Abstract
Most studies of long-term potentiation (LTP) have focused on potentiation induced by the activation of postsynaptic NMDA receptors (NMDARs). However, it is now apparent that NMDAR-dependent signaling processes are not the only form of LTP operating in the brain (Malenka and Bear, 2004). Previously, we have observed that LTP in leech central synapses made by the touch mechanosensory neurons onto the S interneuron was NMDAR-independent (Burrell and Sahley, 2004). Here we examine the cellular mechanisms mediating T-to-S (T→S) LTP and find that its induction requires activation of metabotropic glutamate receptors (mGluRs), voltage-dependent Ca2+ channels (VDCCs) and protein kinase C (PKC). Surprisingly, whenever LTP was pharmacologically inhibited, long-term depression (LTD) was observed at the tetanized synapse, indicating that LTP and LTD were activated at the same time in the same synaptic pathway. This co-induction of LTP and LTD likely plays an important role in activity-dependent regulation of synaptic transmission.
Keywords: metabotropic glutamate receptor, voltage-dependent Ca2+ channel, protein kinase C, neuroplasticity, invertebrate
NMDAR-dependent long-term potentiation (LTP) and long-term depression (LTD) are thought to be critical cellular substrates for mediating learning and memory because their initiation requires coincident activity in both the pre- and postsynaptic neurons (activity dependence) and the resulting changes are restricted to the co-activated synapses (synapse specificity). However, it is now clear that other molecules can perform coincidence-detection in place of NMDARs for both LTP and LTD (Malenka and Bear, 2004; Anwyl, 2006). This heterogeneity in cellular mechanisms mediating LTP and LTD, along with the structural complexity of the vertebrate brain, complicates efforts to determine the functional contribution of synaptic changes to learning-related changes in behavior. The medicinal leech (Hirudo medicinalis) has a number of properties that make it a useful model for studies of LTP and LTD. Most neurons in the leech CNS are large and easily visualized and there are far fewer neurons in the leech CNS (~400 neurons/ganglion with 21 body ganglia plus the head and tail ganglia (Muller et al., 1981)) compared to a mammalian brain. Therefore, it is possible to record from the same, identifiable neuron from one animal to the next and to link changes in a given neuron to a specific behavioral function (Burrell and Sahley, 2005; Kristan et al., 2005). Furthermore, the cellular and molecular properties between leech and vertebrates neurons are highly conserved (Burrell and Sahley, 2001), so discoveries about neural function in invertebrates are relevant to understanding processes in vertebrate neurons.
LTP and LTD have been observed in two different synaptic connections in the leech CNS; those made by the touch (T) sensitive cells onto the S interneuron (S-cell) and by pressure (P) sensitive onto the same S-cell. The S-cell is thought to be critical for certain types of learning in the leech (Modney et al., 1997; Burrell et al., 2003). LTP in the P→S synapse is NMDAR-dependent, synapse-specific, and expressed postsynaptically (Burrell and Sahley, 2004). At the T→S synapse (Fig. 1A), which is the focus of this paper, tetanic stimulation simultaneously induces homosynaptic LTP (homLTP) in the tetanized synapse and heterosynaptic LTD (hetLTD) in the non-tetanized synapse (Fig. 1B; also see Burrell and Sahley, 2004). This pattern of homLTP and hetLTD (synapses consisting of different presynaptic cells, but the same postsynaptic target) has been observed in the CA1 (Lynch et al., 1977), CA3 (Kosub et al., 2005) and dentate gyrus (Abraham and Goddard, 1983) regions of the hippocampus, the amygdala (Royer and Pare, 2003) and the visual cortex (Tsumoto and Suda, 1979). HomLTP at the T→S synapse is NMDAR-independent while T→S hetLTD is NMDAR-dependent (Burrell and Sahley, 2004). In this study, we examined underlying homLTP at the T→S synapse and discovered that inhibition of homLTP uncovered LTD in the same synapse that was apparently initiated in parallel with LTP in the tetanized pathway.
Figure 1.
