Abstract
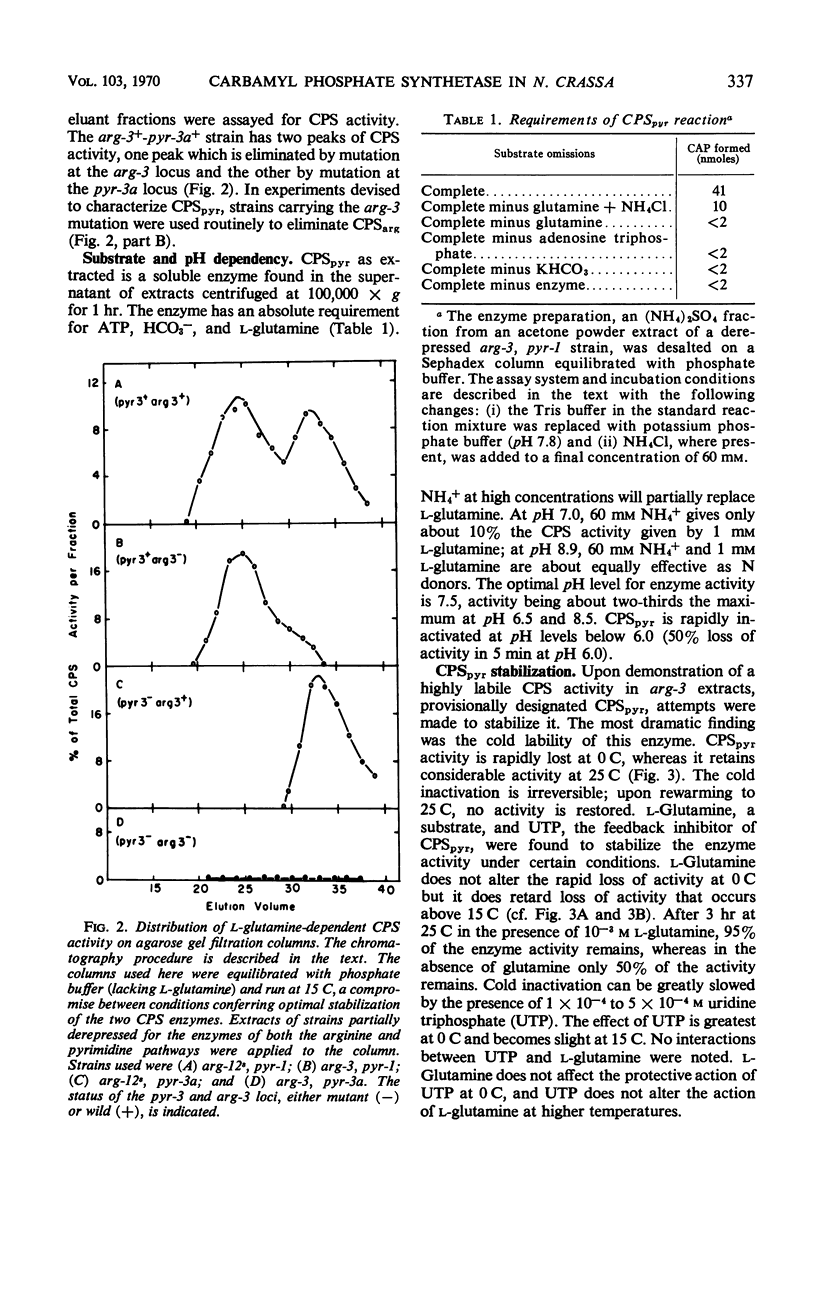
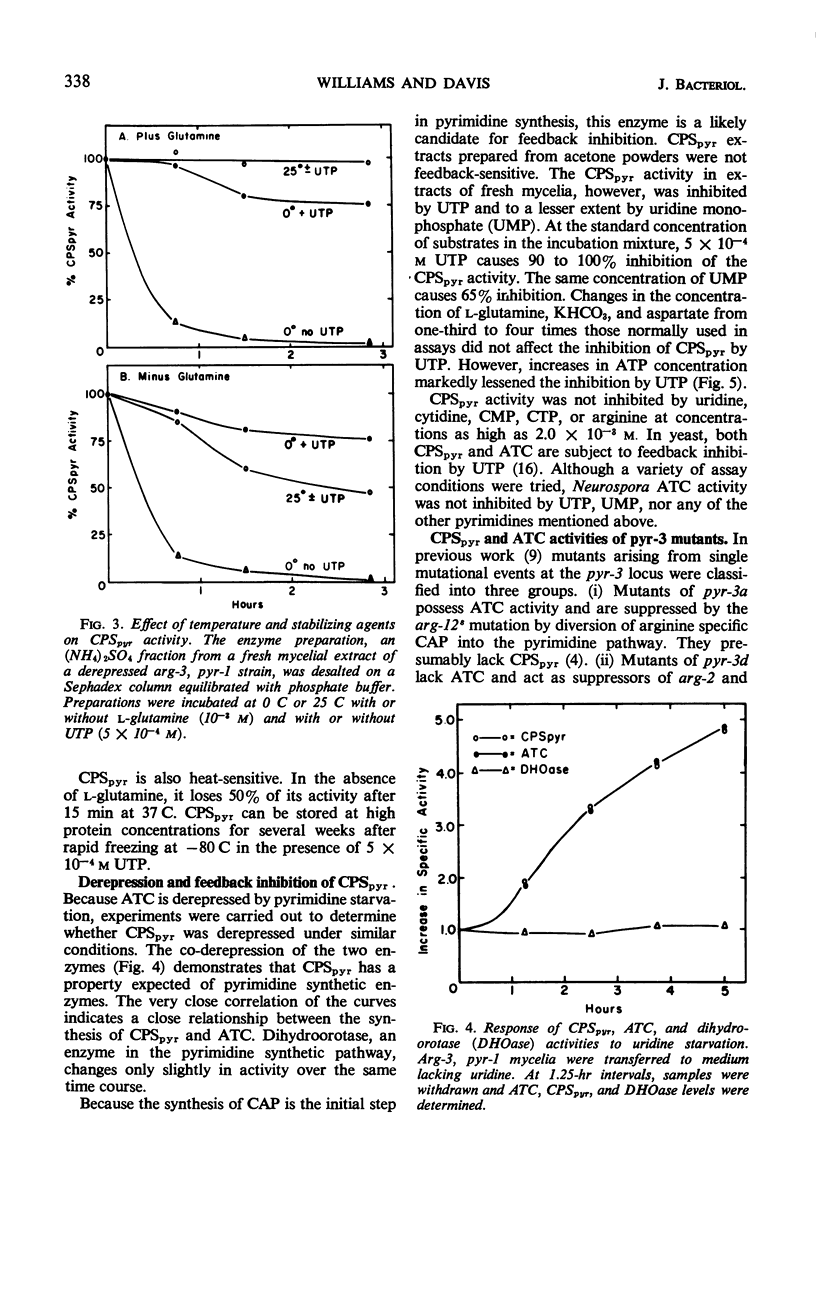
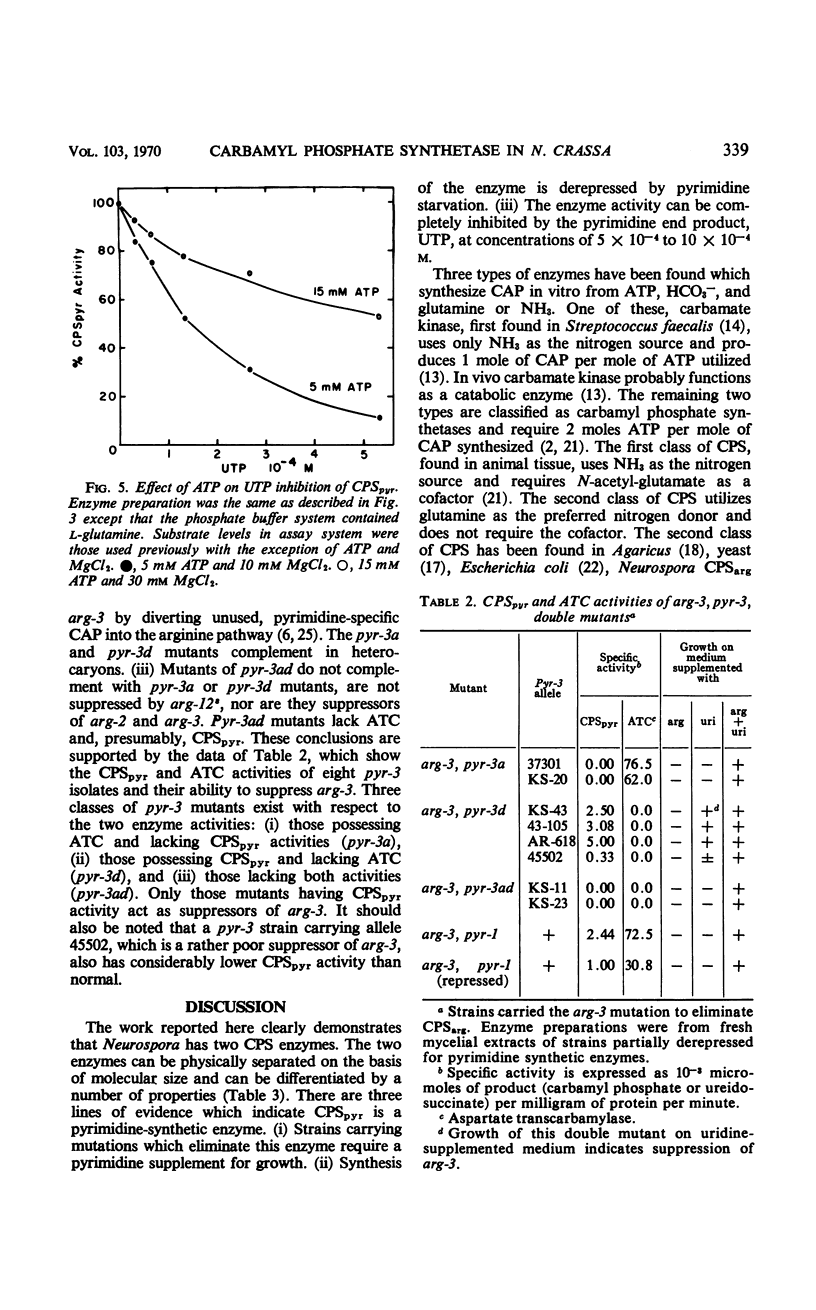
Two carbamyl phosphate synthetases, the first an arginine-synthetic enzyme (CPSarg) and the second a pyrimidine-synthetic enzyme (CPSpyr), are shown to be present in Neurospora. The two enzymes can be separated on the basis of size and are distinguished by several different properties. Both CPSpyr and CPSarg have substrate requirements of adenosine triphosphate, HCO3−, and l-glutamine, although NH4+ in high concentration will partially replace glutamine. CPSpyr activity can be completely inhibited by 5 × 10−4 to 10 × 10−4m uridine triphosphate (UTP). CPSpyr is cold-labile and can be protected against cold inactivation by UTP. The synthesis of CPSpyr and aspartate transcarbamylase (ATC), the initial enzymatic steps of the pyrimidine pathway, are co-derepressed by pyrimidine starvation. Mutations affecting CPSpyr and ATC all map at the same locus, pyr-3. Three classes of mutants with respect to the two activities were found: CPS+ATC−, CPS−ATC+, and CPS−ATC−. The distribution of these mutants on the genetic map, together with other data, indicate that the two activities are carried by a bifunctional protein.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. M., Marvin S. V. Effect of allosteric effectors and adenosine triphosphate on the aggregation and rate of inhibition by N-ethylmaleimide of carbamyl phosphate synthetase of Escherichia coli. Biochemistry. 1970 Jan 6;9(1):171–178. doi: 10.1021/bi00803a022. [DOI] [PubMed] [Google Scholar]
