Abstract
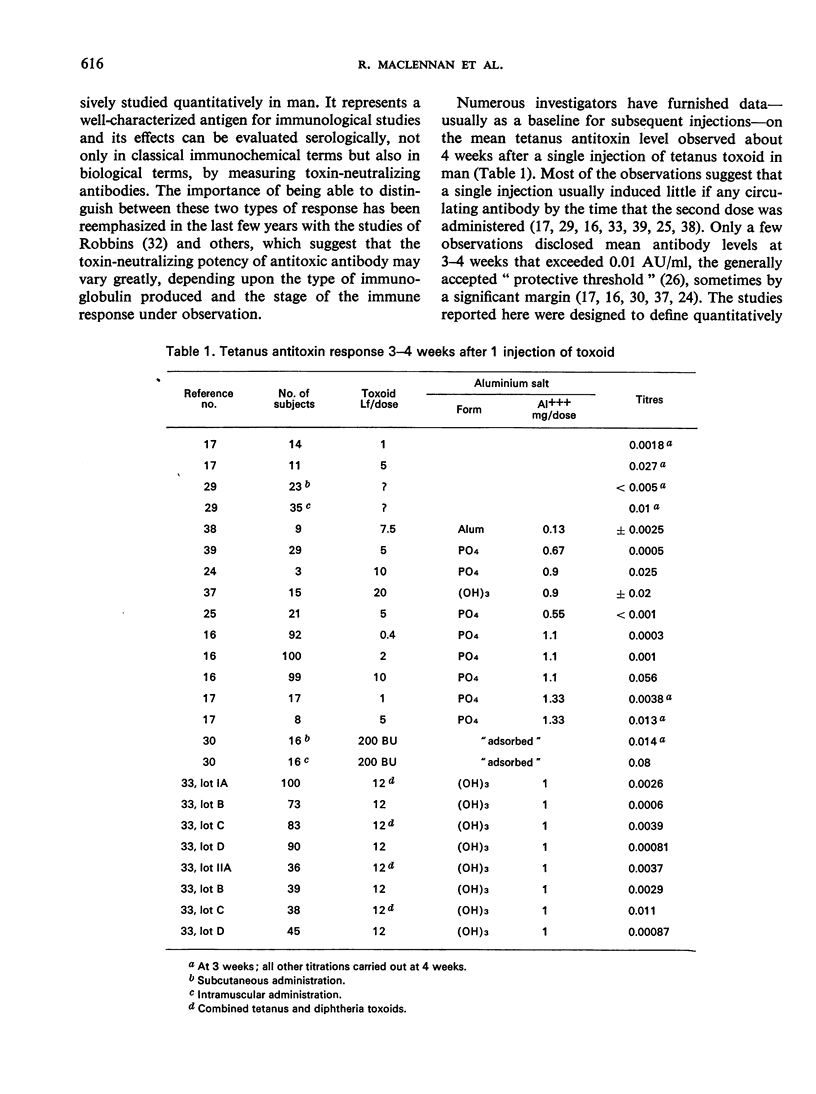
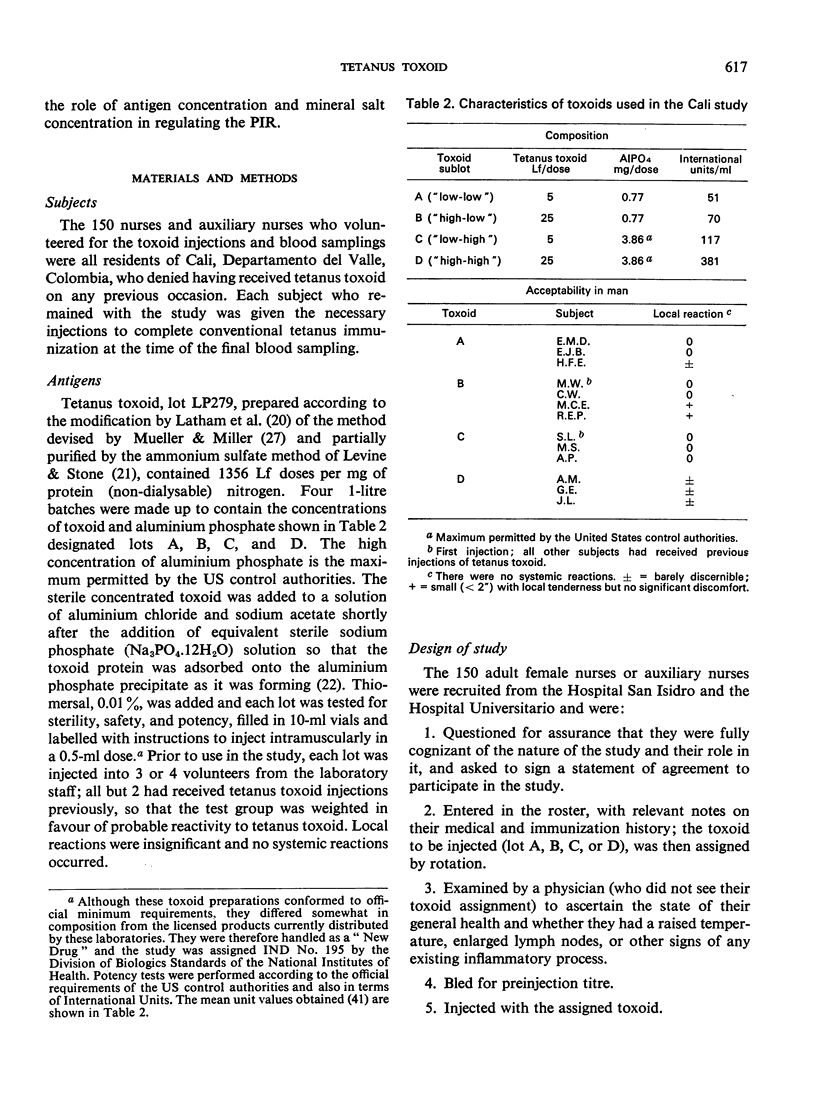
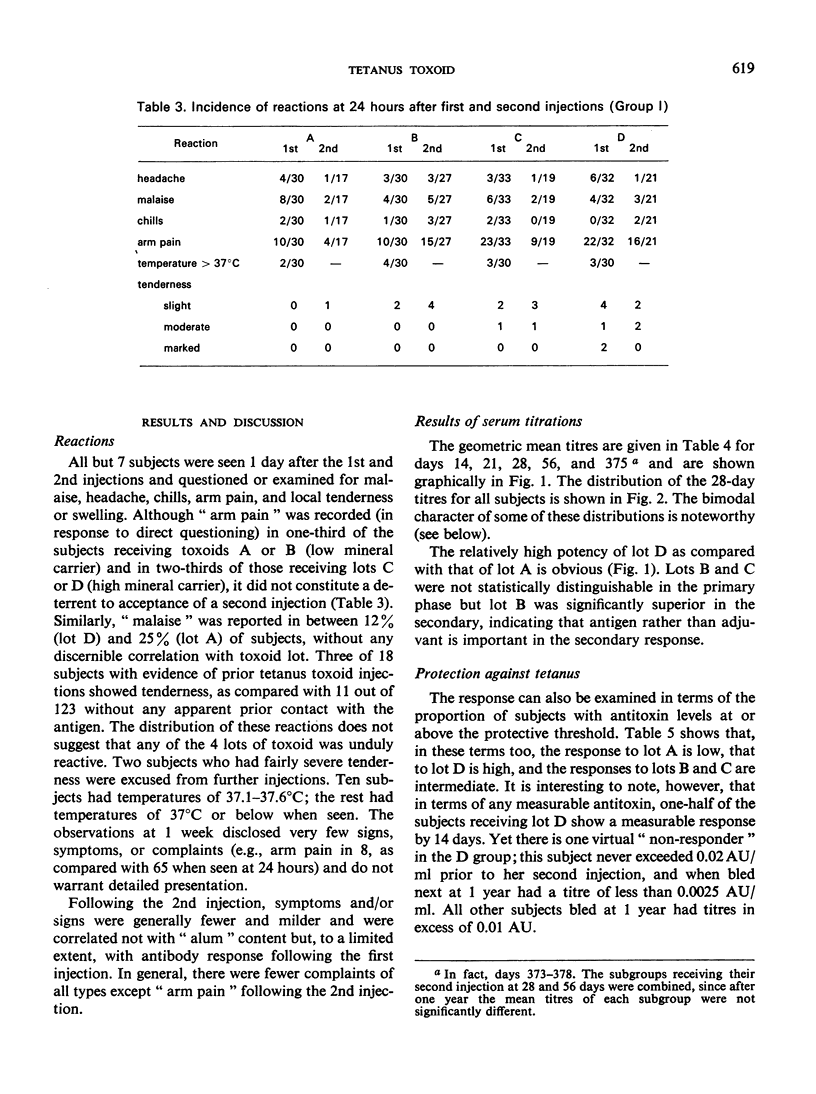
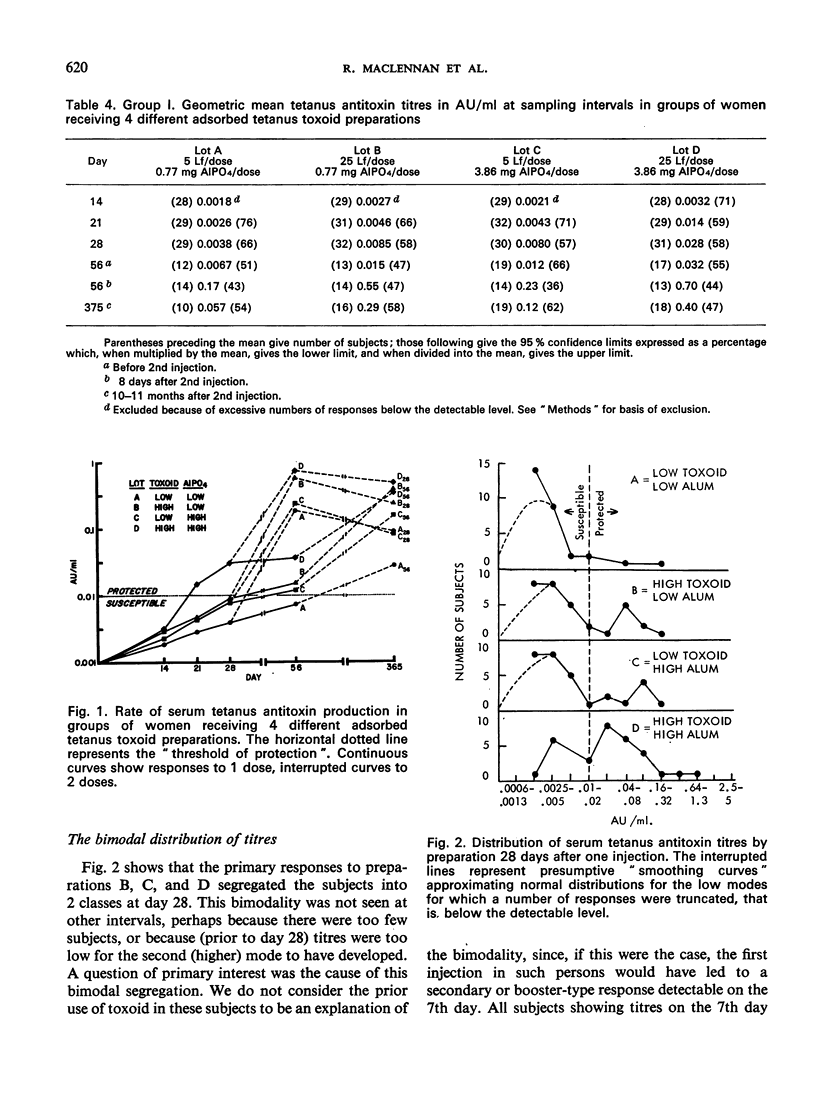
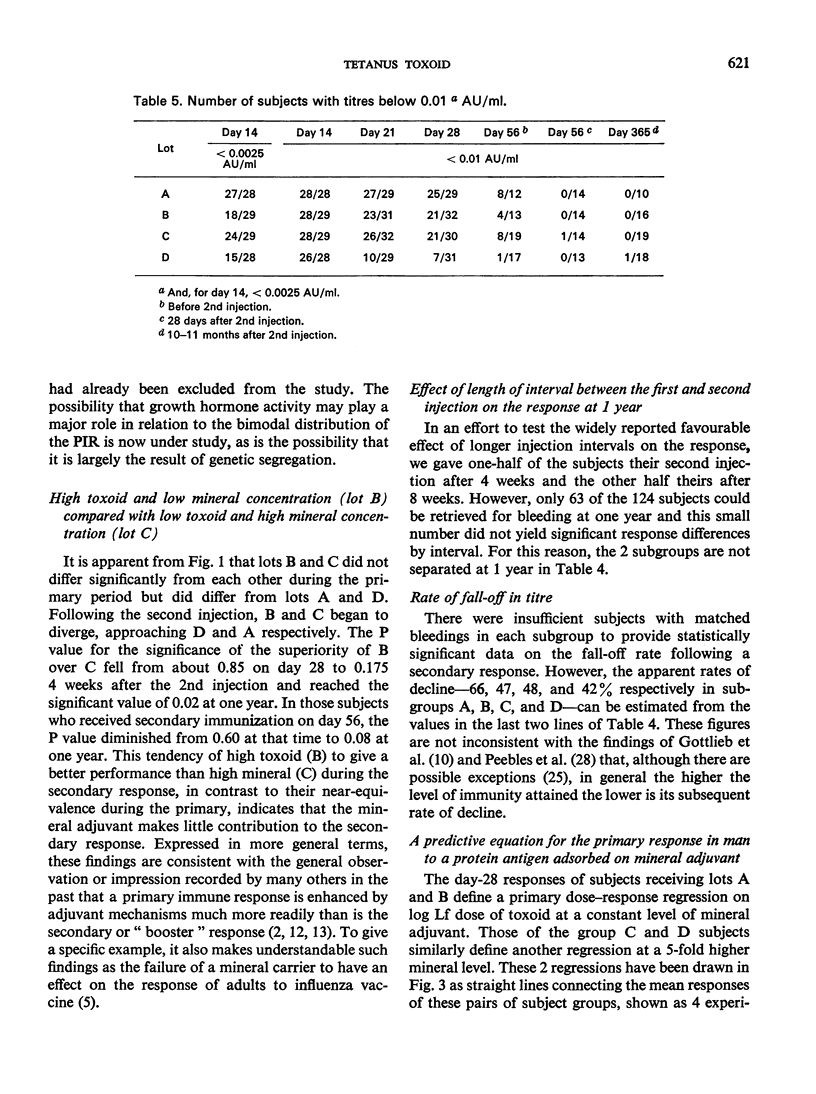
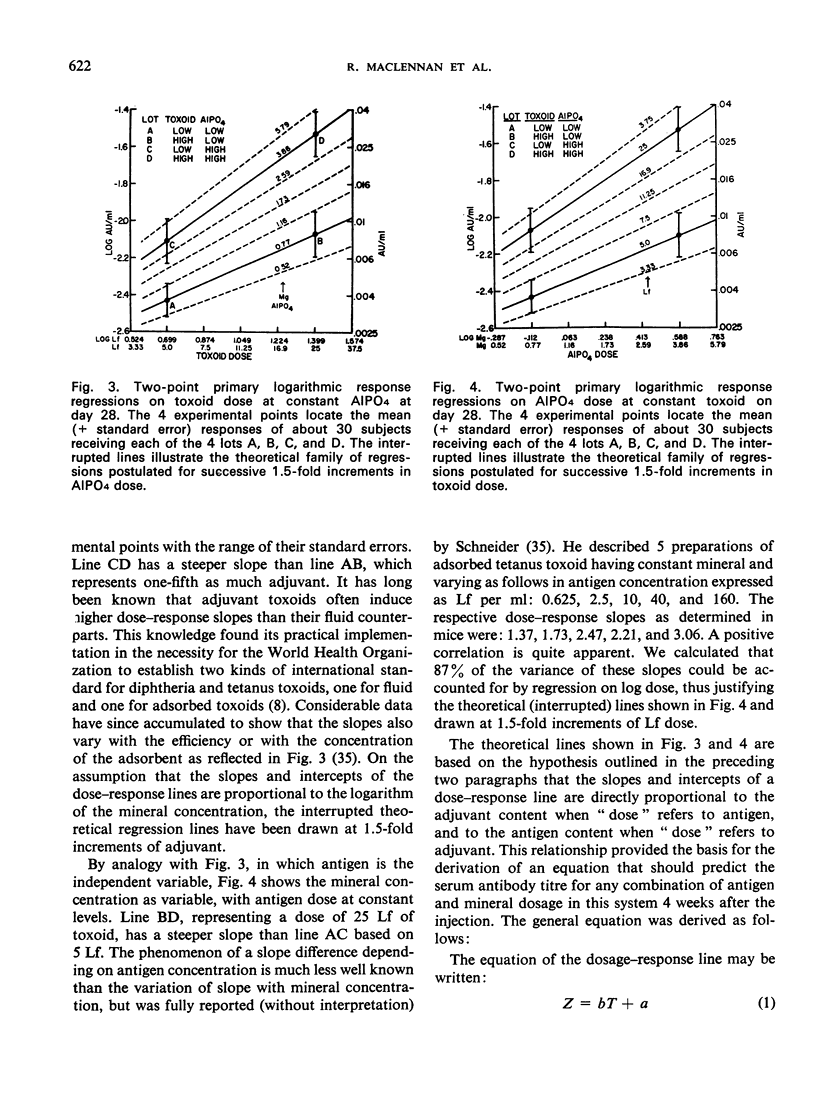
A quantitative study was performed to determine the effect of toxoid concentration and aluminium salt concentration on the primary immune response (PIR) and the secondary response induced by tetanus toxoid in human volunteers. Four toxoid preparations having 5-fold differences in toxoid concentration, aluminium salt concentration, or both, were administered to four comparable groups of human volunteers. Antitoxin titres in the serum of each volunteer were determined at intervals. The PIR was found to be a function of the antigen concentration, the mineral concentration, and the interaction of both. The secondary response was a function of the antigen concentration; increase in mineral adjuvant concentration had no significant effect. The data