Abstract
Production of prostaglandins involved in renal salt and water homeostasis is modulated by regulated expression of the inducible form of cyclooxygenase-2 (COX-2) at restricted sites in the rat renal cortex. Because inflammatory COX-2 is suppressed by glucocorticoids, and prostaglandin levels in the kidney are sensitive to steroids, the sensitivity of COX expression to adrenalectomy (ADX) was investigated. By 2 weeks after ADX in mature rats, cortical COX-2 immunoreactivity increased 10-fold in the cortical thick ascending limb and macula densa. The constitutive isoform, COX-1, was unchanged. The magnitude of the changes and specificity of COX-2 immunoreactivity were validated by in situ hybridization histochemistry of COX-2 mRNA and Western blot analysis. Increased COX-2 activity (>5-fold) was documented by using a specific COX-2 inhibitor. The COX-2 up-regulation in ADX rats was reversed by replacement therapy with either corticosterone or deoxycorticosterone acetate. In normal rats, inhibition of glucocorticoid receptors with RU486 or mineralocorticoid receptors with spironolactone caused up-regulation of renal cortical COX-2. These results indicate that COX-2 expression in situ is tonically inhibited by adrenal steroids, and COX-2 is regulated by mineralocorticoids as well as glucocorticoids.
Prostaglandins, cyclic oxygenated derivatives of arachidonic acid, mediate signaling in inflammation and many normal biological processes. Individual prostanoid species are generated by specific synthases, but initial common precursors are produced by the rate-limiting enzyme prostaglandin G2/H2 synthase, also known as cyclooxygenase (COX). In the late 1980s, it became apparent that basal levels of COX activity could be distinguished from dynamic levels induced by cytokines and endotoxins and suppressed by glucocorticoids (1). Shortly thereafter, two distinct COX genes were described: “constitutive” COX-1 encoding a 2.7- to 2.9-kb transcript and “inducible, glucocorticoid-sensitive” COX-2 encoding a 4.0- to 4.5-kb transcript (2, 3).
Whereas much research has focused on characterization of COX-2 regulation in cultured and/or inflammatory cells, exceptions to transient inducibility and steroid suppression have been detected in control animals at restricted epithelial sites. Sustained high levels of COX-2 expression were demonstrated in immature rat kidney (4) and adult rat vas deferens (5). At these sites, sustained COX-2 expression was not suppressed even by excess exogenous steroids; in fact, continued expression of COX-2 in the vas deferens was testosterone dependent.
In mammalian kidneys, prostaglandins are known to regulate renal hemodynamics and salt/water homeostasis. Studies with COX inhibitors such as aspirin and indomethacin demonstrated important roles for prostaglandins in regulating the renin-angiotensin system through signals generated at the macula densa, but these data remained enigmatic because the predominant isoform, COX-1, had been localized elsewhere; i.e., to the papillary and cortical collecting ducts, arteries, and arterioles (6). By using improved methods of antigen preservation, our laboratories detected COX-2 immunoreactivity (COX-2-ir) in cells of the macula densa and cortical thick ascending limb (cTAL) of Henle's loop (7), and demonstrated up-regulation after dietary salt restriction. Continuing studies showed that renal cortical COX-2 expression was up-regulated by other physiologic experiments in situ; e.g., partial renal ablation (8) and disruption of angiotensin signaling (9). Other investigators have shown that COX-2 is less apparent in the macula densa of immature rats (10) but appears to increase in the cTAL and macula densa of human kidneys with aging (11).
Because glucocorticoid regulation was a hallmark of many early studies of COX-2 expression, it was natural to postulate that glucocorticoids could regulate renal COX-2; our pilot studies showing up-regulation after adrenalectomy (ADX) indicated that under control conditions, renal cortical COX-2 was tonically suppressed by glucocorticoids as had been demonstrated for macrophages in vivo (12). However, additional complexity was possible because the kidney is sensitive to both glucocorticoids and mineralocortcoids, and both are eliminated by ADX. In cultured cells of renal origin, either glucocorticoids or mineralocorticoids down-regulated COX-2 expression (13), but other studies found no evidence for steroid regulation of renal COX-2 in situ (10, 14).
To resolve apparent contradictions and investigate the differential contributions of glucocorticoids and mineralocorticoids, we further studied the effects of maturity and steroids. Experimental replacement of individual steroids after ADX, and inhibition of steroid receptors in control intact animals, revealed that mineralocorticoids play predominant roles in regulation of renal cortical COX-2 expression in mature adult rats.
Materials and Methods
Animals.
