Abstract
Purpose
Central to the use of murine models of disease is the ability to derive reproducible data. The purpose of this study was to determine factors contributing to variability in our murine model of small bowel resection (SBR).
Methods
Male C57Bl/6 mice were randomized to sham or 50% SBR. The effect of housing type (pathogen-free vs standard housing), nutrition (reconstituted powder vs tube feeding formulation), and correlates of intestinal morphology with gene expression changes were investigated. Multiple linear regression modeling or 1-way analysis of variance was used for data analysis.
Results
Pathogen-free mice had significantly shorter ileal villi at baseline and demonstrated greater villus growth after SBR compared to mice housed in standard rooms. Food type did not affect adaptation. Gene expression changes were more consistent and significant in isolated crypt cells that demonstrated adaptive growth when compared with crypts that did not deepen after SBR.
Conclusion
Maintenance of mice in pathogen-free conditions and restricting gene expression analysis to individual animals exhibiting morphologic adaptation enhances sensitivity and specificity of data derived from this model. These refinements will minimize experimental variability and lead to improved understanding of the complex process of intestinal adaptation.
Key words: Short gut syndrome, Surgery models, Mice, Adaptation
After massive small bowel resection (SBR), the remaining bowel compensates by a process termed adaptation. Adaptation is characterized by increases in villus height, crypt depth, accelerated rates of enterocyte proliferation and apoptosis, and overall enhanced nutrient absorption and digestion per unit surface area of mucosa. Clinically, adaptation is heralded by increasing tolerance of enteral nutrition over time. If this important response is incomplete, the patient will be committed to a lifetime of parenteral nutritional support and its allied complications. Understanding the complex process of resection-induced adaptation is therefore a prerequisite toward the development of therapeutic targets intended to magnify this response, thus weaning patients from parenteral nutrition more efficiently.
For the past decade, our laboratory has used a model for small bowel resection (SBR) in mice [1] that has revealed much useful information regarding genes that are necessary for adaptive alterations in enterocyte proliferation [2] and apoptosis [3]. This in vivo model has been invaluable with regard to translation of a complex, multifactorial adaptation response into identification of key molecular targets for subsequent, more mechanistic experiments using cell culture [4], [5]. During this period, several modifications in operative technique, perioperative animal care, and refinements in basic science method have evolved. As a direct consequence of improved skills in multiple molecular methods and assays, we have noted significant variability in our experimental data derived from this in vivo model. Some mice have demonstrated no morphologic evidence for adaptation, whereas others have had an adaptation response that was magnified after extensive enterectomy.
In an effort to maintain consistency in data derived from this model, we sought to systematically evaluate the direct effect of experimental conditions that have changed over time in our murine model of SBR-induced adaptation. The specific purpose of these experiments was to identify experimental variables that significantly affect the magnitude of adaptation responses, thus minimizing variability in experimental outcomes. Not only does this provide invaluable insight into the complexity of the model, but it also in turn highlights the need for meticulous standards and protocols, thereby paving the way toward greater accuracy and reproducibility.
1. Methods
1.1. Experimental design
Male C57BL/6 mice were obtained from Charles River Laboratories (Wilmington, Mass) and housed in the Cincinnati Children's Hospital Research Foundation (CCHRF) animal facilities (Ohio). A protocol for this study was approved by the CCHRF Institutional Animal Care and Use Committee (no. 5D04022).
The first set of experiments sought to determine the effect of environmental conditions on adaptation. To evaluate this variable, mice were housed under either normal condition in our animal facility (considered dirty room) or within a pathogen-free environment (clean room). Mice from each environment were subjected to sham or SBR procedures and then harvested after 3 days. Sentinel mice housed in the dirty room, which does not require any special entry access for animal workers, had positive serologic results for the following murine pathogen strains: coronavirus, minute virus of mice, norovirus, and parvovirus. In contrast, access to the mice in the clean room requires donning of masks, head and foot coverage, gowns, and gloves; all sentinel mice were documented to be pathogen-free.
