Abstract
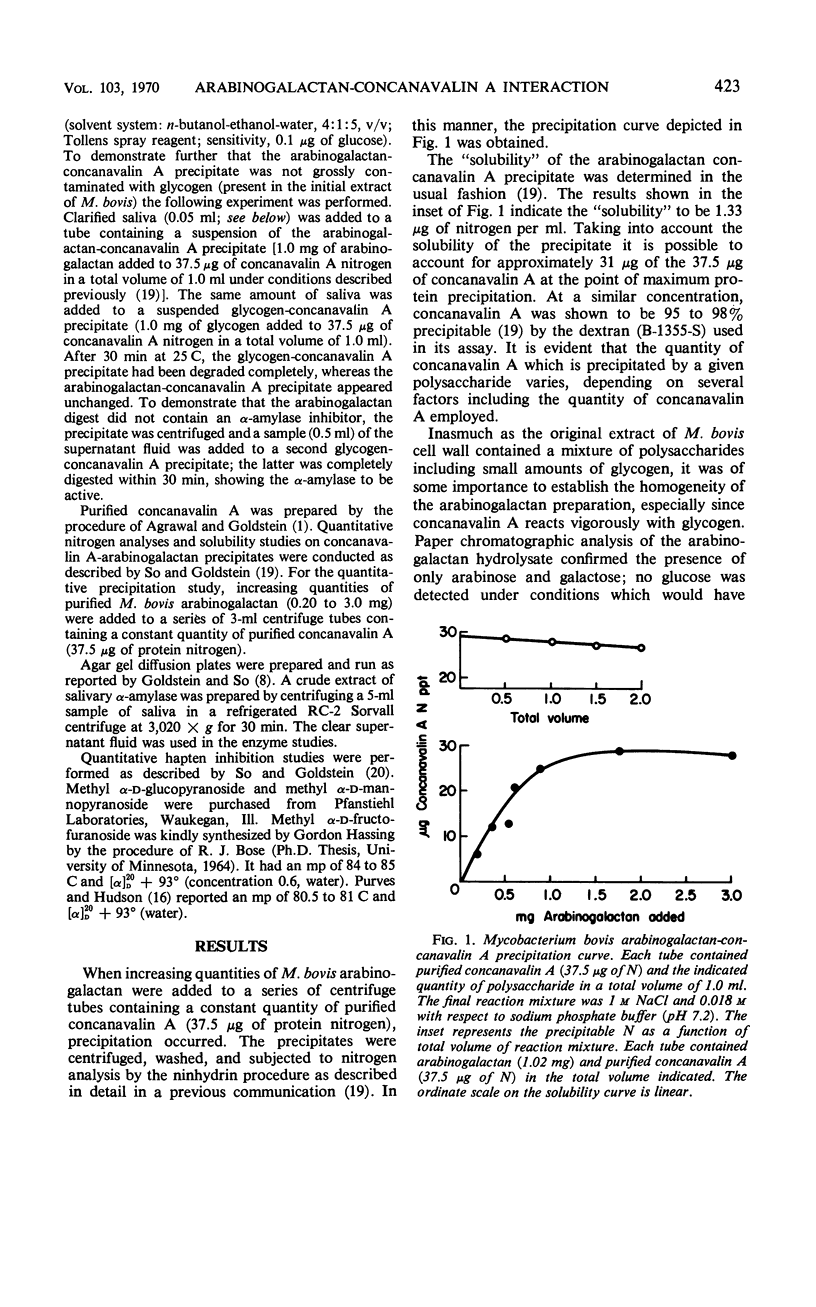
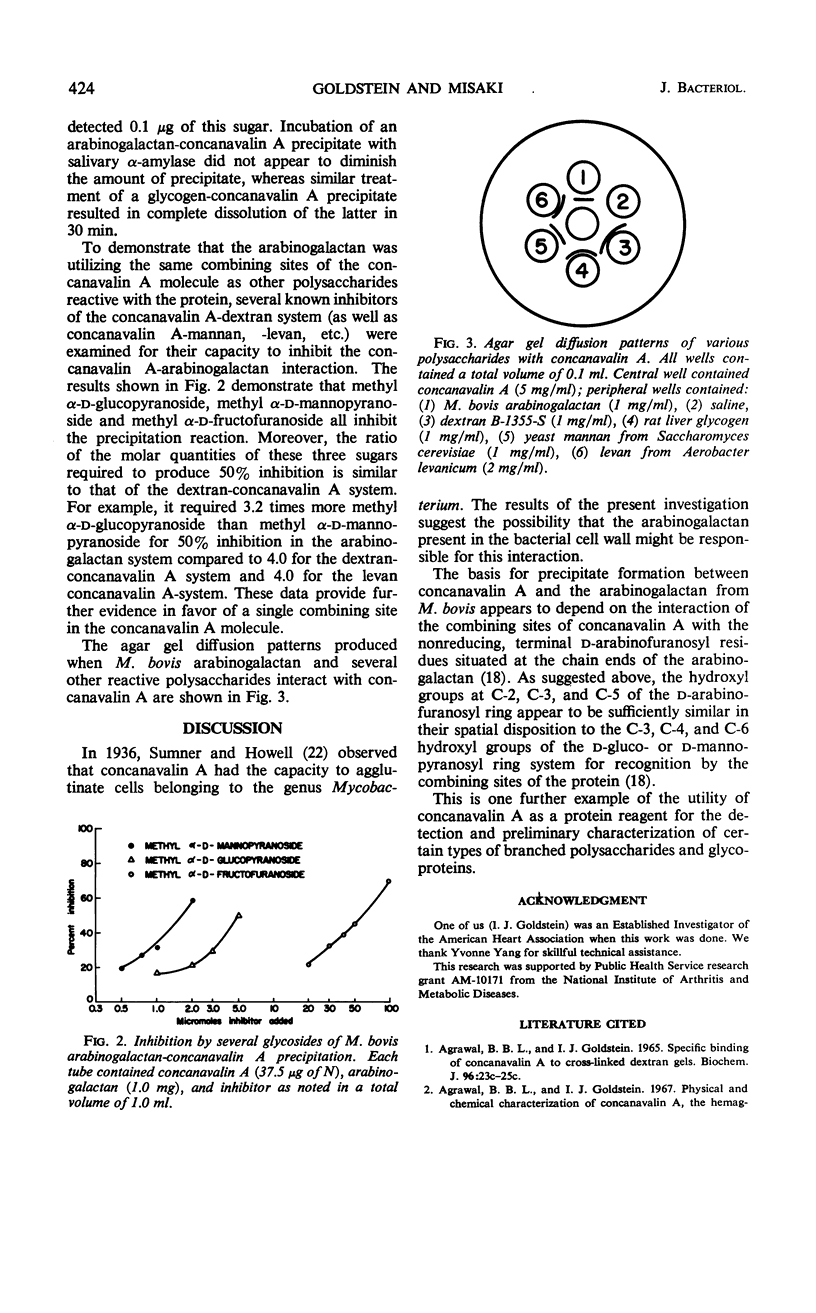
The arabinogalactan isolated from the cell wall of Mycobacterium bovis reacts to form a precipitate with concanavalin A, the phytohemagglutinin of the jack bean. A typical precipitin-like curve is obtained when increasing amounts of the polysaccharide are added to a constant quantity of concanavalin A. Inhibitor studies suggest that concanavalin A reacts with the arabinogalactan by interacting with the C-2, C-3, and C-5 hydroxyl groups of the α-d-arabinofuranosyl residues situated at the chain ends of this polysaccharide.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal B. B., Goldstein I. J. Physical and chemical characterization of concanavalin A, the hemagglutinin from jack bean (Canavalia ensiformis). Biochim Biophys Acta. 1967 Feb 21;133(2):376–379. doi: 10.1016/0005-2795(67)90081-5. [DOI] [PubMed] [Google Scholar]
- CUMMINS C. S. Chemical composition and antigenic structure of cell walls of Corynebacterium, Mycobacterium, Nocardia, Actinomyces and Arthrobacter. J Gen Microbiol. 1962 Apr;28:35–50. doi: 10.1099/00221287-28-1-35. [DOI] [PubMed] [Google Scholar]
- CUMMINS C. S., HARRIS H. Studies on the cell-wall composition and taxonomy of Actinomycetales and related groups. J Gen Microbiol. 1958 Feb;18(1):173–189. doi: 10.1099/00221287-18-1-173. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN I. J., HOLLERMAN C. E., MERRICK J. M. PROTEIN-CARBOHYDRATE INTERACTION. I. THE INTERACTION OF POLYSACCHARIDES WITH CONCANAVALIN A. Biochim Biophys Acta. 1965 Jan 4;97:68–76. doi: 10.1016/0304-4165(65)90270-9. