Abstract
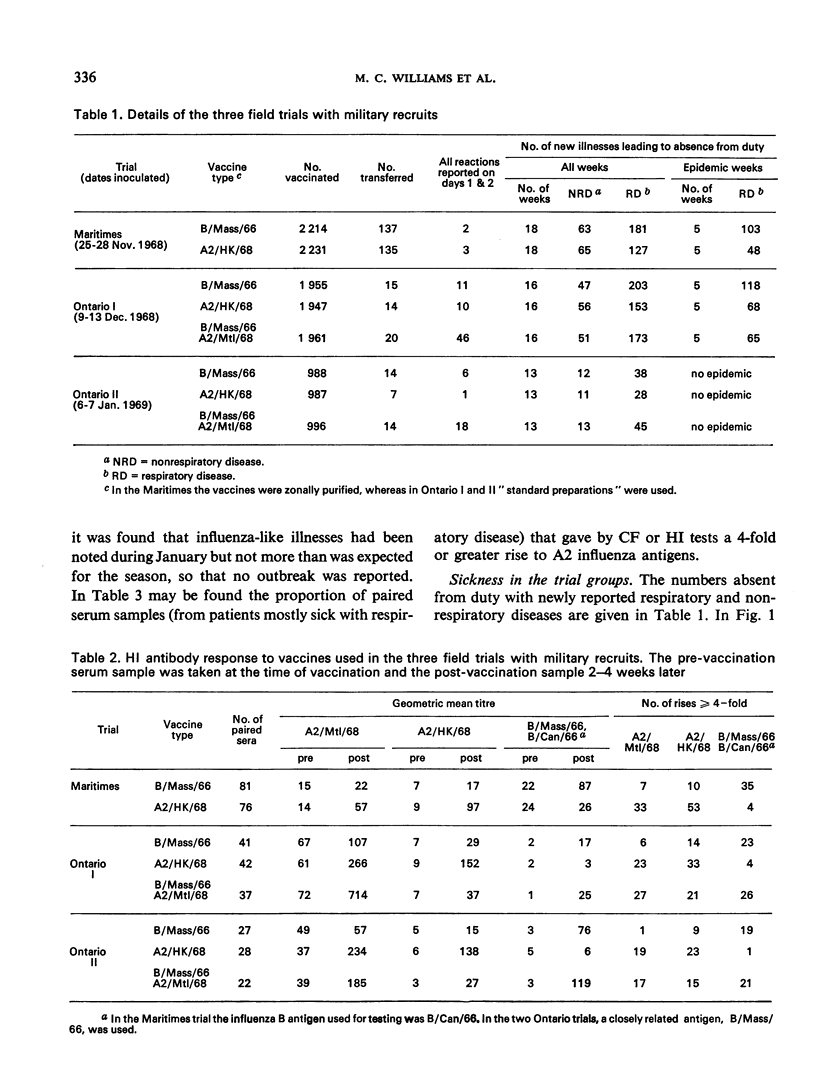
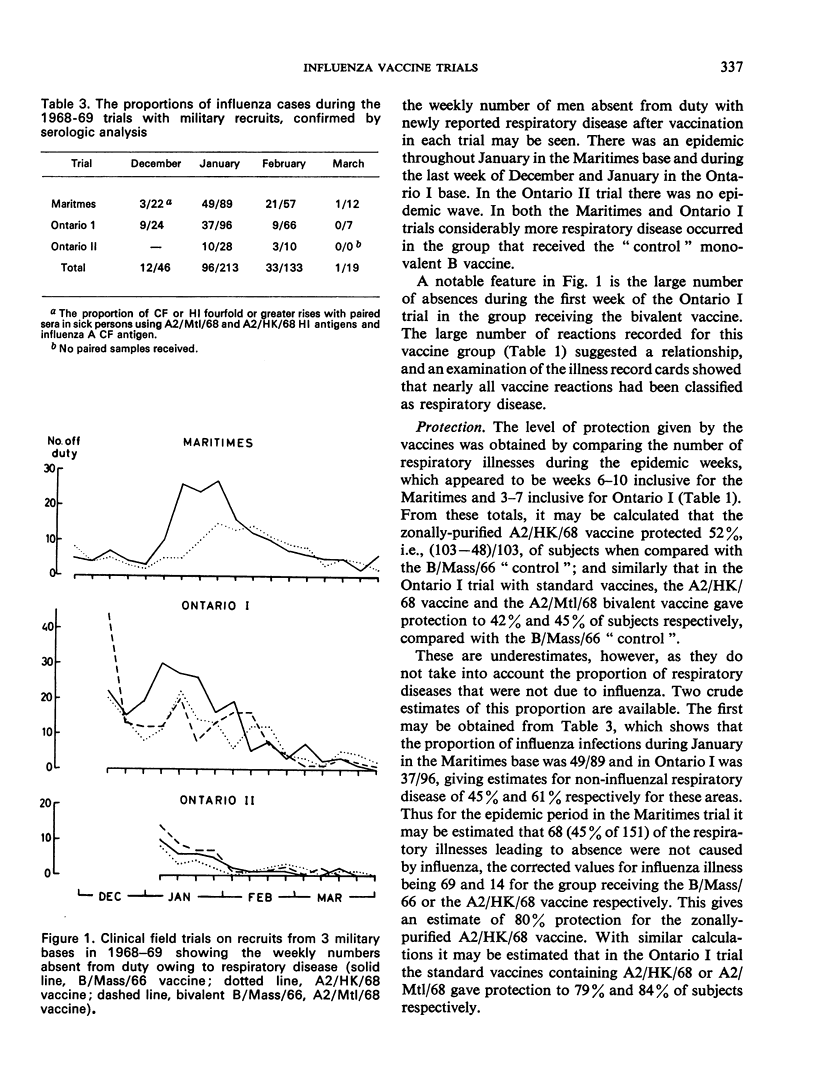
The appearance of the pandemic A/Hong Kong/1/68 (H3N2) influenzavirus strain provided an opportunity for a clinical field trial of influenza vaccines in Canada during the winter of 1968-69. As by November 1968 there were reports of influenza B activity and as supplies of A2/HK/68 vaccines were limited, it was decided to make a series of strictly randomized double-blind trials comparing A2/HK/68 vaccines not only with B/Mass/66 vaccines but also with a bivalent vaccine that was already in production and contained B/Mass/66 and A2/Mtl/68, the latter a strain isolated in Canada during January 1968. In 4 trials, a total of 13 729 military personnel and 4 795 primary schoolchildren were vaccinated. Reported vaccine reactions were less than 0.1% with zonally-purified vaccines and 2.6% with the “standard” aqueous killed bivalent vaccine. Three children had serious reactions. Surveillance detected an outbreak of influenza in the first two trials on the military. The 3 vaccines containing A2 strains gave similar clinical protection conservatively estimated at 42-55% but probably about 80%. The effectiveness of the A2/Mtl/68 vaccine, which was in production before the Hong Kong variant had been isolated, was unexpected. In the absence of a vaccine specific to a new pandemic strain, it should not be assumed that a vaccine made from another recent strain could not be useful.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eddy T. S., Davies N. A. The effect of vaccine on a closed epidemic of Hong Kong influenza. S Afr Med J. 1970 Feb 21;44(8):214–216. [PubMed] [Google Scholar]
- Eickhoff T. C., Meiklejohn G. Protection against Hong Kong influneza by adjuvant vaccine containing A2-Ann Arbor-67. Bull World Health Organ. 1969;41(3):562–563. [PMC free article] [PubMed] [Google Scholar]
