Abstract
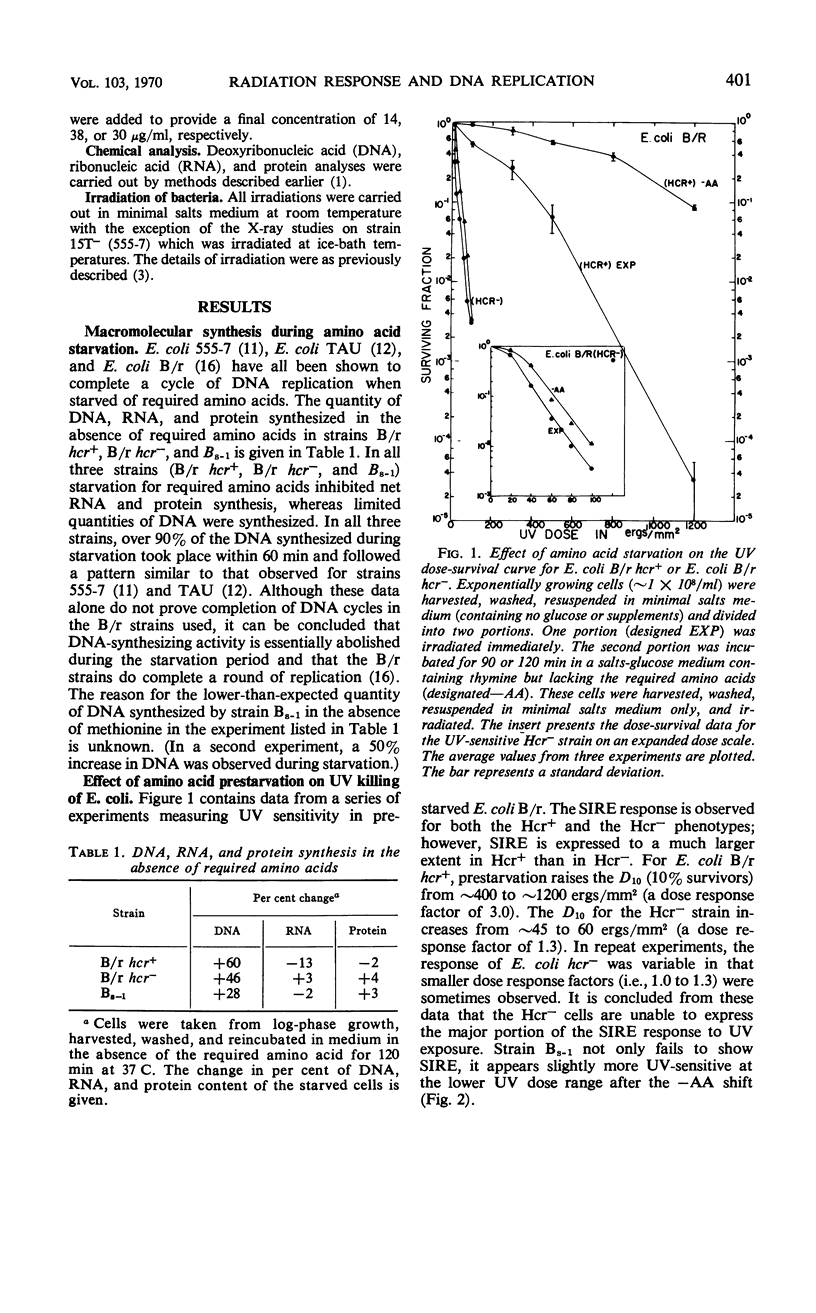
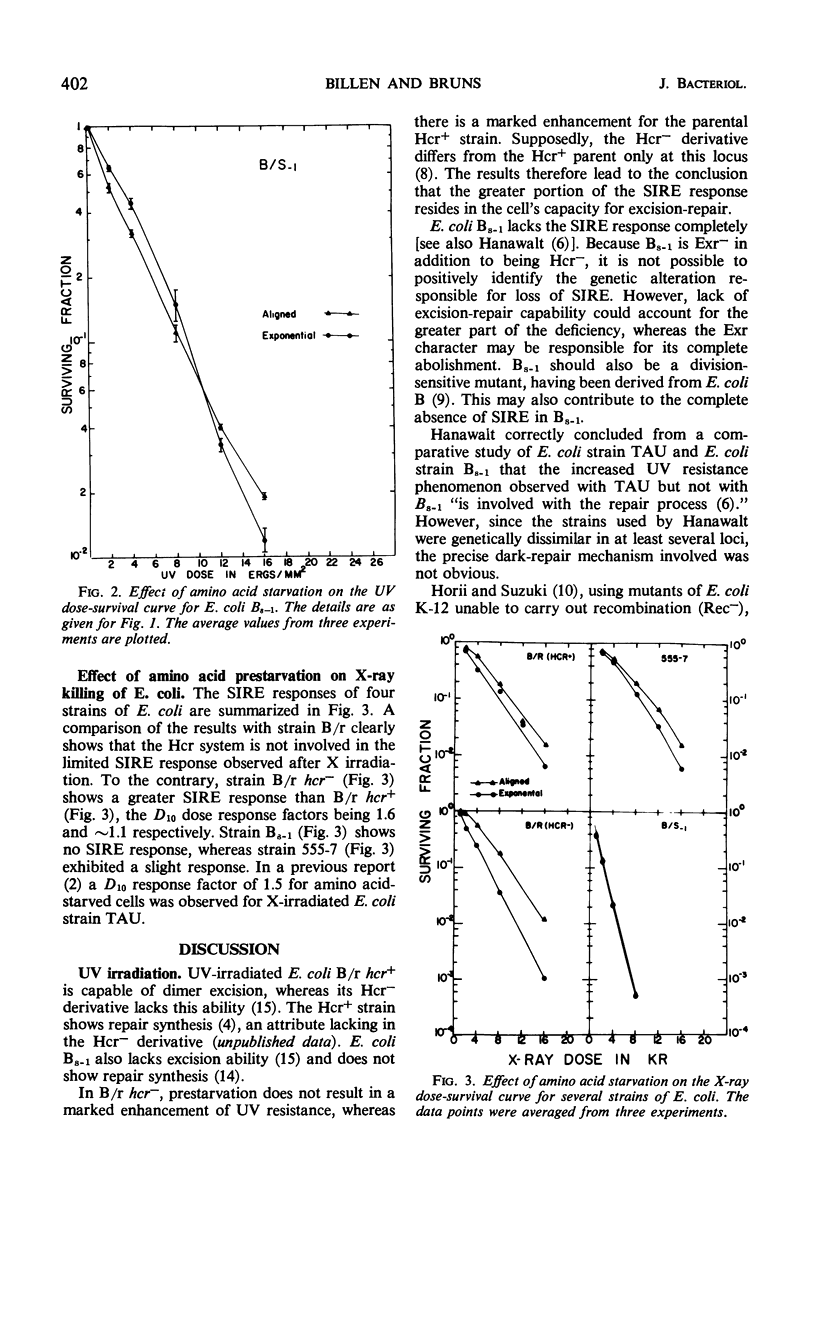
Prestarvation of Escherichia coli for required amino acids results in a marked enhancement in both ultraviolet light (UV) or X-ray resistance for selective strains. Preventing protein synthesis by starvation for required amino acids results in completion of the cycle of chromosomal replication then underway. We have investigated the relationship between starvation-induced resistance enhancement (SIRE) and the excision-repair (Hcr) system in several E. coli strains including E. coli B/r hcr+ and its isogenic mutant E. coli B/r hcr−. The following observations were made. (i) The Hcr system is the major component of SIRE in UV-irradiated strain B/r. By using the Hcr+ strain, SIRE increases the 10% survival dose from ∼400 ergs to ∼1,200 ergs/mm2. With the Hcr cells, the increase is from ∼45 ergs to 60 ergs/mm2. (ii) Although prestarvation leads to a moderate enhancement of resistance to X irradiation, this effect is not dependent on the Hcr system. (iii) The double mutant, E. coli Bs–1 (hcr−exr−) is completely unable to express SIRE whether studied with UV or X irradiation. It is concluded that the Hcr system is the major system responsible for SIRE in UV-treated cells, whereas Exr (resistance to X rays) may be involved to a minor extent. The Exr character appears to be required for SIRE expression in X-ray