Male rats of Sprague-Dawley and Long-Evans strains, as well as F1 hybrids (LE-SD), were used. Under sterile conditions and nembutal anesthesia, bilateral ADX was performed via a single dorsal incision. After surgery, ADX and sham-operated control rats received 1% NaCl in tap water ad libitum to prevent volume depletion. Glucocorticoid or mineralocorticoid replacement was achieved with subcutaneous pellets (50% cholesterol) of deoxycorticosterone acetate (DOCA) or corticosterone (CS), or daily injections to achieve a dose of 15 mg/kg/day. The glucocorticoid receptor (GR) antagonist, RU486, and mineralocorticoid receptor (MR) antagonist, spironolactone, were given at the dose of 7.5 and 20 mg/kg/day, respectively, either by daily injections or subcutaneous pellets.
Immunohistochemistry.
In general, at the termination of an experiment, one kidney of each rat was removed for Western blot analysis; the other was perfused with fixative in situ for histological examination. Under deep anesthesia with nembutal (70 mg/kg, i.p.), rats were exsanguinated with 50 ml/100 g heparinized saline (0.9% NaCl/2 units/ml heparin/0.02% sodium nitrite) through a transcardial aortic cannula and fixed with glutaraldehyde-periodate-acetate-saline as described (15). Glutaraldehyde-periodate-acetate-saline provides excellent preservation of tissue structure, COX-2 antigenicity, and mRNA. The fixed kidney was dehydrated through a graded series of ethanols, embedded in paraffin, sectioned (4 μm for immunohistochemistry, 10 μM for in situ hybridization), and mounted on glass slides. COX-2-ir was immunolocalized with rabbit polyclonal anti-murine COX-2 antibody (Cayman Chemicals, Ann Arbor, MI) at a 2.5 μg/ml dilution. The primary antibodies were localized by using Vectastain ABC-Elite (Vector, Burlingame, CA) with diaminobenzidine as chromogen, followed by a light counterstain with toluidine blue. The specificity of our COX-2 immunolocalization was confirmed by two fundamental tests (16). Staining was eliminated by preabsorption of the primary serum with COX-2 protein purified from the rat distal vas deferens epithelium (5); COX-2-ir colocalized with COX-2 mRNA detected by in situ hybridization.
Immunoblotting.
Homogenates of kidney cortex (10% wt/vol) were prepared in 20 mM Tris⋅Cl, pH 8.0/1 mM EGTA/1 mM EDTA/1 mM PMSF with proteinase inhibitor mixture (Boehringer Mannheim). After a 10-min centrifugation at 10,000 × g, the supernatant was centrifuged for 60 min at 100,000 × g to prepare microsomes as described (7). The microsomes were resuspended in homogenizing buffer, mixed with an equal volume of 2× SDS sample buffer, and boiled for 5 min. The proteins were separated on a 10% SDS gel under reducing conditions and transferred to Immobilon-P transfer membranes (Millipore). The blots were blocked overnight with 20 mM Tris⋅Cl pH 7.4/500 mM NaCl/5% nonfat milk/0.05% Tween-20, followed by incubation for 3 h at room temperature with the rabbit polyclonal antiserum raised against murine COX-2 (Cayman Chemicals) at a 2.5 μg/ml dilution. The primary antibodies were detected with goat anti-rabbit IgG-horseradish peroxidase (Santa Cruz Biotechnology) and exposed on film by using enhanced chemiluminescence (Amersham International).
In Situ Hybridization.
Beginning with the saline exsanguination, all solutions for this protocol were prepared with deionized autoclaved water containing 0.1% diethyl pyrocarbonate. Glutaraldehyde-periodate-acetate-saline, as described above, was as good or better than all other fixatives examined. Before hybridization, sections were deparaffinized, treated with proteinase K (5 μg/ml) for 20 min at room temperature, washed with PBS, refixed in 3.7% formaldehyde, and treated with 0.1 M triethanolamine, pH 8.0, plus acetic anhydride (0.25% vol/vol), and then dehydrated through a graded series of ethanols.
The sense and antisense probes were synthesized by linearizing the 1.3-kb 3′-UTR rat COX-2 fragment ligated into pBSK(−) and transcribing from the flanking T7 or T3 promoters in the presence of digoxigenin-UTP. The probes were hybridized to sections at 50°C for 18 h as described (4). No signal was detected in parallel hybridizations with sense RNA.
Microsome COX Activity Assay.
