Abstract
Lack of fragile X mental retardation protein (FMRP) causes Fragile X Syndrome, the most common form of inherited mental retardation. FMRP is an RNA-binding protein and is a component of messenger ribonucleoprotein complexes, associated with brain polyribosomes, including dendritic polysomes. FMRP is therefore thought to be involved in translational control of specific mRNAs at synaptic sites. In mice lacking FMRP, protein synthesis-dependent synaptic plasticity is altered and structural malformations of dendritic protrusions occur. One hypothesized cause of the disease mechanism is based on exaggerated group I mGluR receptor activation. In this study, we examined the effect of the mGluR5 antagonist MPEP on Fragile X related behavior in Fmr1 KO mice. Our results demonstrate a clear defect in prepulse inhibition of startle in Fmr1 KO mice, that could be rescued by MPEP. Moreover, we show for the first time a structural rescue of Fragile X related protrusion morphology with two independent mGluR5 antagonists.
Keywords: Fragile X syndrome, spines, dendrite branching, MPEP, fenobam, prepulse inhibition of startle, metabotropic glutamate receptor, primary hippocampal neuron culture
Introduction
Fragile X syndrome (FXS) is the most common heritable form of mental retardation. The syndrome is caused by a lack of expression of FMRP (fragile X mental retardation protein), which is the protein product of the FMR1 gene. In most cases, the lack of expression is caused by expansion of a CGG repeat (>200 units) in the 5′ UTR of the FMR1 gene, leading to methylation of both the CGG repeat and the promoter region, accompanied by transcriptional silencing. FMRP is an RNA binding protein that associates with polyribosomes and is localized in neurons in the form of granules that move in a microtubule dependent manner with the speed of RNA transport (Antar et al., 2005; De Diego Otero et al., 2002; Wang et al., 2007). Moreover, FMRP has been shown to influence the translation efficacy of several of its target mRNAs (reviewed in (Bagni and Greenough, 2005; Bardoni et al., 2006; Zalfa et al., 2007), which also implicates local translation at synaptic sites (Greenough et al., 2001; Muddashetty et al., 2007; Weiler et al., 1997; Weiler et al., 2004). In most cases, FMRP acts as a translational repressor (Laggerbauer et al., 2001; Lu et al., 2004). Therefore, FMRP is thought to be involved in the transport and/or the regulation of local mRNA translation at synaptic sites (Bagni and Greenough, 2005; Miyashiro et al., 2003; Weiler et al., 1997; Weiler et al., 2004). The presumed loss of translational regulation at synaptic sites might underlie the cognitive impairment in FXS (Huber et al., 2000).
Over the last few years, the metabotropic glutamate receptor (mGluR) theory of FXS has gained much support (Bear et al., 2004). The mGluR theory states that AMPA receptor internalization triggered by mGluR5 stimulation (Snyder et al., 2001), is exaggerated in Fmr1 KO mice, accounting for the enhanced hippocampal LTD found in knockout mice (Bear et al., 2004; Huber et al., 2002). Recently it was shown that FMRP deficient dendrites indeed show aberrant AMPA receptor trafficking resulting in a significantly reduced number of AMPA receptors at the plasma membrane (Nakamoto et al., 2007). Moreover, Fmr1 KO mice that are crossbred with mice that have a 50% reduction in mGluR5 expression were shown to be rescued in several phenotypic aspects (Dolen et al., 2007). It is hypothesized that FMRP normally is involved in the inhibition of the translation of several local mRNAs that are important for the mediation of AMPA receptor internalization. Since the amount of AMPA receptors in the postsynaptic density is correlated with protrusion shape, this might also explain the immature protrusion morphology that has been found in different brain areas of both fragile X patients and Fmr1 KO mice (Comery et al., 1997; Galvez and Greenough, 2005; Grossman et al., 2006; Hinton et al., 1991; Koekkoek et al., 2005; Nimchinsky et al., 2001). The mGluR theory has also boosted the search for therapeutic targets for FXS. An antagonist of mGluR5 receptors would theoretically counteract the increased amount of AMPA receptor internalization in Fmr1 KO neurons. Behavioral studies have shown that Fmr1 KO mice treated with the mGluR5 antagonist MPEP (2-methyl-6-(phenylethynyl)-pyridine hydrochloride) clearly display less sensitivity to audiogenic seizures and more wild type-like behavior in an open field test compared with untreated mice (Yan et al., 2005). Also in a Drosophila model based on loss of function of dfmr1, the single homolog of the FXR family of genes in the Drosophila genome, MPEP was able to rescue courtship behavior and mushroom body defects (McBride et al., 2005). However, the molecular mechanisms behind the effects of MPEP have not been elucidated.
In the present study, we show a defect in prepulse inhibition of acoustic startle (PPI) in Fmr1 KO mice compared to wild type littermates and a rescue of this behavioral phenotype by the mGluR5 antagonist MPEP. In addition, we demonstrate an altered protrusion morphology in Fmr1 KO primary hippocampal neurons that could be rescued using two different mGluR5 antagonists, MPEP and fenobam, rendering protrusion densities indistinguishable from wild type neurons.
Materials and methods
Mouse models
Fmr1 KO mice were generated in our lab as described previously (Bakker et al., 1994; Mientjes et al., 2006). Both lines were used and were backcrossed to C57Bl/6J mice at least seven times. No differences were observed between both Fmr1 KO lines.
