryptophan residues in complexes of mutated Escherichia coli single-stranded DNA binding proteins with single-stranded polynucleotides. Biophys. J. 55:927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shrivastava, I. H., and I. Bahar. 2006. Common mechanism of pore opening shared by five different potassium channels. Biophys. J. 90:3929–3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grottesi, A., C. Domene, B. Hall, and M. S. P. Sansom. 2005. Conformational dynamics of M2 helices in KirBac channels: helix flexibility in relation to gating via molecular dynamics simulations. Biochemistry. 44:14586–14594. [DOI] [PubMed] [Google Scholar]
- 47.Johansson, J. S., H. Zou, and J. W. Tanner. 1999. Bound volatile general anesthetics alter both local protein dynamics and global protein stability. Anesthesiology. 90:235–245. [DOI] [PubMed] [Google Scholar]
- 48.Ueda, I., and A. Suzuki. 1998. Irreversible phase transition of firefly Luciferase: contrasting effects of volatile anesthetics and myristic acid. Biochim. Biophys. Acta. 1380:313–319. [DOI] [PubMed] [Google Scholar]
- 49.Eckenhoff, M. F. 2001. Promiscuous ligands and attractive cavities: how do the inhaled anesthetics work? Mol. Interv. 1:258–268. [PubMed] [Google Scholar]
- 50.Law, R. J., R. H. Henchman, and J. A. McCammon. 2005. A gating mechanism proposed from a simulation of a human 7 nicotinic acetylcholine receptor. Proc. Natl. Acad. Sci. USA. 102:6813–6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morton, A., and B. W. Matthews. 1995. Specificity of ligand binding in a buried nonpolar cavity of T4 lysozyme: linkage of dynamics and structural plasticity. Biochemistry. 34:8576–8588. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.