Kidney microsomes, purified as described above, were preincubated at 37°C in the presence of 4 μM hematin with COX inhibitors at appropriate concentrations as determined (8, 17, 18). After 15 min, an equal volume containing 100 μM arachidonic acid was added to initiate the reaction. After 10 min at 37°C, the reaction was stopped by boiling 1 min. Production of prostaglandin E2 (PGE2) was determined immediately by enzyme immunoassay (Amersham International).
Micrography.
Bright-field images from the Leitz Orthoplan microscope with DCV digital RGB video camera were digitized by the bioquant tcw image analysis system and saved as computer files. Contrast and color-level adjustment (Adobe Photoshop) were performed for the entire image; i.e., no region- or object-specific editing or enhancements were performed.
Results
COX-2 Expression After ADX.
Under control conditions with intact adrenal glands, kidneys from mature adult rats (males >250 g) immunostained to localize COX-2 were indistinguishable from those illustrated in Fig. 1 A and B (7). Intense COX-2-ir was apparent in one or two isolated cells in some cTAL near the macula densa but rarely in the macula densa itself. COX-2-ir also was detected in interstitial macrophages that accounted for 10–20% of the total cortical COX-2 in normal specimens.
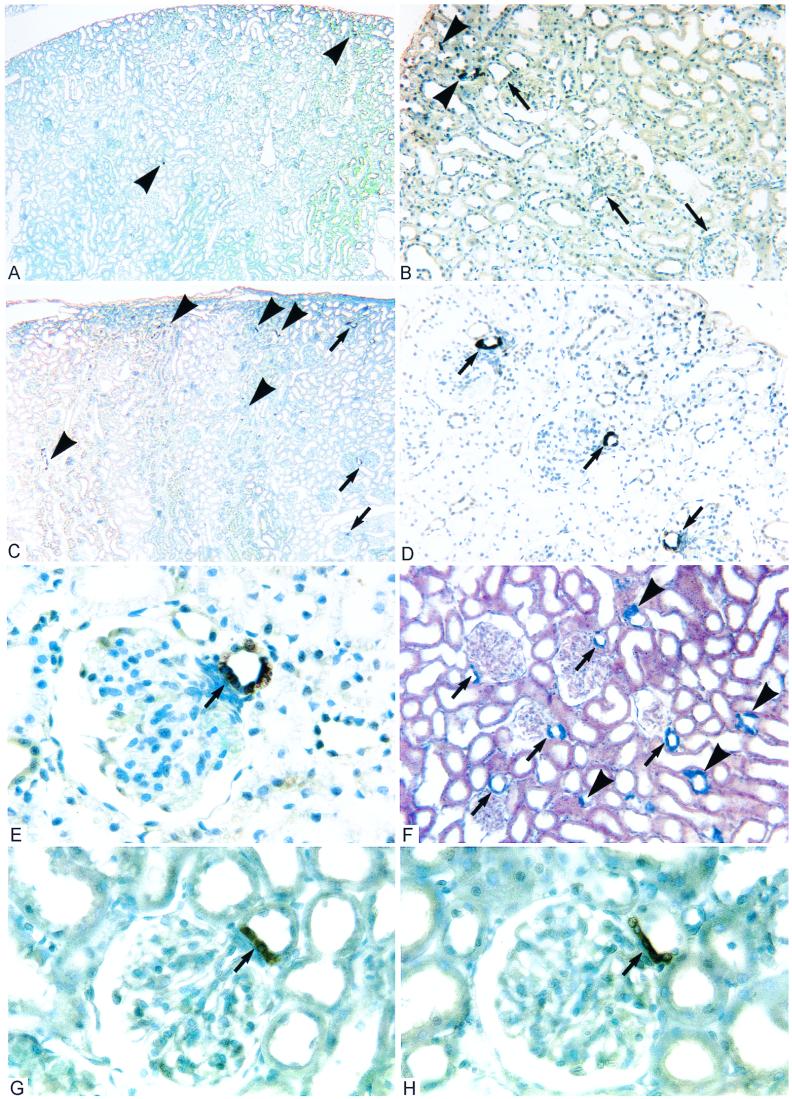
Figure 1.
Histological sections of kidney cortex from adult rats (>250 g). (A and B) After ADX combined with CS replacement for 2 weeks, intense COX-2-ir is observed in macrophages (▸) and a very few scattered cTAL cells, but generally is absent from the macula densa (➞). These images are indistinguishable from controls. (C and D) ADX littermate without CS replacement shows intense COX-2-ir in some cTAL (▸) and nearly all macula densa (➞). (E) At higher magnification, it is apparent that COX-2-ir fills the cytoplasm of macula densa cells (➞) as well as cTAL cells in the opposite wall. (F) In situ hybridization of ADX kidney demonstrates COX-2 mRNA at sites identical to COX-2-ir. (G and H) COX-2 expression is observed at the macula densa (➞) in control rats administered RU486 (G) or spironolactone (H) to block steroid receptors (GR and MR, respectively). (Figure widths: A and C, 2.4 mm; B, D, and F, 600 μm; E, G, and H = 180 μm.)