- Fazekas de St Groth Evolution and hierarchy of influenza viruses. Arch Environ Health. 1970 Sep;21(3):293–303. doi: 10.1080/00039896.1970.10667241. [DOI] [PubMed] [Google Scholar]
- Finklea J. F., Sandifer S. H., Peck F. B., Manos J. P. A clinical and serologic comparison of standard and purified bivalent inactivated influenza vaccines. J Infect Dis. 1969 Dec;120(6):708–712. doi: 10.1093/infdis/120.6.708. [DOI] [PubMed] [Google Scholar]
- Mostow S. R., Schoenbaum S. C., Dowdle W. R., Coleman M. T., Kaye H. S. Studies with inactivated influenza vaccines purified by zonal centrifugation. 1. Adverse reactions and serological responses. Bull World Health Organ. 1969;41(3):525–530. [PMC free article] [PubMed] [Google Scholar]
- PAVILANIS V., FRAPPIER A., SOMLO F., BOUDREAULT A., CLAVEAU P. Evaluation of the effectiveness of anti-influenza vaccination. Can Med Assoc J. 1958 Oct 1;79(7):527–532. [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum S. C., Mostow S. R., Dowdle W. R., Coleman M. T., Kaye H. S. Studies with inactivated influenza vaccines purified by zonal centrifugation. 2. Efficacy. Bull World Health Organ. 1969;41(3):531–535. [PMC free article] [PubMed] [Google Scholar]
- Stuart-Harris C. H. The prevention of influenza by influenza vaccine. Proc R Soc Med. 1967 Jul 7;60(7):659–662. [PMC free article] [PubMed] [Google Scholar]
- Tyrrell D. A., Buckland R., Rubenstein D., Sharpe D. M. Vaccination against Hong Kong influenza in Britain, 1968-9. A report to the Medical Research Council Committee on Influenza and other Respiratory Virus Vaccines. J Hyg (Lond) 1970 Sep;68(3):359–368. doi: 10.1017/s0022172400042261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman R. H., Bond J. O., Levitt L. P., Hartwig E. C., Prather E. C., Baratta R. L., Neill J. S., Small P. A., Jr An evaluation of influenza immunization: influence of route of administration and vaccine strain. Bull World Health Organ. 1969;41(3):543–548. [PMC free article] [PubMed] [Google Scholar]