- Anderson P. M., Meister A. Evidence for an activated form of carbon dioxide in the reaction catalyzed by Escherichia coli carbamyl phosphate synthetase. Biochemistry. 1965 Dec;4(12):2803–2809. doi: 10.1021/bi00888a034. [DOI] [PubMed] [Google Scholar]
- Caroline D. F. Pyrimidine synthesis in Neurospora crassa: gene-enzyme relationships. J Bacteriol. 1969 Dec;100(3):1371–1377. doi: 10.1128/jb.100.3.1371-1377.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS R. H. A mutant form of ornithine transcarbamylase found in a strain of Neurospora carrying a pyrimidine-proline suppressor gene. Arch Biochem Biophys. 1962 Apr;97:185–191. doi: 10.1016/0003-9861(62)90063-2. [DOI] [PubMed] [Google Scholar]
- DAVIS R. H. NEUROSPORA MUTANT LACKING AN ARGININE-SPECIFIC CARBAMYL PHOSPHOKINASE. Science. 1963 Dec 27;142(3600):1652–1654. doi: 10.1126/science.142.3600.1652. [DOI] [PubMed] [Google Scholar]
- DAVIS R. H., WOODWARD V. W. The relationship between gene suppression and aspartate transcarbamylase activity in pyr-3 mutants of Neurospora. Genetics. 1962 Aug;47:1075–1083. doi: 10.1093/genetics/47.8.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. H. Carbamyl phosphate synthesis in Neurospora crassa. I. Preliminary characterization of arginine-specific carbamyl phosphokinase. Biochim Biophys Acta. 1965 Aug 24;107(1):44–53. doi: 10.1016/0304-4165(65)90387-9. [DOI] [PubMed] [Google Scholar]
- Davis R. H. Carbamyl phosphate synthesis in Neurospora crassa. II. Genetics, metabolic position, and regulation of arginine-specific carbamyl phosphokinase. Biochim Biophys Acta. 1965 Aug 24;107(1):54–68. doi: 10.1016/0304-4165(65)90388-0. [DOI] [PubMed] [Google Scholar]