Two weeks after ADX, widespread increases in COX-2-ir were apparent in the cTAL (Fig. 1C) and macula densa (Fig. 1D). However, the histological pattern of COX-2 cells was different in these two loci. In the cTAL, groups of unstained cells were interspersed with stained cells (Fig. 1E); i.e., COX-2 was expressed in some cells but was not generalized to the cTAL epithelium. At the macula densa, intense COX-2-ir generally was exhibited by all of the macula densa cells (Fig. 1E).
To examine COX-2 up-regulation at the level of transcription and confirm the specificity of the increased immunoreactivity after ADX, in situ hybridization was used to detect COX-2 mRNA. In control rats, COX-2 mRNA exhibited a sparse distribution in renal cortex similar to that seen for COX-2-ir (not illustrated). Two weeks after ADX, up-regulation was apparent in the cTAL and strong signals were detected consistently in the macula densa (Fig. 1F).
Subcellular fractions prepared from renal cortex were subjected to Western blot analysis; pilot experiments determined that virtually all COX-2 was contained in the microsome fraction. In control specimens, the COX-2-ir band at 73 kDa was weak (Fig. 2, lane 1); 1 week after ADX, the COX-2 band had increased moderately (lane 2), and by 2 weeks after ADX, the COX-2 signal was maximal (lane 3). Western blots of COX-2 customarily display a doublet representing variable posttranslational modifications (7); we have been unable to correlate consistent changes in the ratio of these bands with ADX. Identical samples immunoblotted for the constitutive isoform, COX-1, showed no changes in response to ADX (not illustrated).
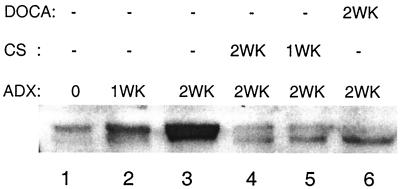
Figure 2.
Effects of ADX and steroid replacement therapy on COX-2 expression in rat kidney cortex. Immunoblots of microsomes: lane 1, faint signal in sham-operated control rat; lane 2, increased signal after ADX for 1 week; lane 3, maximum signal after 2 weeks ADX; lane 4, 2 weeks ADX with concurrent CS replacement; lane 5, 2 weeks ADX with CS replacement after 1 week; and lane 6, 2 weeks ADX with concurrent DOCA replacement.
Hormone Replacements.
Glucocorticoids, the adrenal steroids that have been shown to suppress COX-2 up-regulation in inflammatory cells, are eliminated by ADX. To test for similar effects in renal cortex, CS, the predominant glucocorticoid in rats, was administered to ADX rats via daily injection or subcutaneous pellets. Identical results were obtained for both methods of administration. In a representative experiment shown in Fig. 2, five of six sibling male rats were adrenalectomized on the same day and maintained for 2 weeks on normal food supplemented with 1% salt water to prevent volume depletion. Kidneys from the ADX specimen that received no additional treatment exhibited maximal COX-2 up-regulation as described above (lane 3).
CS was administered according to two schedules. To determine whether CS prevented COX-2 up-regulation, one rat received CS replacement from the time of ADX through the 2 weeks survival (lane 4). Cortical COX-2 remained down-regulated, approximating control levels. To determine whether up-regulated COX-2 could be down-regulated by exogenous CS, another rat (lane 5) received no steroid during the first week following ADX. Presumably, during this period, renal cortical COX-2 in this rat was up-regulated to levels shown for the 1 week ADX specimen (lane 2). On receiving CS replacement during the second week of its 2 weeks ADX, cortical COX-2 expression in this rat had returned to control levels (lane 5). Parallel histologic data were obtained by perfusing one kidney from each rat for immunohistochemical staining. ADX kidneys receiving steroid replacement exhibited sparse scattered COX-2 cells in the cTAL near the macula densa (Fig. 1 A and B) and were indistinguishable from controls.