We next determined the effect of type of liquid diet formulation within each group under the different housing conditions. Our original model consisted of feeding a liquid rodent diet (Test Diet LD 101, Purina, Richmond, Ind) in the postoperative period. This was supplied in powder form and required reconstitution. Over time, we transitioned to a standard tube feeding formulation (Jevity 1, Ross Laboratories, Columbus, Ohio) that was provided in liquid form, thus eliminating the need for reconstitution. Mice underwent sham or SBR procedures after being housed in dirty or clean rooms and were then randomly allocated to one of the 2 feeding regimens. Although not specifically pair-fed, oral intake of the 2 different feeding regimens was not different between the 2 groups.
A subsequent series of experiments was designed to determine the frequency of inadequate, normal, and enhanced adaptation in a large number of mice in both housing and feeding conditions after 50% SBR or sham operation (transection of the bowel with reanastomosis alone). All mice were killed after 3 days, and the magnitude of adaptation was determined by villus height measurements.
We next tested the hypothesis that relevant protein expression alterations during adaptation require isolation of enterocytes from crypts (similar to villi) that have demonstrated morphologic adaptation as gauged by increases in crypt depth. To test this, we performed Western blotting for retinoblastoma (Rb) protein phosphorylation patterns from protein isolated from crypt cells harvested from adapting vs nonadapting crypts after SBR.
1.2. Intestinal resection procedures in mice
The facilities are kept in 12-hour day/night cycles, and when not within the perioperative period, the mice are fed standard laboratory solid diet. The murine model for 50% proximal SBR and sham operations were performed as described previously [1]. Water was provided ad libitum for the first 24 hours after surgery. Thereafter, the mice from all experimental groups were fed with liquid diet or Jevity until sacrifice.
1.3. Enterocyte isolation
At the time of sacrifice, the small bowel was excised, immediately flushed, and placed in cold phosphate buffered saline. The first centimeter of the segment distal to the anastomosis was discarded, the next 2 cm was used for histologic examination, and the subsequent 6 cm was used for enterocyte isolation. For enterocyte isolation, the bowel was cut longitudinally along the antimesenteric border and transferred into tubes containing 5 mL of ice-cold isolation buffer (1.5 mmol/L of KCl, 96 mmol/L of NaCl, 27 mmol/L of Na citrate, 8 mmol/L of KH2PO4, 5.6 mmol/L of Na2HPO4, 15 mmol/L of EDTA, 1 mmol/L of dithiothreitol (DTT) with protease inhibitor tablet [Roche Diagnostics, Indianapolis, Ind]). The tissue was vortexed at 4°C at maximum speed for 5 minutes (preisolation); at this step, luminal contents have been removed. Tissue was then transferred to a new tube with 5 mL isolation buffer and vortexed again at 4°C for another 7 to approximately 10 minutes. This was then filtered through a 70-μm pore cell strainer to capture the muscle and villus fraction. The crypts passed through the filter while the muscle and villi were captured. The muscle was then removed and villi collected. The crypt and villus fractions were examined under light microscope to ensure accuracy of separation. Typically, the crypts demonstrated a U-shaped structure with Paneth cells in the bottom, whereas the villi showed a cylindrical structure resembling what is typically seen with traditional H&E staining. The enterocytes were then spun down at 1000g for 10 minutes at 4°C. The pellet was washed with 1 mL Tris buffer (50 mmol/L of Tris-HCl [pH 7.5], 150 mmol/L of NaCl, 1 mmol/L of EDTA) and resuspended in 1 mL of the same buffer.
1.4. Histologic analysis
Harvested ileum was embedded in paraffin, and 5-μm-thick slices were mounted and stained with H&E. Microscopic measurements were performed for total villus height and crypt depth using a digital camera and microscope computer program (Metamorph 6.2.6, Universal Imaging Corp, Downingtown, Pa) as we have previously reported [6], [7]. A minimum of 20 villi and crypts were counted per animal; the investigators were blinded according to treatment group.
1.5. Western blot analysis
Small intestine enterocytes were isolated as mentioned previously; the cells were then lysed with 1× sodium dodecyl sulfate (SDS) sample loading buffer. The lysate was heated at 100°C for 5 minutes, and the protein concentration was determined using the RC DC kit (Bio-Rad, Hercules, Calif) following the manufacturer's protocol. The proteins were then separated on 10% polyacrylamide gels and transferred to nitrocellulose membranes and detected with appropriate antibodies by Western blot following standard method.