suggested that the higher the post-secondary response, the slower the rate of decline over the ensuing 10 months. The distribution of primary responses at day 28 tended to be bimodal. The response to the best preparation suggested that a single-dose toxoid might be developed to immunize populations that may be difficult to retrieve for multiple injections.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambuel J. P., Graham B. D., Ertel P. Y., Grove M. L. Immunizations. Comprehensive health care and changing professional roles. Am J Dis Child. 1969 Nov;118(5):677–679. [PubMed] [Google Scholar]
- Aprile M. A., Wardlaw A. C. Aluminium compounds as adjuvants for vaccines and toxoids in man: a review. Can J Public Health. 1966 Aug;57(8):343–360. [PubMed] [Google Scholar]
- Benacerraf B., McDevitt H. O. Histocompatibility-linked immune response genes. Science. 1972 Jan 21;175(4019):273–279. doi: 10.1126/science.175.4019.273. [DOI] [PubMed] [Google Scholar]
- Davenport F. M., Hennessy A. V., Askin F. B. Lack of adjuvant effect of A1PO4 on purified influenza virus hemagglutinins in man. J Immunol. 1968 May;100(5):1139–1140. [PubMed] [Google Scholar]
- Dugdale A. E. The "built-in failure-rate" of immunisation at infant-health clinics. Lancet. 1969 Feb 22;1(7591):409–411. doi: 10.1016/s0140-6736(69)91371-3. [DOI] [PubMed] [Google Scholar]
- Gottlieb S., Martin M., McLaughlin F. X., Panaro R. J., Levine L., Edsall G. Long-term immunity to diphtheria and tetanus: a mathematical model. Am J Epidemiol. 1967 Mar;85(2):207–219. doi: 10.1093/oxfordjournals.aje.a120684. [DOI] [PubMed] [Google Scholar]
- Hardegree M. C., Barile M. F., Pittman M., Schofield F. D., Maclennan R., Kelly A. Immunization against neonatal tetanus in New Guinea. Bull World Health Organ. 1970;43(3):439–451. [PMC free article] [PubMed] [Google Scholar]
- Hennessy A. V., Patno M. E., Davenport F. M. Effect of A1PO4 on antibody response. Proc Soc Exp Biol Med. 1971 Nov;138(2):396–398. doi: 10.3181/00379727-138-35905. [DOI] [PubMed] [Google Scholar]