In experiments originally conceived as negative controls, the mineralocorticoid DOCA, a long-lived aldosterone analog, was administered to ADX rats. Surprisingly, DOCA also prevented up-regulation of COX-2 in the renal cortex (lane 6) and/or down-regulated COX-2 after up-regulation (not illustrated). Thus, COX-2 expression in renal cortex was suppressed by ADX replacement therapy with mineralocorticoids as well as glucocorticoids. Furthermore, because glucocorticoids can also stimulate MR, these data raised the possibility that predominant regulation of cortical COX-2 was through the MR pathway.
Receptor Antagonists.
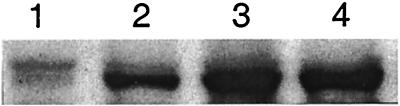
To determine whether renal cortical COX-2 expression normally is suppressed by basal levels of steroids acting through the MR and/or GR pathways, competitive inhibitors of the steroid receptors were administered daily for 2 weeks to mature rats with intact adrenals. Compared with control (Fig. 3, lane 1), some up-regulation of COX-2 was apparent after treatment with RU486, a putative GR antagonist (lane 2). Strong up-regulation of COX-2 (equivalent to ADX) was induced by treatment with the MR antagonist spironolactone (lane 3), and no further increase in COX-2 could be appreciated with RU486 and spironolactone together (lane 4). Histologically, the COX-2 up-regulation induced by the inhibitors was focused at the macula densa. In the specimens treated with RU486, COX-2-ir was observed at approximately 30% of the macula densa (Fig. 1G); in spironolactone specimens, COX-2-ir was observed in nearly every macula densa and was more intense (Fig. 1H). In comparison with ADX rats, fewer COX-2 cells were observed in cTAL after inhibitor treatment.
Figure 3.
Effects of steroid receptor inhibitors on renal cortical COX-2 expression. Immunoblots of microsomes after a 2-week treatment: lane 1, control; lane 2, RU486; lane 3, spironolactone; and lane 4, RU486 + spironolactone.
Further experiments were undertaken to test whether the inhibitor effects could be overwhelmed by exogenous steroids. Normal rats treated with either RU486 or spironolactone as above also received either CS or DOCA at doses adequate to suppress COX-2 up-regulation in ADX. Exogenous DOCA strongly inhibited the induction of COX-2 by spironolactone and totally abolished the effects of RU486, whereas CS partially inhibited the effects of spironolactone but had less influence on RU486.
COX Activity.
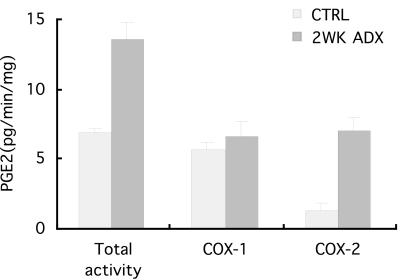
To determine whether increases in immunoreactive COX-2 protein represented increased enzymatic activity, PGE2 production was assayed in vitro. Total COX activity was assayed as PGE2 production in the absence of COX inhibitors; this activity was totally inhibited by the nonspecific COX inhibitor, indomethacin (10−4 M). SC-58236, a COX inhibitor that reportedly displays >1,700-fold selectivity for COX-2 >COX-1, was used at 10−5 M to block COX-2 activity with negligible effects on COX-1 activity. COX-1 activity was assayed as PGE2 produced in the presence of SC-58236; COX-2 activity was the amount of PGE2 production suppressed by SC-58236. In comparison with control rats, total renal cortical COX activity increased 2-fold 2 weeks after ADX (6.85 ± 0.3 vs. 13.50 ±1.29 PGE2 ng/min/mg); COX-1 activity did not change appreciably (5.60 ± 0.57 vs. 6.60 ± 1.07 PGE2 ng/min/mg), but COX-2 activity increased 6-fold (1.25 ± 0.57 vs. 6.90 ±1.07 PGE2 ng/min/mg) (Fig. 4). Thus, the increases in renal cortical COX activity after 2 weeks ADX were due primarily to increased COX-2, but not COX-1, activity. The enzymatic assays complemented immunohistochemical, Western blot, and in situ hybridization data to demonstrate that COX-2 in renal cortex increased significantly after 2 weeks ADX.
Figure 4.
COX-2 activity of renal cortical microsomes was assayed as PGE2 produced by equivalent samples. Two weeks after ADX, total activity doubled; COX-1 activity (determined as PGE2 produced in the presence of specific COX-2 inhibitor) remained unchanged; COX-2 activity (the difference between Total and COX-1 activities) increased 6-fold.