1.6. Statistics
Statistical differences were determined using 1-way analysis of variance or multiple linear regression modeling with the SigmaStat (SPSS, Chicago, Ill) program. Statistical significance was established at a P value of less than .05.
2. Results
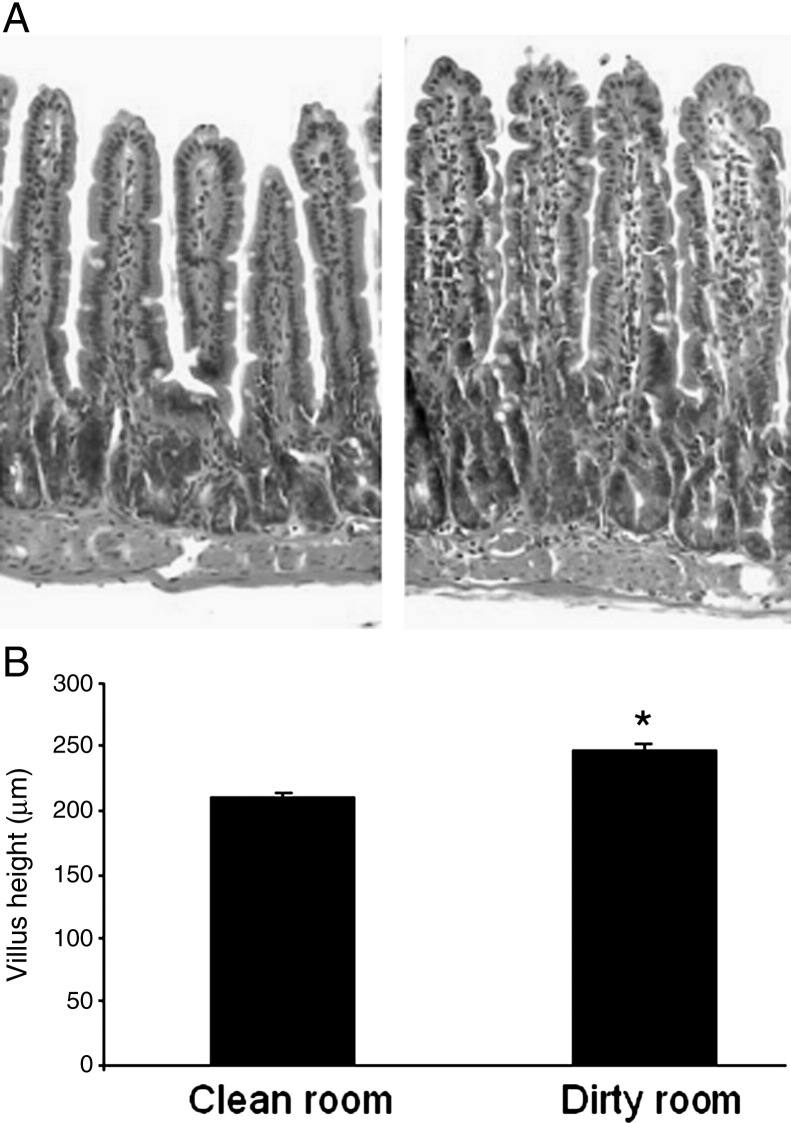
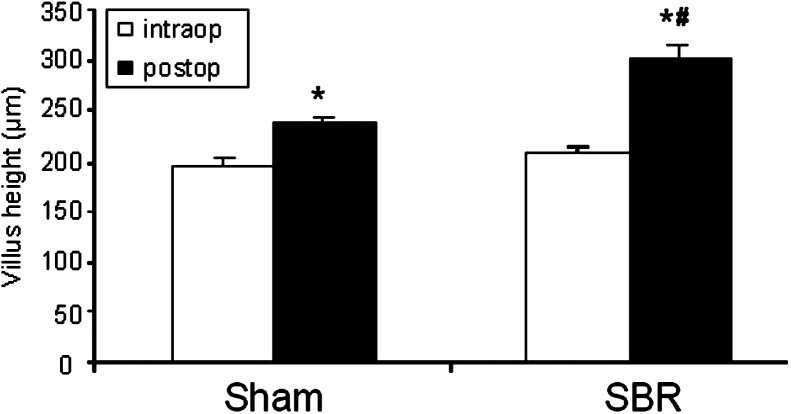
We first evaluated baseline villus height within segments of intestine excised at the time of SBR in mice housed in both clean and dirty rooms. Baseline villus height in unoperated mice was found to be significantly taller when measured in the ileum of mice housed in the dirty room (Fig. 1A and B ). As such, we removed a small (less than 3 mm) segment of intestine from sham-operated mice at the time of sham operation and compared this intraoperative segment with the villus height at the time of harvest 3 days later. Similarly, we measured baseline villus heights within the ileum removed from the SBR animals and compared this with the villus heights of the adapted bowel at the time of sacrifice. As revealed in Fig. 2 , sham operation was associated with a slight (albeit statistically significant) degree of villus growth. As expected, the magnitude of villus growth was greatest in the animals subjected to SBR. These data underscore the importance of comparing villus height differences between SBR and sham groups, thereby, controlling for the contribution of sham operation-induced villus growth. On the other hand, because measurements of protein expression are best done in individual animals, recording the baseline villus height within the intraoperative segment and comparing this with measurements at the time of animal sacrifice permits selection of tissues for further analysis only in mice that have demonstrated morphologic evidence for adaptation.