Prepulse inhibition of startle
Prepulse inhibition of startle (PPI) was measured by analysis of eye blink reactions of mice to acoustic stimuli, based on the magnetic distance measurement technique (MDMT) used for eye blink conditioning (Koekkoek et al., 2002; Koekkoek et al., 2005). Adult Fmr1 KO mice (n=8) and wild type littermates (n=9) were anesthetized with an oxygenated mixture of nitrous oxide and isoflurane. A dental acrylic pedestal was placed on the skull and animals were allowed to recover for three days. Prior to the experiment the mice were very briefly sedated using the isoflurane/nitrous oxide mixture. A sensorholder with an airchannel and a magnetsensor was attached to the pedestal. A small neobdimium iron borium magnet (0.8*1.6*0.2 mm) was glued to the lower eyelid with a minute drop of cyanoacryllate and a silicon body harness was put on to protect the mice from strain on the pedestal. Mice were placed inside their own cages within soundproof training chambers and allowed to recover until normal behavior (grooming, eating) returned, usually this was within 15 minutes. To test and calibrate the MDMT system air puffs were given as a measure of full eyelid closure.
A background noise level of 60 dB white noise was present. Subsequently, the mice were presented with a white noise startle stimulus of 90 dB, which in the prepulse inhibition condition was preceded by a 70 dB white noise prepulse, 50 ms before the startle stimulus.
Each mouse was subjected to seven blocks of trials consisting of one air puff and three repeated measures of a startle stimulus followed fifty seconds later by a prepulse/startle stimulus with a fifty seconds intertrial interval. The next day the same mice were analyzed again in the same way after MPEP treatment. MPEP treatment was administered by i.p. injection of 20 mg/kg MPEP dissolved in PBS, 30 minutes before the experiment. Percentages of PPI of startle were compared by non-parametric Mann Whitney U test.
Primary hippocampal neuron culture
In short, E18 wild type and Fmr1 KO mice litters were planned on the same day. Embryos were decapitated after which hippocampi were removed and dissociated by trypsin and mechanical treatment. Neurons were plated on poly-L-lysine (100 μg/ml, Sigma) and laminin (50 μg/ml, Sigma) coated 30mm glass coverslips. The neurons were attached to the substrate in a drop of Neurobasal medium (Gibco), containing penicillin/streptomycin (Gibco), Glutamax (Gibco) and B-27 (Gibco) supplements. After 2 hours, medium volume was adjusted to 2ml per coverslip in 6-well plates. After 20 days in vitro cells were transfected, using Lipofectamine 2000 (Invitrogen), with an mCherry construct under control of a chicken β-actin promoter to ensure neuron-specific expression. One day after transfection, cells were treated for four hours with 200 μm MPEP (Sigma), 300 μm fenobam (Sigma), 100 μm D-AP-5 (Sigma) or left untreated in supplemented Neurobasal medium. After treatment, neurons were fixed in 4% formaldehyde in PBS, washed in PBS and mounted in Mowiol mounting solution (Mowiol 4-88, Hoechst).
Quantification of protrusion density and dendrite branching
Images of β-actin-mCherry transfected neurons were acquired using a Zeiss LSM510 confocal microscope. Twenty to forty neurons from three independent experiments were imaged for each condition. For each neuron, a z-stack of 10 × 0,3μm was made. The projected images were analyzed for protrusions with Metamorph software (Molecular Devices, Sunnyvale, CA). Two distal dendritic segments of 70-100 μm were chosen per neuron for protrusion morphometric analysis. For each protrusion, the length and the width were measured. The length was defined as the distance from the base to the tip of the protrusion; width was defined as the maximum distance perpendicular to the long axis of the protrusion. In order to make an objective distinction between spines and filopodia, we calculated the ratio of the width and the length for each protrusion. Protrusions with a ratio above or equal to 0,5 were considered as spines and conversely, protrusions with a ratio below 0.5 were considered as filopodia (Okamura et al., 2004). Averages of protrusion densities of three independent experiments were compared with unpaired two-tailed Student's T-tests.
Dendrite branching of β-actin-mCherry transfected neurons was quantified by performing Sholl analyses of stacked Zeiss confocal generated images (40× objective, stack of 20 × 0,2μm). With Metamorph software, concentric equally spaced circles (every 20μm) were drawn around the cell soma of each neuron and subsequently, the amount of dendrite crossings were counted per circle. Averages of counts of three independent experiments were compared with unpaired two-tailed Student's T-tests.