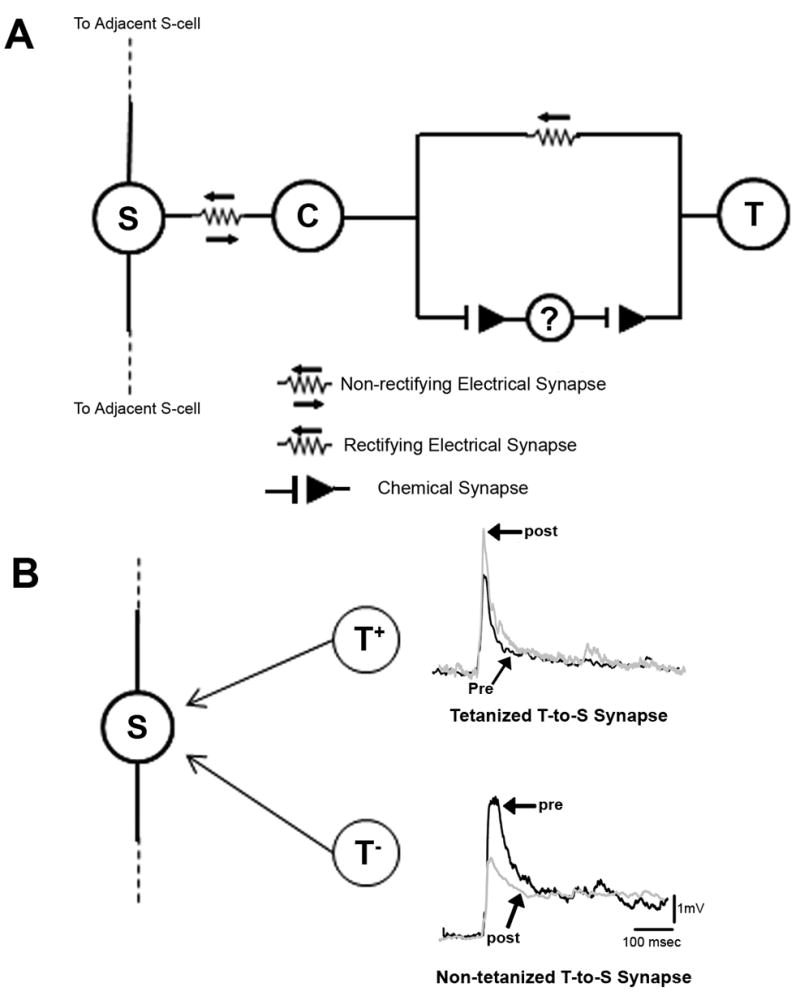
(A) T→S synaptic circuit. The T→S synapse has both a monosynaptic electrical and polysynaptic chemical (glutamatergic) component (Muller and Scott, 1981; Li and Burrell, 2006). The “?” indicates the unknown neuron(s) that mediate the polysynaptic, chemical component of the T→S synapse. Nearly all synaptic input to the S-cell is routed through the coupling (C) interneuron. The S- and the C-cells are linked by a non-rectifying electrical synapse and the level of electrical coupling is so strong that EPSPs elicited in the C-cell are carried to the S-cell with minimal attenuation or delay, acting as monosynaptic EPSPs (Muller and Scott, 1981). The C-cells are not directly recorded from because they are on the opposite (dorsal) side of the ganglion. (B) Changes in T→S EPSP at the tetanized and non-tetanized synapse. Left Diagrammatic representation of convergent inputs by the two T-cells, the tetanized (T+) and non-tetanized (T−), onto a single postsynaptic S-cell. Right Traces labeled “pre” were recorded prior to tetanic stimulation and those labeled “post” were recorded 60min after tetanus. Tetanization of the DP nerve elicited homLTP in the tetanized T→S synapse and simultaneously elicited hetLTD in the non-tetanized T→S synapse (same postsynaptic S-cell, different presynaptic T-cells).