Discussion
The present studies demonstrated that COX-2 expression in the renal cortex of mature rats is regulated by adrenal steroids. Although little COX-2 was apparent in control rats, substantial increases were noted after ADX. Both mRNA and protein were up-regulated in the cTAL and macula densa, and COX-2 activity in cortical microsome fractions increased 6-fold. The effects of ADX were prevented or reversed by steroid replacement, and mimicked in control rats by treatment with steroid receptor antagonists. This report identifies factors that regulate COX-2 expression in the macula densa, and demonstrates that renal cortical COX-2 is under tonic regulation by circulating adrenal corticosteroids. Furthermore, the data suggest that mineralocorticoids are the dominant adrenal steroids regulating expression of COX-2 in the renal cortex.
In considering the roles of renal COX-2, we are reminded that prostaglandins serve as feedback messengers controlling the renin-angiotensin-aldosterone system in the kidney. Renin, the specific proteinase that generates the peptide angiotensin that stimulates aldosterone release from the adrenal, is synthesized and stored in granule cells at the vascular pole of the glomerulus and released in response to signals from the macula densa. Renin release was previously shown to be suppressed by nonselective COX inhibitors such as aspirin or indomethacin, and stimulated by exogenous prostaglandins (19).
Detection of COX-2 protein and mRNA at the macula densa under conditions of increased renin expression and release suggested a role for macula densa COX-2 in mediation of renin release (7, 20–22). Although COX-2-ir is rarely observed in macula densa cells under control conditions, it is possible that adequate COX-2 activity normally is below the limits of immunohistochemical detection. Recent experiments demonstrating that specific COX-2 inhibitors suppress renin levels in the plasma and kidney (8, 9, 23) further support the concept that COX-2 at the macula densa plays a role in regulating renin synthesis and secretion.
The present studies confirmed original reports of Harris et al. (7) that COX-2-ir in mature rats under control conditions is detected in a small population of cells located mostly in the cTAL with a minor component in the macula densa. Two weeks after ADX, an increased number of COX-2 cells was apparent in the cTAL, but the most dramatic changes involved the macula densa. COX-2 was up-regulated in the macula densa of nearly every nephron; the COX-2-positive cells were tightly packed and included most of the macula itself. Based on this evidence, we postulated that under control conditions, physiologic levels of adrenal steroids tonically suppressed COX-2 expression in the cTAL and macula densa. This hypothesis was supported by experiments demonstrating that COX-2 up-regulation in ADX rats was in large part prevented by replacement of missing glucocorticoids with CS via subcutaneous pellets. If the replacement therapy was delayed for 2 weeks after ADX to allow maximal up-regulation of COX-2, treatment with exogenous CS for 2 subsequent weeks down-regulated renal cortical COX-2 nearly to control levels. It is well known that glucocorticoids suppress COX-2 expression in inflammatory cells (14, 24–27), and the results of our ADX and CS replacement experiments were consistent with that pattern.
The kidney is a major target of aldosterone, the highly conserved mineralocorticoid that stimulates salt retention in nearly all vertebrates (28, 29); as demonstrated in transgenic mice, the MRs are essential for survival (30) The kidney is well endowed with MR; they have been localized to all segments of the nephron except the proximal tubules (31). However, unlike GR, MRs are nonselective and equivalently activated by glucocorticoids. Specificity of MR action is achieved through inactivation of glucocorticoids by 11β-hydroxysteroid dehydrogenase type II (11β-HSD2). High levels of 11β-HSD2 found in the cortical collecting duct imply that MRs in the collecting duct are strictly sensitive to aldosterone. On the other hand, lower levels of 11β-HSD2 are detected in the cTAL and macula densa, suggesting that glucocorticoids might stimulate the MR at these sites (31). Thus, the high circulating levels of CS achieved in replacement therapy could also have effected COX-2 down-regulation by activating the MR pathway in the cTAL and macula densa. Therefore, we postulated that renal COX-2 regulation might involve mineralocorticoids and the MR.
Our laboratories previously reported COX-2 up-regulation in the macula densa and cTAL in response to angiotensin-converting enzyme inhibitors and angiotensin receptor antagonists that interfere with production or activation of angiotensin II (9). One consequence of these inhibitors is to prevent stimulation of aldosterone release from the adrenal cortex without interfering with glucocorticoid production. Thus, when aldosterone activation of MR was compromised, normal tonic levels of glucocorticoids were insufficient to suppress COX-2 expression in the macula densa. Therefore, in addition to any direct effects of angiotensin II and the AT1 receptors on regulation of COX-2 expression, inhibition of circulating mineralocorticoids may have contributed to the increases in COX-2 seen in these studies.