- IPSEN J., Jr Immunization of adults against diphtheria and tetanus. N Engl J Med. 1954 Sep 16;251(12):459–466. doi: 10.1056/NEJM195409162511202. [DOI] [PubMed] [Google Scholar]
- Iványi J., Valentová V., Cerný J. The dose of antigen required for the suppression of the IgM and IgG antibody response in chickens. I. The kinetics and characterization of serum antibodies. Folia Biol (Praha) 1966;12(3):157–167. [PubMed] [Google Scholar]
- LATHAM W. C., BENT D. F., LEVINE L. Tetanus toxin production in the absence of protein. Appl Microbiol. 1962 Mar;10:146–152. doi: 10.1128/am.10.2.146-152.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINE L., STONE J. L. The purification of tetanus toxoid by ammonium sulfate fractionation. J Immunol. 1951 Sep;67(3):235–242. [PubMed] [Google Scholar]
- LEVINE L., STONE J. L., WYMAN L. Factors affecting the efficiency of the aluminum adjuvant in diphtheria and tetanus toxoids. J Immunol. 1955 Oct;75(4):301–307. [PubMed] [Google Scholar]
- LEVINE L., WYMAN L., BRODERICK E. J., IPSEN J., Jr A field study in triple immunization (diphtheria, pertussis, tetanus). Estimation of 3 antibodies in infant sera from a single heel puncture using agglutination techniques. J Pediatr. 1960 Dec;57:836–843. doi: 10.1016/s0022-3476(60)80134-5. [DOI] [PubMed] [Google Scholar]
- MCCOMB J. A. THE PROPHYLACTIC DOSE OF HOMOLOGOUS TETANUS ANTITOXIN. N Engl J Med. 1964 Jan 23;270:175–178. doi: 10.1056/NEJM196401232700404. [DOI] [PubMed] [Google Scholar]
- MUELLER J. H., MILLER P. A. Variable factors influencing the production of tetanus toxin. J Bacteriol. 1954 Mar;67(3):271–277. doi: 10.1128/jb.67.3.271-277.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan R., Schofield F. D., Pittman M., Hardegree M. C., Barile M. F. Immunization against neonatal tetanus in New Guinea. Antitoxin response of pregnant women to adjuvant and plain toxoids. Bull World Health Organ. 1965;32(5):683–697. [PMC free article] [PubMed] [Google Scholar]