- GERHART J. C., PARDEE A. B. The enzymology of control by feedback inhibition. J Biol Chem. 1962 Mar;237:891–896. [PubMed] [Google Scholar]
- Hager S. E., Jones M. E. A glutamine-dependent enzyme for the synthesis of carbamyl phosphate for pyrimidine biosynthesis in fetal rat liver. J Biol Chem. 1967 Dec 25;242(24):5674–5680. [PubMed] [Google Scholar]
- Hill J. M., Woodward V. W. Genetic control of aspartate transcarbamylase by the pyr-3 locus of Neurospora crassa. Arch Biochem Biophys. 1968 Apr;125(1):1–12. doi: 10.1016/0003-9861(68)90631-0. [DOI] [PubMed] [Google Scholar]
- JONES M. E. Carbamyl phosphate. Science. 1963 Jun 28;140(3574):1373–1379. [PubMed] [Google Scholar]
- Kaplan J. G., Duphil M., Lacroute F. A study of the aspartate transcarbamylase activity of yeast. Arch Biochem Biophys. 1967 Mar;119(1):541–551. doi: 10.1016/0003-9861(67)90489-4. [DOI] [PubMed] [Google Scholar]
- LEVENBERG B. Role of L-glutamine as donor of carbamyl nitrogen for the enzymatic synthesis of citruline in Agaricus bisporus. J Biol Chem. 1962 Aug;237:2590–2598. [PubMed] [Google Scholar]
- Lacroute F., Piérard A., Grenson M., Wiame J. M. The biosynthesis of carbamoyl phosphate in Saccharomyces cerevisiae. J Gen Microbiol. 1965 Jul;40(1):127–142. doi: 10.1099/00221287-40-1-127. [DOI] [PubMed] [Google Scholar]
- Lacroute F. Regulation of pyrimidine biosynthesis in Saccharomyces cerevisiae. J Bacteriol. 1968 Mar;95(3):824–832. doi: 10.1128/jb.95.3.824-832.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- METZENBERG R. L., HALL L. M., MARSHALL M., COHEN P. P. Studies on the biosynthesis of carbamyl phosphate. J Biol Chem. 1957 Dec;229(2):1019–1025. [PubMed] [Google Scholar]