To test the signaling pathways that mediate T→S LTP, individual ganglia were dissected from 3g leeches obtained from a commercial supplier (Leeches USA Ltd.) and aintained in pond water (0.5 g/1 L H2O Hirudo salt from Leeches USA Ltd.) at 18°C with a 12h:12h light/dark cycle. Dissections and recordings were carried out in normal leech saline (in mM: 115 NaCl, 4 KCl, 1.8 CaCl2, 1.0 MgCl2 and 10 HEPES). Following dissection, ganglia were placed in a recording chamber under constant perfusion. Intracellular recordings from identified T- and S-cells were made using glass sharp microelectrodes connected to a bridge amplifier (BA-1; National Precision Instruments). A detailed protocol for inducing homLTP and hetLTD in the T→S synapse are described in Burrell and Sahley (2004). In brief, unitary excitatory postsynaptic potentials (EPSPs) were elicited in the S-cell by stimulation of the presynaptic neuron (the T-cell) prior to (pre-test) and 1 hr after (post-test) tetanic stimulation of the dorsal posterior nerve root. Ten tetani were delivered at 10sec intervals, with each tetanus consisting of five stimuli delivered at 25Hz (STG 1004 Programmable Stimulator; Multichannel Systems). Drugs were applied after the pre-test for 10mins with the tetanizing stimuli applied at the end of this period (all drugs were obtained from Sigma). T→S synaptic transmission was tested at the tetanized synapse (T+) and the non-tetanized connection (T−). Only two recordings (pre- and post-tetanus) were made because chronic (>10–15min) recordings of the S-cell damage the interneuron (Burrell and Sahley, 2004).
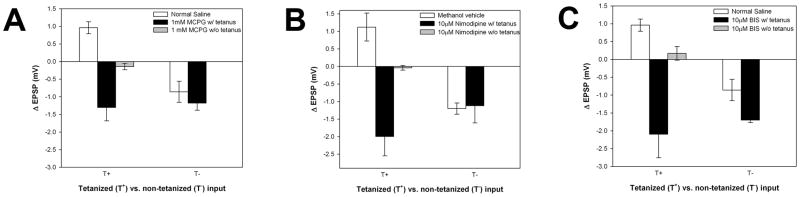
In normal saline, tetanic stimulation elicited homLTP in the tetanized T→S synapse and hetLTD in the non-tetanized connection (Fig. 1B and 2), replicating the results obtained in Burrell and Sahley (2004). The T→S synapse is glutamatergic (Li and Burrell, 2006) and since T→S LTP is NMDAR-independent, the potential involvement of mGluRs was investigated. HomLTP was blocked in ganglia treated with 1 mM alpha-methyl-4-carboxyphenylglycine (MCPG, an antagonist of mGluR1, 2 and 5) during tetanic stimulation. MCPG did not block hetLTD, indicating that mGluRs contribute only to T→S homLTP and not to hetLTD (Fig. 2A), which has already been shown to be NMDAR-dependent (Burrell and Sahley, 2004). Surprisingly, homosynaptic LTD (homLTD) was observed in the tetanized T→S synapses (T+) of the MCPG-treated ganglia in addition to the hetLTD at the non-tetanized synapse (T−; Fig. 2A). Apparently, the same tetanus that elicits mGluR-dependent homLTP in the tetanized T→S synapse simultaneously initiates homLTD in the same synapse that can only be observed when homLTP is blocked.
Figure 2.
Cellular mechanisms of T→S LTP. All data are expressed as a ΔEPSP between an initial pretest and a posttest 60min later. T+ represents the tetanized T→S synapse, while T− represents the non-tetanized pathway. Under control conditions (saline (N=8) or methanol vehicle (N=4)), tetanization induces homLTP in the T+ pathway and hetLTD in the T− pathway. (A) Treatment with the mGluR antagonist, MCPG, blocked homLTP and homLTD was observed at the T+ synapse instead (N=7). MCPG did not affect hetLTD at T− and MCPG applied without tetanus did not alter synaptic transmission (N=4). (B) Treatment with the VDCC blocker, nimodipine, blocked homLTP and homLTD was observed at the T+ synapse instead (N=4). Nimodipine did not affect hetLTD at T− and nimodipine applied without tetanus did not alter synaptic transmission (N=3). (C) Treatment with the PKC antagonist, bisindolylmaleimide (BIS), blocked homLTP and homLTD was observed at the T+ synapse instead (N=4). BIS did not affect hetLTD at T− and BIS applied without tetanus did not alter synaptic transmission (N=4).