Fig. 1.
Villus height in clean vs dirty room-housed mice. A, H&E–stained villus segments from unoperated mice housed in clean room (left) and dirty room (right). B, Average (±SEM) villus height measurements in microns. *Indicates significant difference with P = .003.
Fig. 2.
Villus height before and after operation. Average (±SEM) villus height from C57BL/6 mice after either sham resection (transection and reanastomosis only) or 50% proximal SBR housed in the clean room. The intraoperative segment (intraop) was removed from the ileum 1 cm distal to the anastomosis at time of operation (sham or SBR) or 3 days later at the time of harvest (postop). *Indicates P < .05 sham postoperative vs sham intraoperative; #, P < .05 SBR postoperative vs sham postoperative.
The effects of housing type (clean vs dirty), operative procedure (sham vs SBR), and diet (liquid vs Jevity) on survival are depicted in Table 1 . Linear regression modeling was performed using these 3 variable groups individually, as well as their interaction variables. There was no significant effect of diet; however, the influence of housing environment and operative procedure were significant at a P value of less than .003 and less than .001, respectively. The calculated R 2 was 0.27, indicating that these 2 factors alone accounted for roughly 27% of the observed change. Overall survival appeared to be optimal when mice were housed under pathogen-free conditions and fed the standard liquid rodent diet. Furthermore and consistent with the survival data, mice fed liquid diet and housed in the clean rooms yielded a significantly greater adaptation response to SBR when compared with mice in other groups. When compared with baseline villus height measurements at the time of operation, SBR was associated with a 48% increase in the clean room, liquid diet group and a 39% increase in the clean room, Jevity group. On the other hand, the mice housed in the dirty room demonstrated a 26% and 20% increase in villus height after SBR when fed Jevity or liquid diet, respectively.
Table 1.
Survival for mice in each group based on housing type, diet, and surgical procedure
| Clean |
Dirty |
|||||||
|---|---|---|---|---|---|---|---|---|
| Jevity |
Liquid |
Jevity |
Liquid |
|||||
| Sham | SBR | Sham | SBR | Sham | SBR | Sham | SBR | |
| Total no. per diet group | 31 | 24 | 44 | 20 | ||||
| Total no. per room type | 55 | 64 | ||||||
| Survival | 15/15 | 11/16 | 11/12 | 10/12 | 16/22 | 16/22 | 8/10 | 7/10 |
| Survival per diet | 26/31 | 21/24 | 32/44 | 15/20 | ||||
| Survival (%) | 100 | 68.8 | 91.7 | 83.3 | 72.7 | 72.7 | 80 | 70 |
| Average % per diet | 84.4 | 87.5 | 72.7 | 75 | ||||
| Average % per room type | 85.95 | 73.85 | ||||||
Taking into account the average villus height increase of 48% in the clean room–housed mice fed liquid diet and subjected to SBR with the SD of 16.8%, we calculated that normal adaptation (expected % change ± 1 SD) was in the range of 31% to 65% villus growth. A super adaptation response was heralded by villus growth of greater than 65% from baseline. Finally, mice with less than 31% villus growth after SBR were considered to have not adapted. Using these newly defined parameters, roughly 60% of mice demonstrated normal adaptation, whereas 20% had minimal adaptation, and 20% had a magnified adaptation response. Based on these values and the ability to use SBR mice as their own control using baseline villus heights obtained in the resected bowel, it was determined that for future experiments, a minimum sample size of 4 would be necessary, with significance set at P < .05.
