Abstract
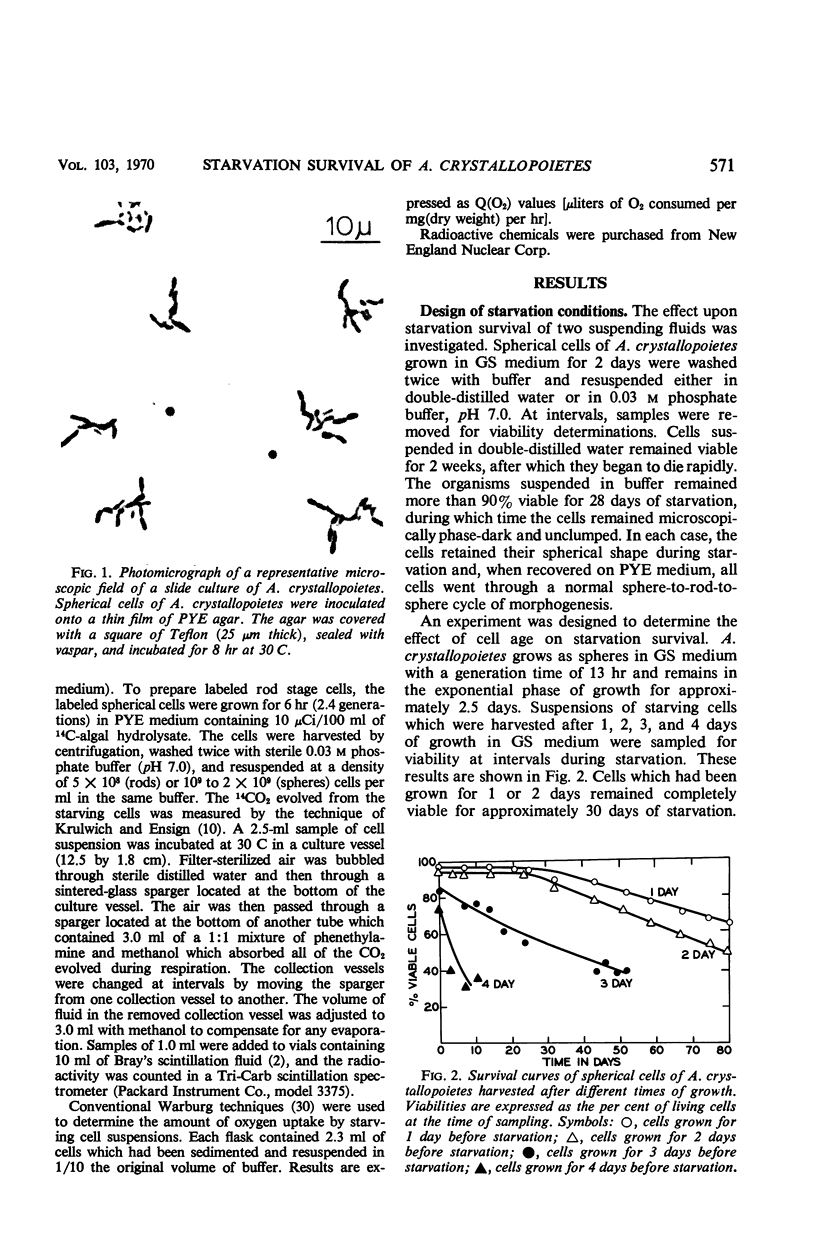
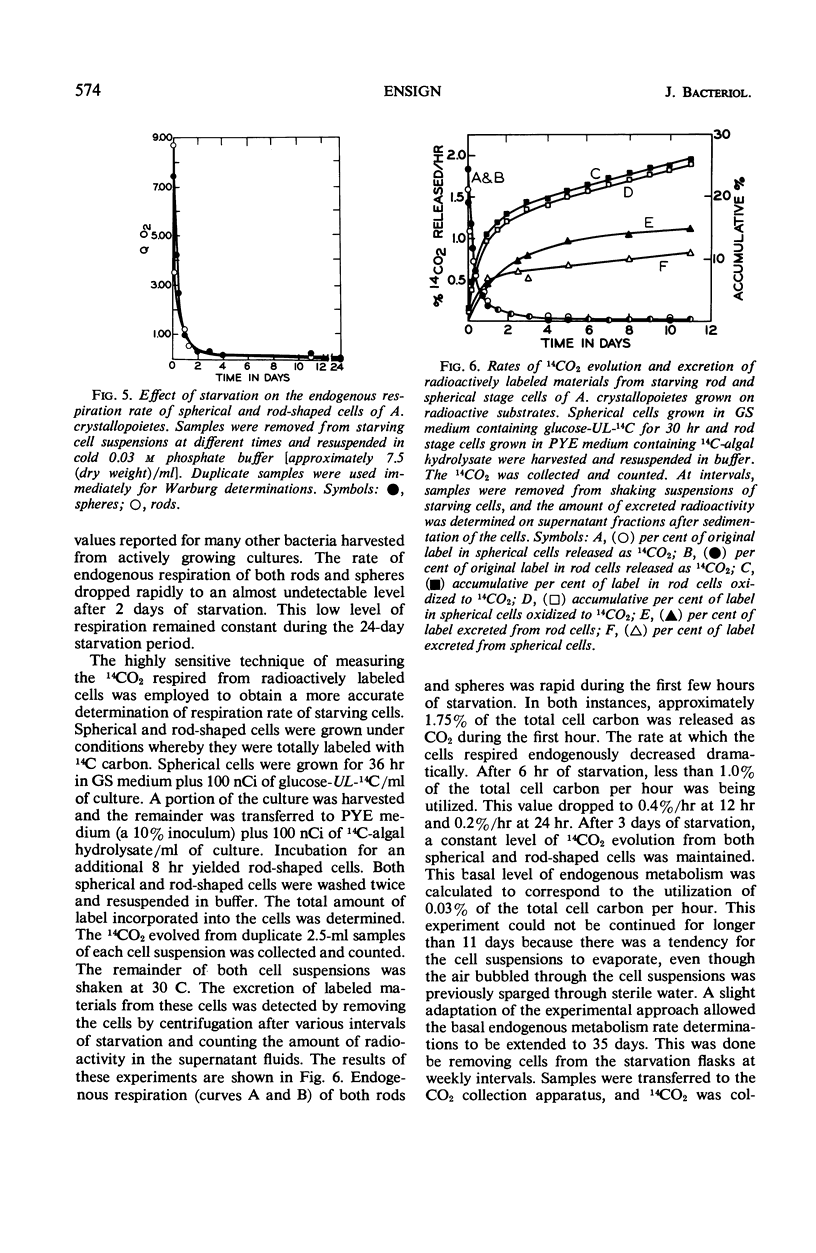
Spherical and rod-shaped cells of Arthrobacter crystallopoietes, harvested during exponential growth, were subjected to total starvation for periods of time as long as 80 days. Viability measurements were made by plate count and slide culture procedures. Both cell forms remained 100% viable for 30 days. Thereafter, viability of rods and spheres decreased equally at a slow rate. After 60 days of starvation, more than 65% of both cell forms were viable. No significant cell lysis occurred as evidenced by microscopic examination, the small amount of 260-nm absorbing material found in the starvation buffer, and stability of radioactively labeled deoxyribonucleic acid in the cells. Endogenous respiration decreased 80-fold during the first 2 days of starvation, accompanied by a 30% decrease in dry weight of the cells. Thereafter, cellular carbon was oxidized to CO2 at the constant level of 0.03%/hr over the remaining 78-day starvation period.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boylen C. W., Ensign J. C. Intracellular substrates for endogenous metabolism during long-term starvation of rod and spherical cells of Arthrobacter crystallopoietes. J Bacteriol. 1970 Sep;103(3):578–587. doi: 10.1128/jb.103.3.578-587.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burleigh I. G., Dawes E. A. Studies on the endogenous metabolism and senescence of starved Sarcina lutea. Biochem J. 1967 Jan;102(1):236–250. doi: 10.1042/bj1020236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton C. E. Aging of Pseudomonas aeruginosa. J Bacteriol. 1967 Dec;94(6):2077–2078. doi: 10.1128/jb.94.6.2077-2078.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn H. J. THE MOST ABUNDANT GROUPS OF BACTERIA IN SOIL. Bacteriol Rev. 1948 Sep;12(3):257–273. doi: 10.1128/br.12.3.257-273.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWES E. A., RIBBONS D. W. STUDIES ON THE ENDOGENOUS METABOLISM OF ESCHERICHIA COLI. Biochem J. 1965 May;95:332–343. doi: 10.1042/bj0950332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENSIGN J. C., WOLFE R. S. NUTRITIONAL CONTROL OF MORPHOGENESIS IN ARTHROBACTER CRYSTALLOPIETES. J Bacteriol. 