If mGluRs are required for T→S LTP, then they likely act in concert with other molecules to form a coincidence detection system that is an alternative to the NMDAR. One possible combination is mGluRs plus VDCCs in which mGluRs detect synaptic input and VDCCs detect depolarization. To test this hypothesis, 20μM nimodipine, an antagonist of L-type Ca2+ channels, was applied during tetanus. As with blockade of mGluRs, nimodipine prevented T→S homLTP and LTD was observed at both the tetanized and non-tetanized pathways (Fig. 2B).
One potential intracellular target of mGluRs and VDCCs is PKC. Group I mGluRs activate phospholipase C that leads to the production of DAG, which along with Ca2+ influx from the VDCCs, activates PKC (De Blasi et al., 2001). To test the involvement of PKC during T→S LTP, the membrane permeable PKC antagonist bisindolylmaleimide (BIS, 10μM) was applied during the tetanus. Again, LTP in the tetanized pathway was blocked and LTD was observed at both the tetanized and non-tetanized synapses (Fig. 2C).
In many synapses LTP is initiated by NMDAR activation, which acts as a coincidence detector of pre- and postsynaptic activity. However, there are a number of forms of LTP and LTD, including T→S LTP, where a combination of mGluRs and VDCCs act as a coincidence detection system (Schrader et al., 2004; Bender et al., 2006; Codazzi et al., 2006). In the case of the T→S synapse, it is likely that mGluR activation and Ca2+ influx via the VDCCs converge on PKC, given that group I mGluRs activate phospholipase C that leads to the production of DAG, which along with Ca2+ influx from the VDCCs, activates PKC (De Blasi et al., 2001). However, MCPG is not a selective mGluR antagonist, so the involvement of group 1 mGluRs has yet to be confirmed. The precise site of LTP in this polysynaptic pathway is not known and therefore it is impossible to say whether T→S LTP manifests as an increase in presynaptic neurotransmitter release, an increase in postsynaptic glutamate receptor density/function or a combination of both pre- and postsynaptic mechanisms.
A surprising result from these experiments is that whenever homLTP was prevented at the T→S synapse, homLTD was observed. The depression observed in the various drug-treated synapses was due to the tetanic stimulation and not to the application of the drugs themselves given that MCPG, BIS, nimodipine and BAPTA treatments without tetanization had no effect on synaptic transmission (Fig. 2). It was already known that the same tetanizing stimulus that induced homLTP in the active T→S synapse simultaneously induced hetLTD in the inactive T→S synapse (same postsynaptic S-cell, different presynaptic T-cells; see Fig. 1B, 2 and Burrell and Sahley, 2004). The data presented here indicate that homLTD is also induced at the active, tetanized synapse, but is masked by homLTP. The signaling mechanisms mediating T→S homLTD at the tetanized synapse are not known, but there are a number of observations that suggest that this LTD is NMDAR-dependent. First, hetLTD produced by the same tetanic stimulation and observed at the same time, but at a different T→S connection, is NMDAR-dependent (Burrell and Sahley 2004). Second, one would expect that if the homLTD was NMDAR-dependent, than the magnitude of the homLTP would increase in the presence of drugs that blocked NMDAR function. This, in fact, was observed by Burrell and Sahley (2004; see Fig. 7A) when LTP was induced in the presence of the NMDAR antagonist APV. Third, T→S homLTD induced by low frequency stimulation (450 stimuli at 1Hz) is blocked by the NMDAR antagonists, APV and MK-801 (unpublished data).