exposed cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BILLEN D. Alterations in the radiosensitivity of Escherichia coli through modification of cellular macromolecular components. Biochim Biophys Acta. 1959 Jul;34:110–116. doi: 10.1016/0006-3002(59)90238-0. [DOI] [PubMed] [Google Scholar]
- BILLEN D. UNBALANCED DEOXYRIBONUCLEIC ACID SYNTHESIS: ITS ROLE IN X-RAY-INDUCED BACTERIAL DEATH. Biochim Biophys Acta. 1963 Aug 20;72:608–618. [PubMed] [Google Scholar]
- Billen D., Hewitt R. R., Lapthisophon T., Achey P. M. Deoxyribonucleic acid repair replication after ultraviolet light or x-ray exposure of bacteria. J Bacteriol. 1967 Nov;94(5):1538–1545. doi: 10.1128/jb.94.5.1538-1545.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billen D., Hewitt R., Jorgensen G. X-ray-induced perturbations in the replication of the bacterial chromosome. Biochim Biophys Acta. 1965 Jul 15;103(3):440–454. doi: 10.1016/0005-2787(65)90137-1. [DOI] [PubMed] [Google Scholar]
- HILL R. F., SIMSON E. A study of radiosensitive and radioresistant mutants of Escherichia coli strain B. J Gen Microbiol. 1961 Jan;24:1–14. doi: 10.1099/00221287-24-1-1. [DOI] [PubMed] [Google Scholar]
- Hanawalt P. C. The U.V. sensitivity of bacteria: its relation to the DNA replication cycle. Photochem Photobiol. 1966 Jan;5(1):1–12. [PubMed] [Google Scholar]