MR involvement was tested directly by administration of the aldosterone analog, DOCA, to ADX rats. Renal cortical COX-2 was suppressed to undetectable levels. Because DOCA has very low GR agonist activity, these data indicated that MR activation alone was sufficient to down-regulate COX-2. The importance of MR was supported further by experiments with steroid receptor antagonists. In control rats with intact adrenals, inhibition of MR with spironolactone produced strong up-regulation of COX-2 in renal cortical microsomes analyzed by Western blots and intense COX-2-ir in nearly every macula densa examined histologically. Spironolactone effects were strongly inhibited by exogenous DOCA and weakly inhibited by exogenous CS. Overall, the spironolactone data are consistent with a strong influence of MR on COX-2 regulation.
Experiments with the GR antagonist RU486 produced similar but less pronounced effects. In control rats, RU486 produced significant but submaximal COX-2 up-regulation. The RU486 effects were abolished by exogenous DOCA but affected minimally by exogenous CS. The response was not dose dependent; i.e., doubling the dose of RU486 did not increase the COX-2 expression. Furthermore, the spironolactone and RU486 effects were not additive; the response to spironolactone was maximal. Thus, macula densa COX-2 expression is down-regulated to a greater degree by mineralocorticoids than glucocorticoids.
In summary, our studies demonstrate that COX-2 in the cTAL and macula densa is under tonic regulation by adrenal steroids, and it is up-regulated under stimuli that call for increased renin synthesis and release. It will be of interest in the future to investigate signaling pathways that regulate COX-2 by directly influencing the steroids and their receptors. Details regarding the transcriptional regulation and roles of COX-2 in kidney function may assume increasing relevance as specific COX-2 inhibitors are employed as clinical anti-inflammatory agents.
Acknowledgments
We thank Dr. Larry Marnett for his collegial critique. This work was performed in the George M. O'Brien Center for Kidney and Urologic Diseases, which is supported by National Institutes of Health Grant DK 39261 and funds from the Department of Veterans Affairs.
Abbreviations
- COX
cyclooxygenase
- COX-2-ir
COX-2 immunoreactivity
- cTAL
cortical thick ascending limb
- ADX
adrenalectomy
- DOCA
deoxycorticosterone acetate
- GR
glucocorticoid receptor
- MR
mineralocorticoid receptor
- PGE2
prostaglandin E2
- CS
corticosterone
References
- 1.Raz A, Wyche A, Fagan D, Needleman P. Adv Exp Med Biol. 1989;259:1–21. doi: 10.1007/978-1-4684-5700-1_1. [DOI] [PubMed] [Google Scholar]
- 2.Kujubu D A, Fletcher B S, Varnum B C, Lim R W, Herschman H R. J Biol Chem. 1991;266:12866–12872. [PubMed] [Google Scholar]
- 3.O'Banion M K, Sadowski H B, Winn V, Young D A. J Biol Chem. 1991;266:23261–23267. [PubMed] [Google Scholar]
- 4.Zhang M Z, Wang J L, Cheng H F, Harris R C, McKanna J A. Am J Physiol. 1997;273:F994–F1002. doi: 10.1152/ajprenal.1997.273.6.F994. [DOI] [PubMed] [Google Scholar]
- 5.McKanna J A, Zhang M Z, Wang J L, Cheng H, Harris R C. Am J Physiol. 1998;275:R227–R233. doi: 10.1152/ajpregu.1998.275.1.R227. [DOI] [PubMed] [Google Scholar]
- 6.Smith W L, Bell T G. Am J Physiol. 1978;235:F451–F457. doi: 10.1152/ajprenal.1978.235.5.F451. [DOI] [PubMed] [Google Scholar]
- 7.Harris R C, McKanna J A, Akai Y, Jacobson H R, DuBois R N, Breyer M D. J Clin Invest. 1994;94:2504–2510. doi: 10.1172/JCI117620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J L, Cheng H F, Zhang M Z, McKanna J A, Harris R C. Am J Physiol. 1998;275:F613–F622. doi: 10.1152/ajprenal.1998.275.4.F613. [DOI] [PubMed] [Google Scholar]