It has been known that certain activity patterns could simultaneously induce multiple forms of synaptic plasticity at different synapses given that similar patterns of homLTP and hetLTD have been observed (Lynch et al., 1977; Tsumoto and Suda, 1979; Abraham and Goddard, 1983; Bradler and Barrionuevo, 1990; Martinez et al., 2002; Royer and Pare, 2003; Kosub et al., 2005). However, recent findings, including those presented here, now indicate that co-induction of LTP and LTD can actually occur in the same synapse (O’connor et al., 2005; Bender et al., 2006; Nevian and Sakmann, 2006; Sjostrom et al., 2007; Tzounopoulos et al., 2007). One potential function of this co-induction of LTP and LTD is that it contributes to spike-timing dependent plasticity (STDP). In STDP different patterns of coincident pre- and postsynaptic activity elicit either LTP or LTD (Dan and Poo, 2004). It has been suggested that STDP utilizes two independent processes of synaptic plasticity, one mediating potentiation and the other depression, and that the relative level of activation of these two processes determines whether LTP or LTD is produced (Bender et al., 2006; Nevian and Sackman, 2006; Tzounopoulos et al., 2007). Alternatively, co-induction of LTP and LTD at the same synapse may allow for a kind of metaplasticity, (Abraham & Bear 1996) where modulation of one form of synaptic plasticity alters the magnitude of the other. For example, modulation that results in a decrease in homLTD would be expected to cause the level of the co-induced homLTP to increase.
The data presented here demonstrate the complexities of understanding the cellular basis of activity-dependent forms of synaptic plasticity. LTP and LTD are often thought to be mediated by NMDAR-dependent processes, but it is increasingly clear that a variety of non-NMDAR-dependent mechanisms exist and are common; examples include LTP and LTD that are mediated by mGluRs, VDCCs, PKC, Ca2+ release from intracellular stores, and endocannabinoid receptors (Malenka and Bear, 2004; Anwyl, 2006). In addition, it is now clear that both LTP and LTD processes can be co-activated with the level of synaptic change determined by the interaction of these two processes (Duguid and Sjostrom, 2006). The leech provides a useful model system for examining the cellular mechanism mediating these processes and their functional relevance from the neural circuit to the behavioral level.
Acknowledgments
The authors thank Dr. Brenda Moss for helpful comments in the preparation of this manuscript. Supported by grants from the National Science Foundation (IBN-0432683, BDB) and by a subproject of the National Institutes of Health grant (P20 RR015567, BDB), which is designated as a Center of Biomedical Research Excellence (COBRE).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Abraham WC, Goddard GV. Asymmetric relationships between homosynaptic long-term potentiation and heterosynaptic long-term depression. Nature. 1983;305:717–719. doi: 10.1038/305717a0. [DOI] [PubMed] [Google Scholar]
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- Anwyl R. Induction and expression mechanisms of postsynaptic NMDA receptor-independent homosynaptic long-term depression. Prog Neurobiol. 2006;78:17–37. doi: 10.1016/j.pneurobio.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Bender VA, Bender KJ, Brasier DJ, Feldman DE. Two coincidence detectors for spike timing-dependent plasticity in somatosensory cortex. J Neurosci. 2006;26:4166–4177. doi: 10.1523/JNEUROSCI.0176-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrell BD, Sahley CL. Learning in simple systems. Curr Opin Neurobiol. 2001;11:757–764. doi: 10.1016/s0959-4388(01)00281-1. [DOI] [PubMed] [Google Scholar]
- Burrell BD, Sahley CL, Muller KJ. Progressive recovery of learning during regeneration of a single synapse in the medicinal leech. J Comp Neurol. 2003;457:67–74. doi: 10.1002/cne.10530. [DOI] [PubMed] [Google Scholar]