Results
MPEP rescues prepulse inhibition of startle defect in Fmr1 KO mice
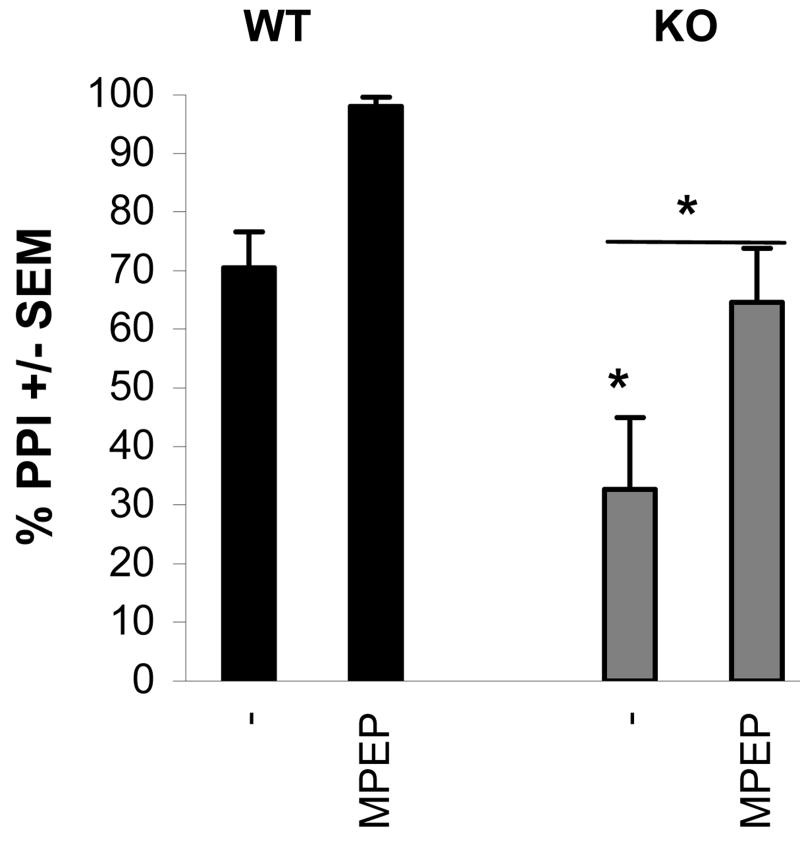
One of the most common clinical features of FXS is heightened sensitivity to sensory stimulation (Frankland et al., 2004; Miller et al., 1999). PPI is a widely used model to study basic sensorimotor processing and has shown to be related to mGluR signaling (Grauer and Marquis, 1999). In our mouse model, we examined PPI in wild type and Fmr1 KO mice. Mice were presented with a startling acoustic stimulus of 90dB, which in the prepulse condition was preceded by a 70dB pulse, 50 ms before the startle stimulus. In wild type mice, the startle response after a prepulse was inhibited by 73% compared to the response after a startle stimulus alone (Fig.1). This indicates good PPI in the wild type mice. In the Fmr1 KO mice however, the startle response was inhibited by only 30% when a prepulse preceded the startle stimulus, illustrating that PPI is defective in Fmr1 KO mice. To study if MPEP can rescue a behavioral FXS phenotype in our mouse model, we examined PPI in wild type and Fmr1 KO mice with or without MPEP treatment. Treatment of Fmr1 KO mice with 20 mg/kg MPEP thirty minutes prior to the experiment, rescued the PPI to a level of 70%, indistinguishable from the wild type PPI response (Fig.1). Interestingly, wild type mice also responded to MPEP treatment with higher PPI levels. This effect was not further examined in this study.
Fig.1. Rescue of prepulse inhibition of startle in Fmr1 KO mice.
Both wild type and Fmr1 KO mice were subjected to prepulse inhibition of startle procedures. Fmr1 KO mice displayed a dramatic impairment of PPI on day 1 (baseline levels). This reduction was rescued to wild type levels on day 2 by injection of 20 mg/kg MPEP 30 minutes prior to training. Interestingly, the wild types showed an equal improvement of PPI performance after injection of MPEP.
Rescue of protrusion phenotype of Fmr1 KO primary hippocampal neurons
Although MPEP has been shown to rescue several behavioral FXS phenotypes in mice and Drosophila (McBride et al., 2005; Yan et al., 2005), the molecular mechanism behind these rescue effects remains elusive. Therefore we decided to study if mGluR5 antagonists are also able to rescue FXS related altered protrusion morphology in an established in vitro model of primary hippocampal neurons.
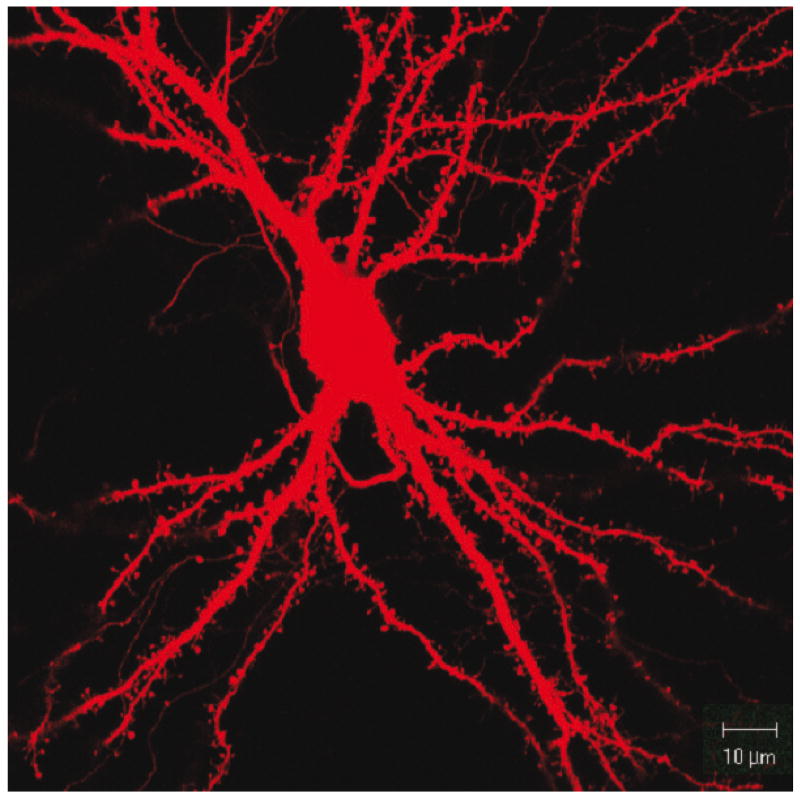
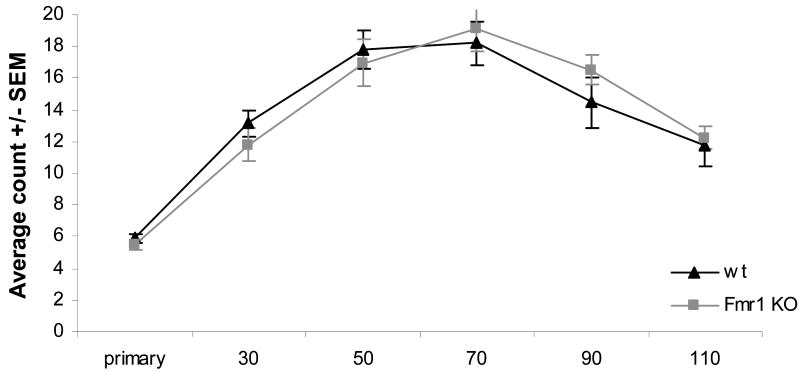
In order to characterize our in vitro model, we first examined the basic neuronal properties of dendrite branching and protrusion morphology of our primary hippocampal cultures. Primary hippocampal neurons of wt and Fmr1 KO mice were cultured in parallel. After twenty days in vitro, neurons were transfected with a βactin-mCherry construct in order to visualize the neurons, including dendritic protrusions. The next day, neurons were fixed, after which transfected neurons were imaged by confocal microscopy (Fig.2). In order to quantify dendrite branching, we used Sholl analysis, which measures the number of dendrite crossings with equally spaced concentric circles around the cell soma. Quantification of three independent experiments comparing dendrite branching of Fmr1 KO neurons and wild type neurons did not reveal any significant difference (Fig.3).