- Peebles T. C., Levine L., Eldred M. C., Edsall G. Tetanus-toxoid emergency boosters: a reappraisal. N Engl J Med. 1969 Mar 13;280(11):575–581. doi: 10.1056/NEJM196903132801102. [DOI] [PubMed] [Google Scholar]
- SCHEIBEL I., TULINIUS S. Simultaneous tetanus immunization and reinforcement of diphtheria immunity in adults, with special reference to the interference phenomenon. Acta Pathol Microbiol Scand. 1961;52:227–240. doi: 10.1111/j.1699-0463.1961.tb03199.x. [DOI] [PubMed] [Google Scholar]
- Snyder H. E., Edsall G., Levine L. Tetanus immunization. J Trauma. 1966 Jul;6(4):529–538. doi: 10.1097/00005373-196607000-00008. [DOI] [PubMed] [Google Scholar]
- TASMAN A., BANGMA P. J., SMITH L. Immunological reactivity of tuberculosis patients. Antonie Van Leeuwenhoek. 1961;27:367–385. doi: 10.1007/BF02538466. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. The regulatory role of macrophages in antigenic stimulation. Adv Immunol. 1972;15:95–165. doi: 10.1016/s0065-2776(08)60684-7. [DOI] [PubMed] [Google Scholar]