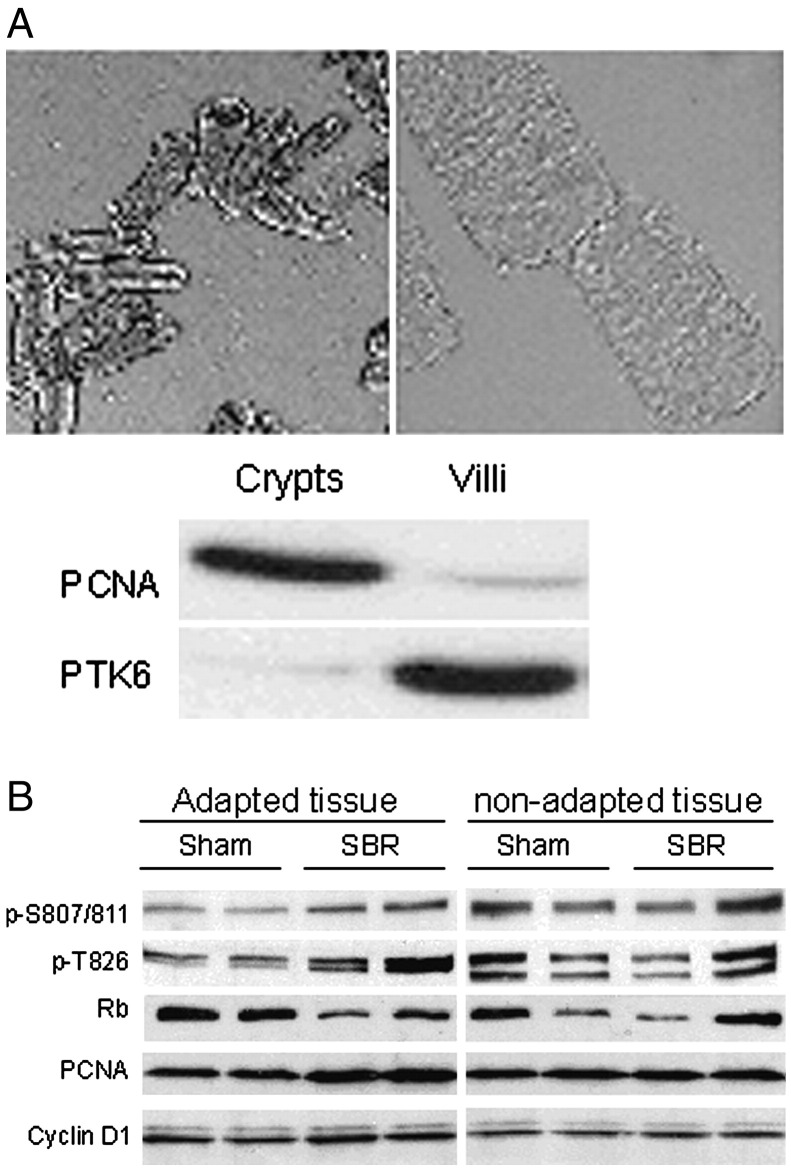
We sought to determine a relationship between the histologic findings and relevant gene expression profiles in our SBR model. The small intestine crypts are a highly dynamic region of the intestinal mucosa and our laboratory has previously established important Rb phosphorylation regulation within prototypical intestinal crypt epithelial cells by epidermal growth factor [4]. The Rb protein plays a central role in regulating cell proliferation. When Rb is in its hypophosphorylated state, cells are maintained in quiescence. Alternatively, Rb hyperphosphorylation has been established as a critical step in the initiation of cell cycle progression. Using immunohistochemistry, we have previously found that Rb phosphorylated at S807/811 and T826 is specifically seen in crypt enterocytes. To specifically look at the proliferation signal changes in the crypts, we developed a new method to isolate crypt and villus enterocytes. The validation of the method was confirmed by Western blot showing the proliferation marker proliferating cell nuclear antigen (PCNA) in crypts and villus marker PTK6 in villi (Fig. 3A). This new method allows us to correlate biochemical Rb signals with histologic crypt changes. Indeed, Rb phosphorylation at S807/811 and T826 shows increase only in histologically deeper SBR crypts when compared to sham crypts (Fig. 3B). These results were verified by immunostaining in tissue sections (Fig. 4 ). Total Rb expression tended to decrease in adapting tissue. Furthermore, the proliferation marker PCNA was increased, whereas cyclin D1 expression does not change in adapting crypts. The mechanism for all these changes is being subjected to further investigation.