Fig.2.
Representative image of a wild type E18 hippocampal mouse neuron (DIV21), transfected with a β-actin-mCherry construct.
Fig.3. Dendrite branching is normal in Fmr1 KO primary hippocampal neurons.
Sholl analysis of wild type and Fmr1 KO primary hippocampal neurons cultured in parallel was performed with Metamorph software. Average of three independent experiments.
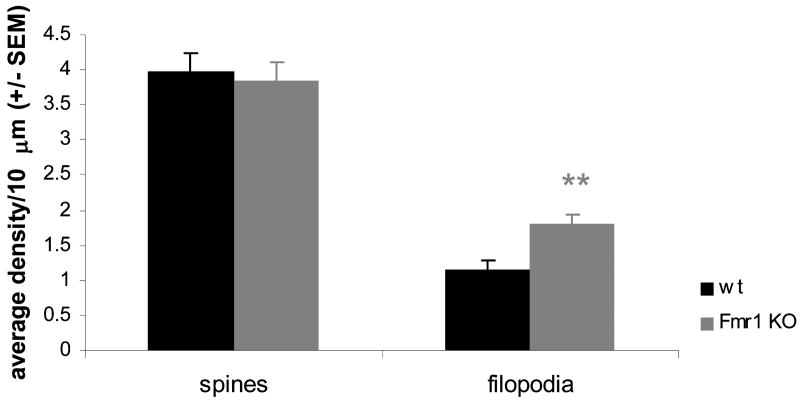
Protrusions were quantified and measured for their length and width with Metamorph software. Based on these measurements, they were classified objectively as spines or filopodia (immature spines). Mature spines have a mushroom shaped appearance with a large spine head, while immature spines or filopodia have a long and thin appearance. Therefore, protrusions whose width was equal to or more than half the size of its length, were judged as standard mature mushroom spines. If this ratio was less than half the size of the length, protrusions were considered to be filopodia. Using the Metamorph software we compared the protrusions of wt and Fmr1 KO neurons. Based on the above described criteria, Fmr1 KO neurons had an excess of filopodia when compared to wt neurons (Fig.4).
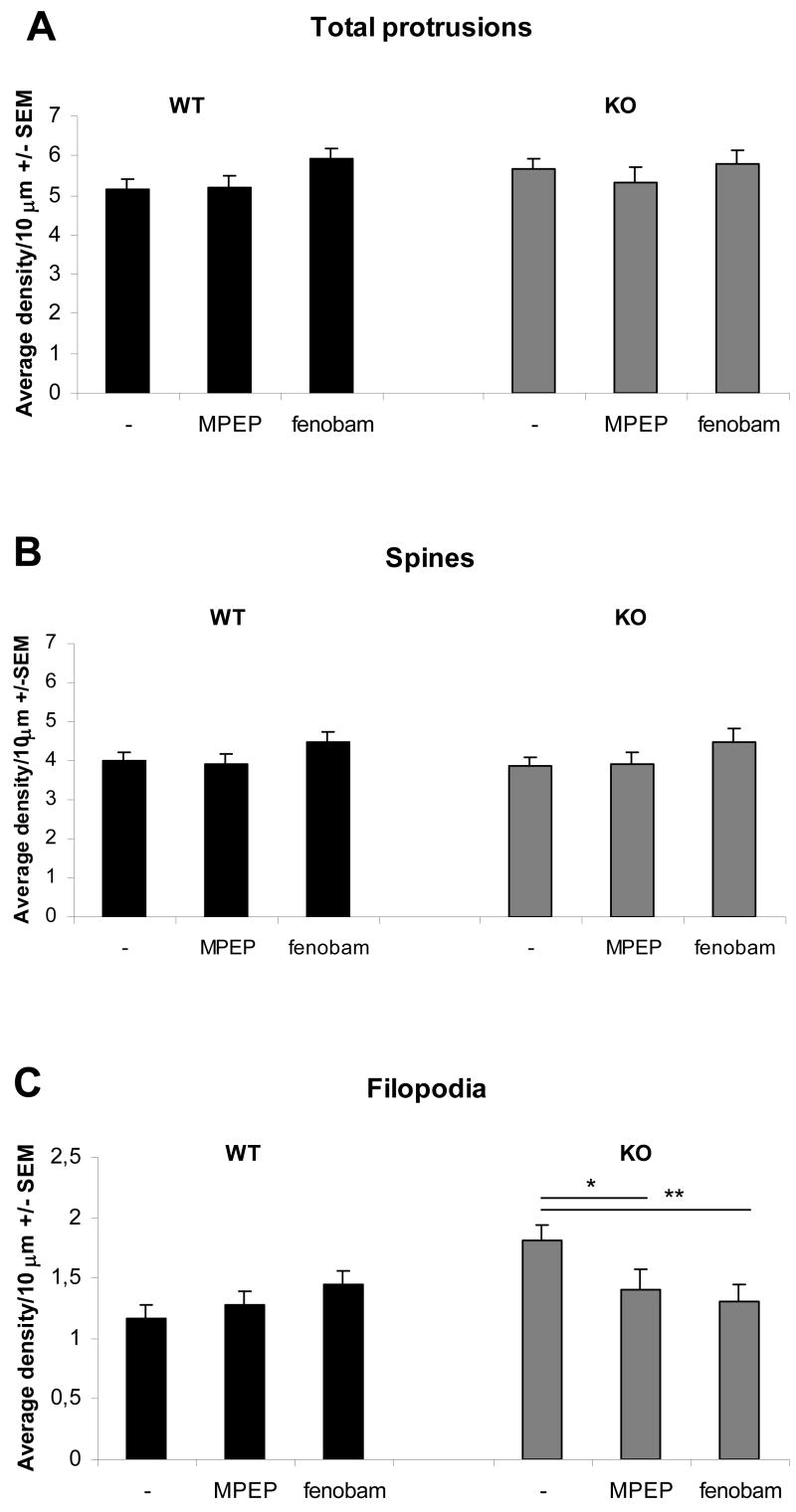
Fig.4. Fmr1 KO primary hippocampal neurons have an immature protrusion phenotype.