- Burrell BD, Sahley CL. Multiple forms of long-term potentiation and long-term depression converge on a single interneuron in the leech CNS. J Neurosci. 2004;24:4011–4019. doi: 10.1523/JNEUROSCI.0178-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrell BD, Sahley CL. Serotonin Mediates Learning-Induced Potentiation of Excitability. J Neurophysiol. 2005;94:4002–4010. doi: 10.1152/jn.00432.2005. [DOI] [PubMed] [Google Scholar]
- Codazzi F, Di Cesare A, Chiulli N, Albanese A, Meyer T, Zacchetti D, Grohovaz F. Synergistic control of protein kinase Cgamma activity by ionotropic and metabotropic glutamate receptor inputs in hippocampal neurons. J Neurosci. 2006;26:3404–3411. doi: 10.1523/JNEUROSCI.0478-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan Y, Poo MM. Spike timing-dependent plasticity of neural circuits. Neuron. 2004;44:23–30. doi: 10.1016/j.neuron.2004.09.007. [DOI] [PubMed] [Google Scholar]
- De Blasi A, Conn PJ, Pin J, Nicoletti F. Molecular determinants of metabotropic glutamate receptor signaling. Trends Pharmacol Sci. 2001;22:114–120. doi: 10.1016/s0165-6147(00)01635-7. [DOI] [PubMed] [Google Scholar]
- Duguid I, Sjostrom PJ. Novel presynaptic mechanisms for coincidence detection in synaptic plasticity. Curr Opin Neurobiol. 2006;16:312–322. doi: 10.1016/j.conb.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Kosub KA, Do VH, Derrick BE. NMDA receptor antagonists block heterosynaptic long-term depression (LTD) but not long-term potentiation (LTP) in the CA3 region following lateral perforant path stimulation. Neurosci Lett. 2005;374:29–34. doi: 10.1016/j.neulet.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Kristan WB, Jr, Calabrese RL, Friesen WO. Neuronal control of leech behavior. Prog Neurobiol. 2005;76:279–327. doi: 10.1016/j.pneurobio.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Lynch GS, Dunwiddie T, Gribkoff V. Heterosynaptic depression: a postsynaptic correlate of long-term potentiation. Nature. 1977;266:737–739. doi: 10.1038/266737a0. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: An embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Modney BK, Sahley CL, Muller KJ. Regeneration of a central synapse restores nonassociative learning. J Neurosci. 1997;17:6478–6482. doi: 10.1523/JNEUROSCI.17-16-06478.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller KJ, Nicholls JG, Stent GS. Neurobiology of the Leech. Cold Spring Harbor Laboratory Press; Cold Spring Habbor: 1981. [Google Scholar]
- Muller KJ, Scott SA. Transmission at a ‘direct’ electrical connexion mediated by an interneurone in the leech. J Physiol. 1981;311:565–583. doi: 10.1113/jphysiol.1981.sp013605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevian T, Sakmann B. Spine Ca2+ signaling in spike-timing-dependent plasticity. J Neurosci. 2006;26:11001–11013. doi: 10.1523/JNEUROSCI.1749-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor DH, Wittenberg GM, Wang SS. Dissection of bidirectional synaptic plasticity into saturable unidirectional processes. J Neurophysiol. 2005;94:1565–1573. doi: 10.1152/jn.00047.2005. [DOI] [PubMed] [Google Scholar]
- Royer S, Pare D. Conservation of total synaptic weight through balanced synaptic depression and potentiation. Nature. 2003;422:518–522. doi: 10.1038/nature01530. [DOI] [PubMed] [Google Scholar]
- Schrader LA, Perrett SP, Ye L, Friedlander MJ. Substrates for coincidence detection and calcium signaling for induction of synaptic potentiation in the neonatal visual cortex. J Neurophysiol. 2004;91:2747–2764. doi: 10.1152/jn.00908.2003. [DOI] [PubMed] [Google Scholar]
- Sjostrom PJ, Turrigiano GG, Nelson SB. Multiple forms of long-term plasticity at unitary neocortical layer 5 synapses. Neuropharmacology. 2007;52:176–184. doi: 10.1016/j.neuropharm.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Tsumoto T, Suda K. Cross-depression: an electrophysiological manifestation of binocular competition in the developing visual cortex. Brain Res. 1979;168:190–194. doi: 10.1016/0006-8993(79)90138-0. [DOI] [PubMed] [Google Scholar]
- Tzounopoulos T, Rubio ME, Keen JE, Trussell LO. Coactivation of pre- and postsynaptic signaling mechanisms determines cell-specific spike-timing-dependent plasticity. Neuron. 2007;54:291–301. doi: 10.1016/j.neuron.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]