Fig. 3.
Rb phosphorylation at S807/811 and T826 is increased in adapting crypts. A, Crypts and villus enterocytes were isolated and verified by light microscopy. Protein was then extracted and analyzed by Western blot to further validate the isolation procedure by showing a crypt-specific proliferation marker PCNA and villus-distinctive marker PTK6. B, Western blot of protein expression of total/phosphorylated Rb, PCNA, and cyclin D1 in isolated crypts in mice after sham or SBR.
Fig. 4.
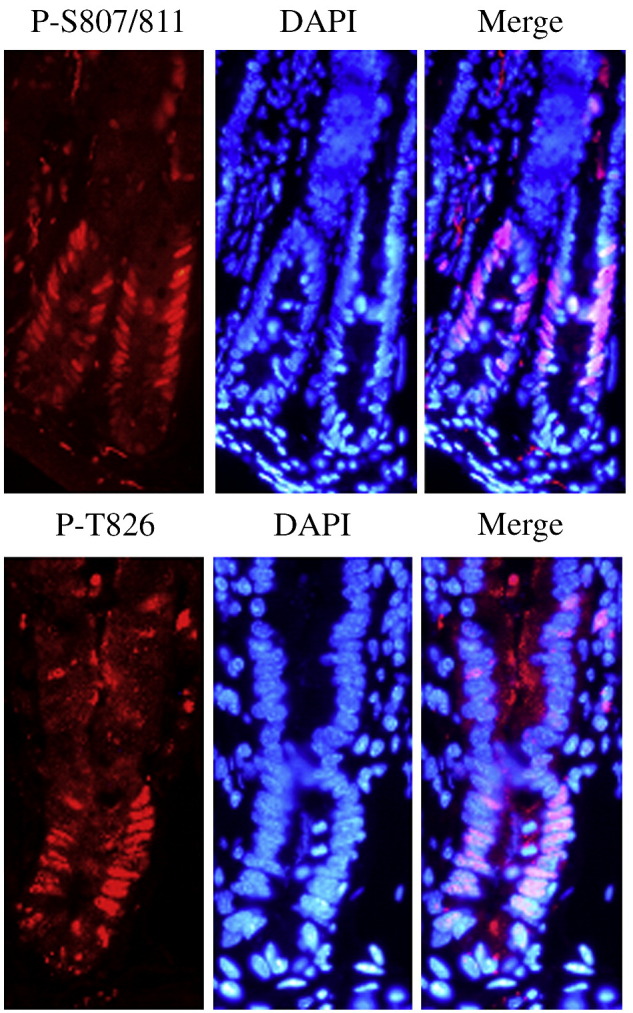
Immunostaining of mouse small intestine epithelium for Rb phosphorylation at sites S807/811 and T826. Phosphorylated Rb at sites S807/811 and T826 are shown in red, nuclei are stained in blue with 4′-6-Diamidino-Z-phenylindole (DAPI). The pink cells in the merged image represent nuclear staining of phosphorylated Rb within the proliferative crypt region.
3. Discussion
The ability to manipulate an in vivo model with precision has tremendous implications for translation into human studies. The expected pitfalls when compared to in vitro work appear further confounded when one considers the effects of the animal's own genetic background, the consequences of which are not currently fully appreciated. From this study, we have been able to draw several practical conclusions regarding our murine SBR model. First, we demonstrated the significant effect of housing on baseline villus height and adaptive growth in a standard facility vs a pathogen-free environment. Next, we showed for the first time that there is a gradient of adaptation within the same mouse strain in response to the same SBR procedure. In our hands, 60% of mice demonstrated the expected adaptation response, whereas 20% had a greater than expected response. The remaining mice showed no histologic evidence for adaptation. Furthermore, we revealed that alterations in specific protein activity and/or expression directly correlated with histologic criteria for adaptation. In addition, the ability to use excised intestinal segments from SBR mice at the time of resection so that they serve as their own controls permits a smaller necessary sample size than we have previously used. These observations will pave the way for more consistency and reliability of this important model.