Protrusion densities of wild type and Fmr1 KO primary hippocampal neurons cultured in parallel were counted with Metamorph software. Fmr1 KO neurons had significantly more filopodia than wild type neurons (p<0,001), corresponding to an immature phenotype. Averages of 3 independent experiments, compared with Student's T tests. The distinction between spines and filopodia was made objectively by using a threshold ratio of 0,5 for the width/length ratio of protrusions.
After characterization of our in vitro model and establishing an Fmr1 KO phenotype in protrusion morphology, we continued to study the effect of MPEP on protrusion morphology. In addition, we also studied the effects of the more specific mGluR5 antagonist fenobam. Fenobam was originally discovered as an anxiolytic agent with unknown molecular target, that later was discovered to be a potent mGluR5 antagonist with an allosteric modularity site shared by MPEP, but different in structure (Porter et al., 2005). In parallel, wild type and Fmr1 KO neurons were subjected to treatment with the mGluR5 antagonists. Treatment of Fmr1 KO neurons with 200 μM MPEP or 300 μM fenobam for four hours, rescued the protrusion phenotype (Fig.5). The number of filopodia in treated Fmr1 KO neurons was significantly lower than that in untreated Fmr1 KO neurons, and indistinguishable from wild type neurons (Fig.5C). Protrusion numbers of wild type neurons were not significantly altered by MPEP or fenobam treatment.
Fig.5. Rescue of protrusion morphology in Fmr1 KO primary hippocampal neurons.
Fmr1 KO and wild type neurons were treated for four hours with 200 μm MPEP or 300 μm fenobam. The total amount of protrusions (A) and the amount of mature spines (B) were unaffected by mGluR5 antagonist treatment. The Fmr1 KO phenotype showing an increased number of filopodia was completely rescued by both mGluR5 antagonists (C). Averages of 3 independent experiments, compared with Student's T tests (*=p<0,05, **=p<0,01).
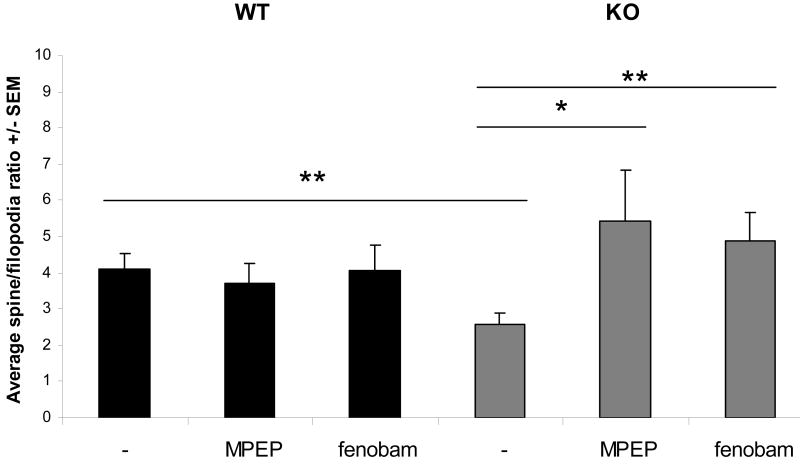
The total protrusion density did not differ significantly between wt and Fmr1 KO neurons with or without treatments (Fig.5A). Although the average number of protrusions classified as spines in Fmr1 KO neurons were not statistically different from wt neurons (Fig.5B), the average percentage of spines compared to filopodia per neuron was significantly lower in Fmr1 KO neurons and was also rescued by either MPEP or fenobam treatment (Fig.6). In other words, mGluR5 antagonist treatment restored the spine/filopodia ratio of Fmr1 KO neurons to wild type levels.
Fig.6. mGluR5 antagonist treatment changes the distribution of spines and filopodia in Fmr1 KO neurons.
The average spine/filopodia ratio changes significantly in Fmr1 KO primary hippocampal neurons after treatment with two independent mGluR5 antagonists. As total protrusion density is not different between wild type and Fmr1 KO neurons, we can conclude that the excess of filopodia in Fmr1 KO neurons can successfully be changed into or replaced by spines.