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN I. J., HOLLERMAN C. E., SMITH E. E. PROTEIN-CARBOHYDRATE INTERACTION. II. INHIBITION STUDIES ON THE INTERACTION OF CONCANAVALIN A WITH POLYSACCHARIDES. Biochemistry. 1965 May;4:876–883. doi: 10.1021/bi00881a013. [DOI] [PubMed] [Google Scholar]
- Goldstein I. J., Iyer R. N. Interaction of concanavalin A, a phytohemagglutinin, with model substrates. Biochim Biophys Acta. 1966 May 26;121(1):197–200. doi: 10.1016/0304-4165(66)90374-6. [DOI] [PubMed] [Google Scholar]
- Goldstein I. J., So L. L. Protein-carbonhydrate interaction. 3. Agar gel-diffusion studies on the interaction of Concanavalin A, a lectin isolated from jack bean, with polysaccharides. Arch Biochem Biophys. 1965 Aug;111(2):407–414. doi: 10.1016/0003-9861(65)90203-1. [DOI] [PubMed] [Google Scholar]
- HEHRE E. J. Contribution of classical immunology to the development of knowledge of dextran structure. Bull Soc Chim Biol (Paris) 1960;42:1581–1585. [PubMed] [Google Scholar]
- Misaki A., Yukawa S. Studies on cell walls of Mycobacteria. II. Constitution of polysaccharides from BCG cell walls. J Biochem. 1966 May;59(5):511–520. doi: 10.1093/oxfordjournals.jbchem.a128335. [DOI] [PubMed] [Google Scholar]
- Misaki A., Yukawa S., Tsuchiya K., Yamasaki T. Studies on cell walls of Mycobacteria. I. Chemical and biological properties of the cell walls and the mucopeptide of BCG. J Biochem. 1966 Apr;59(4):388–396. doi: 10.1093/oxfordjournals.jbchem.a128314. [DOI] [PubMed] [Google Scholar]