Baseline villus height was significantly different between the clean and dirty room mice. Indeed, the morphology of the intestinal mucosa has been previously shown to be affected by microbial flora in several mammalian species including piglets [8], mice [9], and rats [10]. In a comparative study of bacteria-free vs conventionally housed rats, we previously noted a greater proliferation response to SBR in the former [11]. This effect could not be reproduced in the conventional mice when given oral antibiotics. In one report, the effect of pathogen-free housing on mucosal architecture persisted in older rats, despite switching over to conventional housing [12]. It would therefore seem that colonization of the developing intestinal tract is associated with alterations in gene expression profiles that persist for the lifetime of the animal. The specific mechanism for differing responses to SBR and the role of varied intestinal flora are presently speculative. Intestinal microbiota have been shown to facilitate the breakdown of otherwise undigestible polysaccharides in the gut lumen [9], modulate intestinal epithelial cell turnover [13], [14], and affect the immune system [15], among other influences.
We were surprised to note the magnitude of adaptation as measured by SBR-induced villus growth did not occur uniformly among all animals. In reality, only 60% of operated animals demonstrated a typical response, whereas the remaining mice had either no adaptation or a “super” adaptation response. At present, we have no specific explanation for this phenomenon. It is possible that there might have been differences in many factors such as intestinal flora, food intake, level of activity, or subtle variations in genotype between the mice of the same strain. Along these lines, we are presently performing experiments to determine whether the magnitude of adaptation is genetically predetermined. Genomic comparisons between nonadapting and super adapting mice may provide important insight into this aspect. The most important practical and pertinent aspect of this observation to our murine SBR model is that there exists significant intrinsic variability, and this must be used as a screen for subsequent analysis of gene expression profiles.
Isolation of specific crypt and villus enterocyte from the other cells of the intestine was a much more sensitive method to detect patterns of gene expression in these specific cellular regions. We have previously demonstrated this while searching for alterations in messenger RNA expression in crypt and villus enterocytes isolated by laser capture microdissection microscopy [16]. Furthermore, the alterations in gene expression were inconsistent in the crypt zone that did not demonstrate histologic criteria for adaptation. These 2 factors (enterocyte isolation and screening for adapting crypts) markedly enhanced our ability to reliably detect gene expression alterations as a consequence of intestinal resection. In the present study, we looked for site-specific phosphorylation patterns of Rb within crypt enterocytes. Unique patterns of Rb phosphorylation have been observed in the intestinal crypt by immunostaining and is the subject of further study. Furthermore, we have identified a signature Rb phosphorylation profile after epidermal growth factor treatment of intestinal epithelial cells in vitro [4]. It was therefore logical for us to correlate changes in this protein activity with crypt histologic result after SBR.
In summary, through identification of key factors that affect adaptation as well as recognition of a normal distribution of adaptive mucosal growth, we have been able to identify gene expression patterns that are more consistent. Furthermore, we have demonstrated the significance of isolating select cell populations that have shown adaptive growth for subsequent gene expression analysis. These important modifications will serve to minimize variability in data as well as to heighten sensitivity for the recognition of novel genes that are necessary for adaptation to occur.
Acknowledgments
The authors thank Jareen Meinzen-Derr, PhD, for her time spent assisting with the application and interpretation of the statistics used in these studies.
Footnotes
Presented at the 59th Annual Meeting of the Section on Surgery, American Academy of Pediatrics, San Francisco, CA, October 25-27, 2007.
The study is supported by National Institutes of Health, Bethesda, MD, with the following grants: RO1 DK53234, RO1 DK59288 (Dr B Warner), T32 DK07727 (Dr C Martin) and by Digestive Disease Research Development Core Center of Cincinnati, Cincinnati, OH, R24 DK064403.