Discussion
In this study we have shown a clear defect in PPI in Fmr1 KO mice measured by eye blink in response to loud sound. In support of the mGluR theory of FXS, this defect was rescued to wild type levels after treatment of the mice with 20 mg/kg of the mGluR5 antagonist MPEP. The impaired PPI response in Fmr1 KO mice is in line with sensorimotor gating deficits in FXS patients (Frankland et al., 2004). However, in the Frankland study, PPI was found to be increased rather than decreased in Fmr1 KO mice. One explanation could be that the measurement of startle eyelid responses with the magnetic distance measurement technique (MDMT) as performed in our study is more sensitive than standard wholebody startle measurements such as used in the Frankland study. Eyelid measurements of startle include the very first components of the startle response, whereas wholebody startle measurements require induction of very strong startle responses. Therefore, eyelid startle measurement allows for a better dissection of the more subtle differences in startle behavior. In addition, our method allows us to reduce the sound pressure levels necessary for startle induction which is relevant since Fmr1 KO mice react strongly to loud acoustic stimuli and are highly susceptible to audiogenic seizures (Musumeci et al., 2000). In another study, PPI in Fmr1 KO mice was not significantly altered (Spencer et al., 2006). This could also be attributed to differences in the sensitivity of the methods used to measure PPI. Interestingly, we found that wild type mice showed increased PPI after MPEP treatment which is in contrast with earlier studies in rats (Henry et al., 2002; Zou et al., 2007). The underlying molecular mechanisms of the increased PPI in MPEP-treated wild type mice are unknown and beyond the scope of this study. Nevertheless, the rescue of PPI levels in the Fmr1 KO mice underscores the therapeutic potential of MPEP (and/or other mGluR5 antagonists) for treatment of Fragile X related behavior. The PPI as measured in this study has therefore proven to be a valid behavioral test to study mGluR5 targeted therapeutic intervention in FXS patients. In this study, an acute effect of MPEP was measured (thirty minutes after i.p. injection). However, in consideration of potential future therapeutic interventions in patients, it would be interesting to study these effects in a chronic model for MPEP treatment after long-term exposure (e.g. 2 months) of mice to MPEP. In addition, other mGluR5 antagonists that are more specific for the mGluR5 receptor and show less side effects are due to be tested in clinical trials in the future.
In an attempt to study the effect at the cellular level, we have shown altered protrusion morphology of Fmr1 KO neurons in an established in vitro model. Primary hippocampal neurons of E18 wild type and Fmr1 KO mice were cultured for 21 days, a time at which dendritic spines have matured and form synaptic contacts characteristic of those seen in vivo (Papa 1995). Protrusion morphology in Fmr1 KO neurons was significantly different from wild type neurons. Fmr1 KO had more filopodia than wild type neurons, corresponding to a more immature phenotype (Fig.4). This is in accordance with literature for both FXS patients and Fmr1 KO mice (Comery et al., 1997; Galvez and Greenough, 2005; Grossman et al., 2006; Hinton et al., 1991; Koekkoek et al., 2005; Nimchinsky et al., 2001). In primary hippocampal neurons, reported quantities of protrusions tend to differ in literature. One study has even described fewer protrusions in hippocampal cultures of Fmr1 KO mice (Braun 2000). Another more recent study showed increased density of filopodia-like spines in cultured Fmr1 KO hippocampal neurons, but with many more protrusions per distance (3-5 filopodia/10 μm) than in our study (Antar et al., 2006). However, these cultures were not fully matured and different culture methods (such as use of glial cell feeder layers) might influence the protrusion number. In contrast, it was reported that specifically in hippocampal area CA1, Fmr1 KO neurons have more stubby spines as opposed to filopodia (Grossman et al., 2006). In light of all these seemingly different findings, we analyzed our own culture system extensively and used an objective measurement technique to distinguish mature mushroom-like protrusions from immature filopodia-like protrusions. With these criteria, Fmr1 KO neurons in our culture system showed a decreased spine to filopodia ratio. Furthermore, we have shown rescue of this altered protrusion morphology in Fmr1 KO primary hippocampal neurons by two independent mGluR5 antagonists, MPEP and fenobam. As total protrusion density is not different between wild type and Fmr1 KO neurons, we conclude that the excess of filopodia in Fmr1 KO neurons can successfully be changed into or replaced by spines.
Since spine shape is correlated with the number of AMPA receptors in the postsynaptic density (Matsuzaki et al., 2001), these data correlate with the rescue effect of MPEP on AMPA receptor trafficking as shown by Nakamoto et al. (Nakamoto et al., 2007). In the latter study, the concentrations of MPEP used on primary neurons (10-50 μM) differed from our experiments due to different time courses of the experiments. In our study, a higher MPEP concentration was needed to visualize fast effects (within 4 hours) on protrusion morphology, whereas Nakamoto et al. studied MPEP effects after 16 hours and up to three days. In wild type cerebellar Purkinje cells, daily treatment with 30 μM MPEP for ten days changes normal protrusion morphology into a more immature phenotype with more filopodia like protrusions (Catania et al., 2001). In the present study, acute MPEP treatment had no significant effect on the protrusion morphology of wild type hippocampal neurons (Fig. 4).
Others have shown that MPEP can target NMDA receptors at high concentrations (Lea et al., 2005; Popoli et al., 2004). However, it is unlikely that the rescue effect in this study is mediated by NMDA receptors, as we also see the rescue effect with the structurally different, more specific mGluR5 antagonist fenobam. Moreover, we also tested the effect of the NMDA specific antagonist D-AP-5 (100 μM) on protrusion morphology, which did not show rescue of the Fmr1 KO protrusion phenotype (data not shown).