- Smith E. E., Goldstein I. J. Protein-carbohydrate interaction. V. Further inhibition studies directed toward defining the stereochemical requirements of the reactive sites of concanavalin A. Arch Biochem Biophys. 1967 Jul;121(1):88–95. doi: 10.1016/0003-9861(67)90012-4. [DOI] [PubMed] [Google Scholar]
- So L. L., Goldstein I. J. Protein-carbohydrate interaction. IV. Application of the quantitative precipitin method to polysaccharide-concanavalin A interaction. J Biol Chem. 1967 Apr 10;242(7):1617–1622. [PubMed] [Google Scholar]
- So L. L., Goldstein I. J. Protein-carbohydrate interaction. IX. Application of the quantitative hapten inhibition technique to polysaccharide-concanavalin A interaction. Some comments on the forces involved n concanavalin A-polysaccharide interaction. J Immunol. 1967 Jul;99(1):158–163. [PubMed] [Google Scholar]
- Sumner J. B., Howell S. F. Identification of Hemagglutinin of Jack Bean with Concanavalin A. J Bacteriol. 1936 Aug;32(2):227–237. doi: 10.1128/jb.32.2.227-237.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardi A. H., Allen W. S., Turner D. L., Stary Z. Isolation of arabinose-containing hyaluronate peptides and xylose-containing chondroitin sulfate peptides from protease-digested brain tissue. Arch Biochem Biophys. 1966 Oct;117(1):44–53. doi: 10.1016/0003-9861(66)90123-8. [DOI] [PubMed] [Google Scholar]