- Hewitt R., Suit J. C., Billen D. Utilization of 5-bromouracil by thymineless bacteria. J Bacteriol. 1967 Jan;93(1):86–89. doi: 10.1128/jb.93.1.86-89.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R. F. Ultraviolet-induced lethality and reversion to prototrophy in Escherichia coli strains with normal and reduced dark repair ability. Photochem Photobiol. 1965 Jun;4(3):563–568. doi: 10.1111/j.1751-1097.1965.tb09774.x. [DOI] [PubMed] [Google Scholar]
- LARK K. G., REPKO T., HOFFMAN E. J. THE EFFECT OF AMINO ACID DEPRIVATION ON SUBSEQUENT DEOXYRIBONUCLEIC ACID REPLICATION. Biochim Biophys Acta. 1963 Sep 17;76:9–24. [PubMed] [Google Scholar]
- MAALOE O., HANAWALT P. C. Thymine deficiency and the normal DNA replication cycle. I. J Mol Biol. 1961 Apr;3:144–155. doi: 10.1016/s0022-2836(61)80041-7. [DOI] [PubMed] [Google Scholar]
- Mattern I. E., Zwenk H., Rörsch A. The genetic constitution of the radiation-sensitive mutant Escherichia coli Bs-1. Mutat Res. 1966 Oct;3(5):374–380. doi: 10.1016/0027-5107(66)90047-9. [DOI] [PubMed] [Google Scholar]
- PETTIJOHN D., HANAWALT P. EVIDENCE FOR REPAIR-REPLICATION OF ULTRAVIOLET DAMAGED DNA IN BACTERIA. J Mol Biol. 1964 Aug;9:395–410. doi: 10.1016/s0022-2836(64)80216-3. [DOI] [PubMed] [Google Scholar]
- Wolf B., Pato M. L., Ward C. B., Glaser D. A. On the origin and direction of replication of the E. coli chromosome. Cold Spring Harb Symp Quant Biol. 1968;33:575–584. doi: 10.1101/sqb.1968.033.01.064. [DOI] [PubMed] [Google Scholar]