In conclusion, our in vitro model of primary hippocampal neurons and the in vivo measurement of PPI form excellent tools to further study the molecular mechanisms that underlie therapeutic intervention with mGluR5 antagonists in FXS patients and have great potential for testing newly developed drugs.
Acknowledgments
We thank Dr. Casper C. Hoogenraad for helpful discussions and suggestions and Ronald Buijsen and Ingeborg Nieuwenhuizen for their technical assistance. This work was supported by the FRAXA Research Foundation (RW), NIH (NICHD R01 HD38038), (BAO and DLN), ZonMw 912-04-022 (BAO) and ZonMw 912-07-022 (RW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antar LN, et al. Localization of FMRP-associated mRNA granules and requirement of microtubules for activity-dependent trafficking in hippocampal neurons. Genes Brain Behav. 2005;4:350–9. doi: 10.1111/j.1601-183X.2005.00128.x. [DOI] [PubMed] [Google Scholar]
- Antar LN, et al. Local functions for FMRP in axon growth cone motility and activity-dependent regulation of filopodia and spine synapses. Mol Cell Neurosci. 2006;32:37–48. doi: 10.1016/j.mcn.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Bagni C, Greenough WT. From mRNP trafficking to spine dysmorphogenesis: the roots of fragile X syndrome. Nat Rev Neurosci. 2005;6:376–87. doi: 10.1038/nrn1667. [DOI] [PubMed] [Google Scholar]
- Bakker CE, et al. Fmr1 knockout mice: A model to study fragile X mental retardation. Cell. 1994;78:23–33. [PubMed] [Google Scholar]
- Bardoni B, et al. The fragile X syndrome: exploring its molecular basis and seeking a treatment. Expert Rev Mol Med. 2006;8:1–16. doi: 10.1017/S1462399406010751. [DOI] [PubMed] [Google Scholar]
- Bear MF, et al. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–7. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Catania MV, et al. Endogenous activation of group-I metabotropic glutamate receptors is required for differentiation and survival of cerebellar Purkinje cells. J Neurosci. 2001;21:7664–73. doi: 10.1523/JNEUROSCI.21-19-07664.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comery TA, et al. Abnormal dendritic spines in fragile X knockout mice: Maturation and pruning deficits. Proc Natl Acad Sci USA. 1997;94:5401–5404. doi: 10.1073/pnas.94.10.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JC, et al. Fragile X Mental Retardation Protein Targets G Quartet mRNAs Important for Neuronal Function. Cell. 2001;107:489–99. doi: 10.1016/s0092-8674(01)00566-9. [DOI] [PubMed] [Google Scholar]
- Darnell JC, et al. FMRP RNA targets: identification and validation. Genes Brain Behav. 2005;4:341–9. doi: 10.1111/j.1601-183X.2005.00144.x. [DOI] [PubMed] [Google Scholar]
- Darnell JC, et al. The fragile X mental retardation protein, FMRP, recognizes G-quartets. Ment Retard Dev Disabil Res Rev. 2004;10:49–52. doi: 10.1002/mrdd.20008. [DOI] [PubMed] [Google Scholar]
- De Diego Otero Y, et al. Transport of Fragile X Mental Retardation Protein via Granules in Neurites of PC12 Cells. Mol Cell Biol. 2002;22:8332–41. doi: 10.1128/MCB.22.23.8332-8341.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolen G, et al. Correction of Fragile X Syndrome in Mice. Neuron. 2007;56:955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland PW, et al. Sensorimotor gating abnormalities in young males with fragile X syndrome and Fmr1-knockout mice. Mol Psychiatry. 2004;9:417–425. doi: 10.1038/sj.mp.4001432. [DOI] [PubMed] [Google Scholar]
- Galvez R, Greenough WT. Sequence of abnormal dendritic spine development in primary somatosensory cortex of a mouse model of the fragile X mental retardation syndrome. Am J Med Genet A. 2005;135:155–160. doi: 10.1002/ajmg.a.30709. [DOI] [PubMed] [Google Scholar]
- Grauer SM, Marquis KL. Intracerebral administration of metabotropic glutamate receptor agonists disrupts prepulse inhibition of acoustic startle in Sprague-Dawley rats. Psychopharmacology (Berl) 1999;141:405–12. doi: 10.1007/s002130050850. [DOI] [PubMed] [Google Scholar]
- Greenough WT, et al. Synaptic regulation of protein synthesis and the fragile X protein. Proc Natl Acad Sci U S A. 2001;98:7101–6. doi: 10.1073/pnas.141145998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman AW, et al. Local protein synthesis and spine morphogenesis: Fragile X syndrome and beyond. J Neurosci. 2006;26:7151–5. doi: 10.1523/JNEUROSCI.1790-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry SA, et al. The mGluR5 antagonist MPEP, but not the mGluR2/3 agonist LY314582, augments PCP effects on prepulse inhibition and locomotor activity. Neuropharmacology. 2002;43:1199–209. doi: 10.1016/s0028-3908(02)00332-5. [DOI] [PubMed] [Google Scholar]
- Hinton VJ, et al. Analysis of neocortex in three males with the fragile X syndrome. Am J Med Genet. 1991;41:289–294. doi: 10.1002/ajmg.1320410306. [DOI] [PubMed] [Google Scholar]
- Huber KM, et al. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci U S A. 2002;99:7746–50. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, et al. Role for rapid dendritic protein synthesis in hippocampal mGluR- dependent long-term depression. Science. 2000;288:1254–7. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- Koekkoek SK, et al. Monitoring kinetic and frequency-domain properties of eyelid responses in mice with magnetic distance measurement technique. J Neurophysiol. 2002;88:2124–33. doi: 10.1152/jn.2002.88.4.2124. [DOI] [PubMed] [Google Scholar]