Presented at the 59th Annual Meeting of the Section on Surgery of the American Academy of Pediatrics; October 27-29, 2007, San Francisco, Calif.
References
- 1.Helmrath M.A., VanderKolk W.E., Can G. Intestinal adaptation following massive small bowel resection in the mouse. J Am Coll Surg. 1996;183:441–449. [PubMed] [Google Scholar]
- 2.Stern L.E., Falcone R.A., Jr., Kemp C.J. p21 (WAF1/CIP1) is required for the mitogenic response to intestinal resection. J Surg Res. 2000;90:45–50. doi: 10.1006/jsre.2000.5834. [DOI] [PubMed] [Google Scholar]
- 3.Stern L.E., Huang F., Kemp C.J. Bax is required for increased enterocyte apoptosis after massive small bowel resection. Surgery. 2000;128:165–170. doi: 10.1067/msy.2000.107370. [DOI] [PubMed] [Google Scholar]
- 4.Guo J., Sheng G., Warner B.W. Epidermal growth factor-induced rapid retinoblastoma phosphorylation at Ser780 and Ser795 is mediated by ERK1/2 in small intestine epithelial cells. J Biol Chem. 2005;280:35992–35998. doi: 10.1074/jbc.M504583200. [DOI] [PubMed] [Google Scholar]
- 5.Sheng G., Bernabe K.Q., Guo J. Epidermal growth factor receptor-mediated proliferation of enterocytes requires p21waf1/cip1 expression. Gastroenterology. 2006;131:153–164. doi: 10.1053/j.gastro.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Stehr W., Mercer T.I., Bernal N.P. Opposing roles for p21waf1/cip1 and p27kip1 in enterocyte differentiation, proliferation, and migration. Surgery. 2005;138:187–194. doi: 10.1016/j.surg.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 7.Stehr W., Bernal N.P., Erwin C.R. Roles for p21waf1/cip1 and p27kip1 during the adaptation response to massive intestinal resection. Am J Physiol Gastrointest Liver Physiol. 2006;290:G933–G941. doi: 10.1152/ajpgi.00235.2005. [DOI] [PubMed] [Google Scholar]
- 8.Herich R., Levkut M., Bomba A. Differences in the development of the small intestine between gnotobiotic and conventionally bred piglets. Berl Munch Tierarztl Wochenschr. 2004;117:46–51. [PubMed] [Google Scholar]
- 9.Hooper L.V., Midtvedt T., Gordon J.I. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr. 2002;22:283–307. doi: 10.1146/annurev.nutr.22.011602.092259. [DOI] [PubMed] [Google Scholar]
- 10.Sharma R., Schumacher U., Ronaasen V. Rat intestinal mucosal responses to a microbial flora and different diets. Gut. 1995;36:209–214. doi: 10.1136/gut.36.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juno R.J., Knott A.W., Jarboe M.D. Characterization of small bowel resection and intestinal adaptation in germ-free rats. Surgery. 2003;134:582–589. doi: 10.1016/s0039-6060(03)00281-2. [DOI] [PubMed] [Google Scholar]
- 12.Clarke R.M. Diet, mucosal architecture and epithelial cell production in the small intestine of specified-pathogen-free and conventional rats. Lab Anim. 1975;9:201–209. doi: 10.1258/002367775780994600. [DOI] [PubMed] [Google Scholar]
- 13.Lesher S., Walburg H.E., Jr., Sacher G.A., Jr. Generation cycle in the duodenal crypt cells of germ-free and conventional mice. Nature. 1964;202:884–886. doi: 10.1038/202884a0. [DOI] [PubMed] [Google Scholar]
- 14.Rossi F., MacLean H.E., Yuan W. p107 and p130 coordinately regulate proliferation, Cbfa1 expression, and hypertrophic differentiation during endochondral bone development. Dev Biol. 2002;247:271–285. doi: 10.1006/dbio.2002.0691. [DOI] [PubMed] [Google Scholar]
- 15.Macpherson A.J., Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 16.Erwin C.R., Jarboe M.D., Sartor M.A. Developmental characteristics of adapting mouse small intestine crypt cells. Gastroenterology. 2006;130:1324–1332. doi: 10.1053/j.gastro.2006.02.019. [DOI] [PubMed] [Google Scholar]