1964 Apr;87:924–932. doi: 10.1128/jb.87.4.924-932.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gounot A. M. Role biologique des Arthrobacter dans les limons souterrains. Ann Inst Pasteur (Paris) 1967 Dec;113(6):923–945. [PubMed] [Google Scholar]
- Jacobson A., Gillespie D. Metabolic events occurring during recovery from prolonged glucose starvation in Escherichia coli. J Bacteriol. 1968 Mar;95(3):1030–1039. doi: 10.1128/jb.95.3.1030-1039.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krulwich T. A., Ensign J. C. Alteration of glucose metabolism of Arthrobacter crystallopoietes by compounds which induce sphere to rod morphogenesis. J Bacteriol. 1969 Feb;97(2):526–534. doi: 10.1128/jb.97.2.526-534.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noller E. C., Durham N. N. Sealed aerobic slide culture for photomicrography. Appl Microbiol. 1968 Feb;16(2):439–440. doi: 10.1128/am.16.2.439-440.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POSTGATE J. R., CRUMPTON J. E., HUNTER J. R. The measurement of bacterial viabilities by slide culture. J Gen Microbiol. 1961 Jan;24:15–24. doi: 10.1099/00221287-24-1-15. [DOI] [PubMed] [Google Scholar]
- POSTGATE J. R., HUNTER J. R. Acceleration of bacterial death by grown substrates. Nature. 1963 Apr 20;198:273–273. doi: 10.1038/198273a0. [DOI] [PubMed] [Google Scholar]
- POSTGATE J. R., HUNTER J. R. The survival of starved bacteria. J Gen Microbiol. 1962 Oct;29:233–263. doi: 10.1099/00221287-29-2-233. [DOI] [PubMed] [Google Scholar]
- Pirt S. J. The maintenance energy of bacteria in growing cultures. Proc R Soc Lond B Biol Sci. 1965 Oct 12;163(991):224–231. doi: 10.1098/rspb.1965.0069. [DOI] [PubMed] [Google Scholar]
- Robinson J. B., Salonius P. O., Chase F. E. A note on the differential response of arthrobacter spp. and pseudomonas spp. to drying in soil. Can J Microbiol. 1965 Aug;11(4):746–748. doi: 10.1139/m65-100. [DOI] [PubMed] [Google Scholar]