- Koekkoek SK, et al. Deletion of FMR1 in Purkinje Cells Enhances Parallel Fiber LTD, Enlarges Spines, and Attenuates Cerebellar Eyelid Conditioning in Fragile X Syndrome. Neuron. 2005;47:339–52. doi: 10.1016/j.neuron.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Laggerbauer B, et al. Evidence that fragile X mental retardation protein is a negative regulator of translation. Hum Mol Genet. 2001;10:329–338. doi: 10.1093/hmg/10.4.329. [DOI] [PubMed] [Google Scholar]
- Lea PMt, et al. Neuroprotective activity of the mGluR5 antagonists MPEP and MTEP against acute excitotoxicity differs and does not reflect actions at mGluR5 receptors. Br J Pharmacol. 2005;145:527–34. doi: 10.1038/sj.bjp.0706219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R, et al. The fragile X protein controls microtubule-associated protein 1B translation and microtubule stability in brain neuron development. Proc Natl Acad Sci U S A. 2004;101:15201–15206. doi: 10.1073/pnas.0404995101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, et al. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci. 2001;4:1086–92. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride SM, et al. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile x syndrome. Neuron. 2005;45:753–64. doi: 10.1016/j.neuron.2005.01.038. [DOI] [PubMed] [Google Scholar]
- Mientjes EJ, et al. The generation of a conditional Fmr1 knock out mouse model to study Fmrp function in vivo. Neurobiol Dis. 2006;21:549–555. doi: 10.1016/j.nbd.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Miller LJ, et al. Electrodermal responses to sensory stimuli in individuals with fragile X syndrome: a preliminary report. Am J Med Genet. 1999;83:268–79. [PubMed] [Google Scholar]
- Miyashiro KY, et al. RNA Cargoes Associating with FMRP Reveal Deficits in Cellular Functioning in Fmr1 Null Mice. Neuron. 2003;37:417–31. doi: 10.1016/s0896-6273(03)00034-5. [DOI] [PubMed] [Google Scholar]
- Muddashetty RS, et al. Dysregulated metabotropic glutamate receptor-dependent translation of AMPA receptor and postsynaptic density-95 mRNAs at synapses in a mouse model of fragile X syndrome. J Neurosci. 2007;27:5338–48. doi: 10.1523/JNEUROSCI.0937-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musumeci SA, et al. Audiogenic seizures susceptibility in transgenic mice with fragile X syndrome. Epilepsia. 2000;41:19–23. doi: 10.1111/j.1528-1157.2000.tb01499.x. [DOI] [PubMed] [Google Scholar]
- Nakamoto M, et al. Fragile X mental retardation protein deficiency leads to excessive mGluR5-dependent internalization of AMPA receptors. Proc Natl Acad Sci U S A. 2007;104:15537–42. doi: 10.1073/pnas.0707484104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimchinsky EA, et al. Abnormal development of dendritic spines in fmr1 knock-out mice. J Neurosci. 2001;21:5139–46. doi: 10.1523/JNEUROSCI.21-14-05139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura K, et al. Cadherin activity is required for activity-induced spine remodeling. J Cell Biol. 2004;167:961–72. doi: 10.1083/jcb.200406030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoli P, et al. Neuroprotective effects of the mGlu5R antagonist MPEP towards quinolinic acid-induced striatal toxicity: involvement of pre- and post-synaptic mechanisms and lack of direct NMDA blocking activity. J Neurochem. 2004;89:1479–89. doi: 10.1111/j.1471-4159.2004.02448.x. [DOI] [PubMed] [Google Scholar]
- Porter RH, et al. Fenobam: a clinically validated nonbenzodiazepine anxiolytic is a potent, selective, and noncompetitive mGlu5 receptor antagonist with inverse agonist activity. J Pharmacol Exp Ther. 2005;315:711–21. doi: 10.1124/jpet.105.089839. [DOI] [PubMed] [Google Scholar]
- Snyder EM, et al. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nat Neurosci. 2001;4:1079–85. doi: 10.1038/nn746. [DOI] [PubMed] [Google Scholar]
- Spencer CM, et al. Exaggerated behavioral phenotypes in Fmr1/Fxr2 double knockout mice reveal a functional genetic interaction between Fragile X-related proteins. Hum Mol Genet. 2006;15:1884–1894. doi: 10.1093/hmg/ddl121. [DOI] [PubMed] [Google Scholar]
- Wang H, et al. Dynamic Association of the Fragile X Mental Retardation Protein as an mRNP between Microtubules and Polyribosomes. Mol Biol Cell. 2007 doi: 10.1091/mbc.E07-06-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler IJ, et al. Fragile X mental retardation protein is translated near synapses in response to neurotransmitter activation. Proc Natl Acad Sci USA. 1997;94:5395–5400. doi: 10.1073/pnas.94.10.5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler IJ, et al. Fragile X mental retardation protein is necessary for neurotransmitter-activated protein translation at synapses. Proc Natl Acad Sci U S A. 2004;101:17504–17509. doi: 10.1073/pnas.0407533101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan QJ, et al. Suppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology. 2005;49:1053–1066. doi: 10.1016/j.neuropharm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Zalfa F, et al. A new function for the fragile X mental retardation protein in regulation of PSD-95 mRNA stability. Nat Neurosci. 2007 doi: 10.1038/nn1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou D, et al. Metabotropic glutamate subtype 5 receptors modulate fear-conditioning induced enhancement of prepulse inhibition in rats. Neuropharmacology. 2007;52:476–86. doi: 10.1016/j.neuropharm.2006.08.016. [DOI] [PubMed] [Google Scholar]