- 9.Cheng H-F, Wang J-L, Zhang M-Z, Miyazaki Y, Ichikawa I, McKanna J A, Harris R C. J Clin Invest. 1999;103:1–10. doi: 10.1172/JCI5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vio C P, Cespedes C, Gallardo P, Masferrer J L. Hypertension. 1997;30:687–692. doi: 10.1161/01.hyp.30.3.687. [DOI] [PubMed] [Google Scholar]
- 11.Nantel F, Meadows E, Denis D, Connolly B, Metters K M, Giaid A. FEBS Lett. 1999;457:475–477. doi: 10.1016/s0014-5793(99)01088-1. [DOI] [PubMed] [Google Scholar]
- 12.Masferrer J L, Reddy S T, Zweifel B S, Seibert K, Needleman P, Gilbert R S, Herschman H R. J Pharmacol Exp Ther. 1994;270:1340–1344. [PubMed] [Google Scholar]
- 13.Schaefers H J, Goppelt-Struebe M. Biochem Pharmacol. 1996;52:1415–1421. doi: 10.1016/s0006-2952(96)00503-5. [DOI] [PubMed] [Google Scholar]
- 14.Masferrer J L, Seibert K, Zweifel B, Needleman P. Proc Natl Acad Sci USA. 1992;89:3917–3921. doi: 10.1073/pnas.89.9.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKanna J A, Zhang M Z. J Histochem Cytochem. 1997;45:527–538. doi: 10.1177/002215549704500405. [DOI] [PubMed] [Google Scholar]
- 16.Larsson L I. Immunocytochemistry: Theory and Practice. Boca Raton, FL: CRC; 1988. pp. 6–36. [Google Scholar]
- 17.Capdevila J H, Morrow J D, Belosludtsev Y Y, Beauchamp D R, DuBois R N, Falck J R. Biochemistry. 1995;34:3325–3337. doi: 10.1021/bi00010a023. [DOI] [PubMed] [Google Scholar]
- 18.Penning T D, Talley J J, Bertenshaw S R, Carter J S, Collins P W, Docter S, Graneto M J, Lee L F, Malecha J W, Miyashiro J M, et al. J Med Chem. 1997;40:1347–1365. doi: 10.1021/jm960803q. [DOI] [PubMed] [Google Scholar]
- 19.Schnermann J, Briggs J P. In: The Kidney: Physiology and Pathophysiology. Seldin D W, Giebisch G, editors. New York: Raven; 1992. pp. pp.1249–1289. [Google Scholar]
- 20.Jensen B L, Kurtz A. Kidney Int. 1997;52:1242–1249. doi: 10.1038/ki.1997.449. [DOI] [PubMed] [Google Scholar]
- 21.Yang T, Singh I, Pham H, Sun D, Smart A, Schnermann J B, Briggs J P. Am J Physiol. 1998;274:F481–F489. doi: 10.1152/ajprenal.1998.274.3.F481. [DOI] [PubMed] [Google Scholar]
- 22.Hartner A, Goppelt-Struebe M, Hilgers K F. Hypertension. 1998;31:201–205. doi: 10.1161/01.hyp.31.1.201. [DOI] [PubMed] [Google Scholar]
- 23.Harding P, Sigmon D H, Alfie M E, Huang P L, Fishman M C, Beierwaltes W H, Carretero O A. Hypertension. 1997;29:297–302. doi: 10.1161/01.hyp.29.1.297. [DOI] [PubMed] [Google Scholar]
- 24.Kujubu D A, Herschman H R. J Biol Chem. 1992;267:7991–7994. [PubMed] [Google Scholar]
- 25.O'Banion M K, Winn V D, Young D A. Proc Natl Acad Sci USA. 1992;89:4888–4892. doi: 10.1073/pnas.89.11.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newman S P, Flower R J, Croxtall J D. Biochem Biophys Res Commun. 1994;202:931–939. doi: 10.1006/bbrc.1994.2019. [DOI] [PubMed] [Google Scholar]
- 27.Needleman P, Isakson P C. J Rheumatol. 1997;24,Suppl. 49:6–8. [PubMed] [Google Scholar]
- 28.Rossier B C, Palmer G. In: The Kidney: Physiology and Pathophysiology. Seldin D W, Giebisch G, editors. New York: Raven; 1992. pp. pp.1559–1560. [Google Scholar]
- 29.Funder J W, Krozowski Z, Myles K, Sato A, Sheppard K E, Young M. Recent Prog Horm Res. 1997;52:247–260. [PubMed] [Google Scholar]
- 30.Berger S, Bleich M, Schmid W, Cole T J, Peters J, Watanabe H, Kriz W, Warth R, Greger R, Schutz G. Proc Natl Acad Sci USA. 1998;95:9424–9429. doi: 10.1073/pnas.95.16.9424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bostonjoglo M, Reeves W B, Reilly R F, Velazquez H, Robertson N, Litwack G, Morsing P, Dorup J, Bachmann S, Ellison D H. J Am Soc Nephrol. 1998;9:1347–1358. doi: 10.1681/ASN.V981347. [DOI] [PubMed] [Google Scholar]