- Ryan F. J. Spontaneous Mutation in Non-Dividing Bacteria. Genetics. 1955 Sep;40(5):726–738. doi: 10.1093/genetics/40.5.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIERRA G., GIBBONS N. E. Role and oxidation pathway of poly-beta-hydroxybutyric acid in Micrococcus halodenitrificans. Can J Microbiol. 1962 Apr;8:255–269. doi: 10.1139/m62-032. [DOI] [PubMed] [Google Scholar]
- STRANGE R. E., WADE H. E., NESS A. G. The catabolism of proteins and nucleic acids in starved Aerobacter aerogenes. Biochem J. 1963 Feb;86:197–203. doi: 10.1042/bj0860197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobek J. M., Charba J. F., Foust W. N. Endogenous metabolism of Azotobacter agilis. J Bacteriol. 1966 Sep;92(3):687–695. doi: 10.1128/jb.92.3.687-695.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes J. L., Parson W. L. Role of poly-beta-hydroxybutyrate in survival of Sphaerotilus discophorus during starvation. Can J Microbiol. 1968 Jul;14(7):785–789. doi: 10.1139/m68-130. [DOI] [PubMed] [Google Scholar]
- Thomas T. D., Batt R. D. Degradation of cell constituents by starved Streptococcus lactis in relation to survival. J Gen Microbiol. 1969 Nov;58(3):347–362. doi: 10.1099/00221287-58-3-347. [DOI] [PubMed] [Google Scholar]
- Thomas T. D., Batt R. D. Metabolism of exogenous arginine and glucose by starved Streptococcus lactis in relation to survival. J Gen Microbiol. 1969 Nov;58(3):371–380. doi: 10.1099/00221287-58-3-371. [DOI] [PubMed] [Google Scholar]
- Thomas T. D., Batt R. D. Survival of Streptococcus lactis in starvation conditions. J Gen Microbiol. 1968 Mar;50(3):367–382. doi: 10.1099/00221287-50-3-367. [DOI] [PubMed] [Google Scholar]
- Willetts N. S. Intracellular protein breakdown in non-growing cells of Escherichia coli. Biochem J. 1967 May;103(2):453–461. doi: 10.1042/bj1030453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zevenhuizen L. P. Formation and function of the glycogen-like polysaccharide of Arthrobacter. Antonie Van Leeuwenhoek. 1966;32(4):356–372. doi: 10.1007/BF02097485. [DOI] [PubMed] [Google Scholar]
- van Houte J., Jansen H. M. Role of glycogen in survival of Streptococcus mitis. J Bacteriol. 1970 Mar;101(3):1083–1085. doi: 10.1128/jb.101.3.1083-1085.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]