- Manney T. R., Duntze W., Janosko N., Salazar J. Genetic and biochemical studies of partially active tryptophan synthetase mutants of Saccharomyces cerevisiae. J Bacteriol. 1969 Aug;99(2):590–596. doi: 10.1128/jb.99.2.590-596.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manney T. R. Evidence for chain termination by super-suppressible mutants in yeast. Genetics. 1968 Dec;60(4):719–733. doi: 10.1093/genetics/60.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piérard A., Glansdorff N., Mergeay M., Wiame J. M. Control of the biosynthesis of carbamoyl phosphate in Escherichia coli. J Mol Biol. 1965 Nov;14(1):23–36. doi: 10.1016/s0022-2836(65)80226-1. [DOI] [PubMed] [Google Scholar]
- REISSIG J. L. Spectrum of forward mutants in the pyr-3 region of Neurospora. J Gen Microbiol. 1963 Feb;30:327–337. doi: 10.1099/00221287-30-2-327. [DOI] [PubMed] [Google Scholar]
- Radford A. Information from ICR-170-induced mutations on the structure of the pyrimidine-3 locus in Neurospora. Mutat Res. 1969 Nov-Dec;8(3):537–544. doi: 10.1016/0027-5107(69)90071-2. [DOI] [PubMed] [Google Scholar]
- Radford A. Polarised complementation at the pyrimidine-3 locus of Neurospora. Mol Gen Genet. 1969 Jul 3;104(3):288–294. doi: 10.1007/BF02539292. [DOI] [PubMed] [Google Scholar]
- Tatibana M., Ito K. Carbamyl phosphate synthetase of the hematopoietic mouse spleen and the control of pyrimidine biosynthesis. Biochem Biophys Res Commun. 1967 Jan 23;26(2):221–227. doi: 10.1016/0006-291x(67)90238-0. [DOI] [PubMed] [Google Scholar]
- YASHPHE J., GORINI L. PHOSPHORYLATION OF CARBAMATE IN VIVO AND IN VITRO. J Biol Chem. 1965 Apr;240:1681–1686. [PubMed] [Google Scholar]



