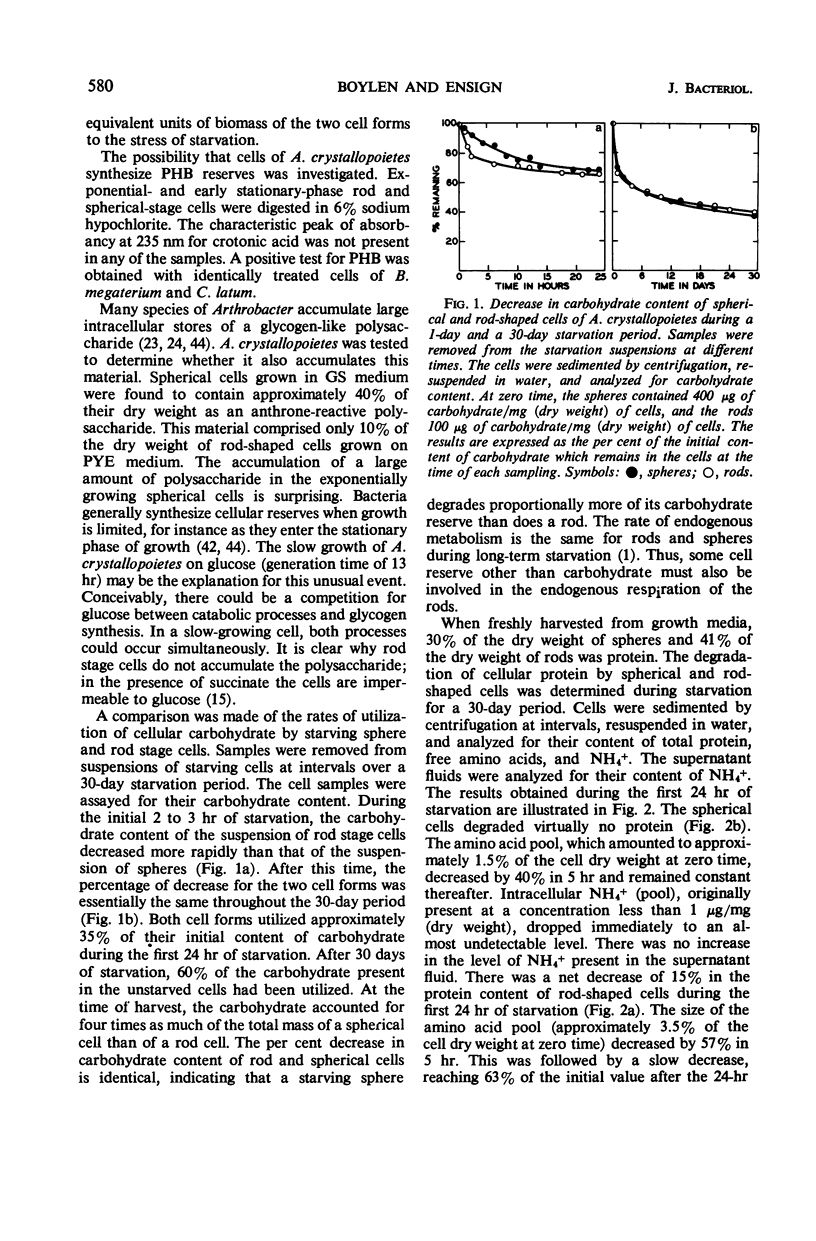
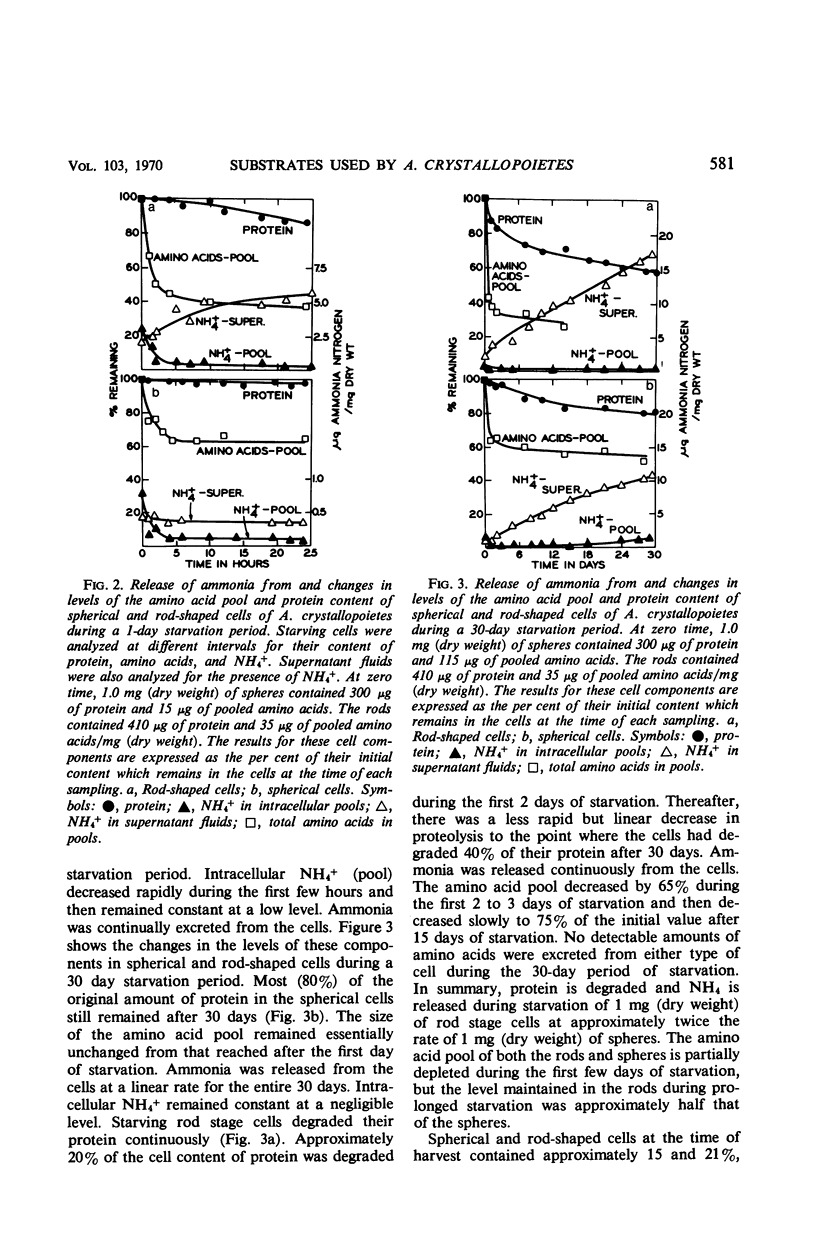
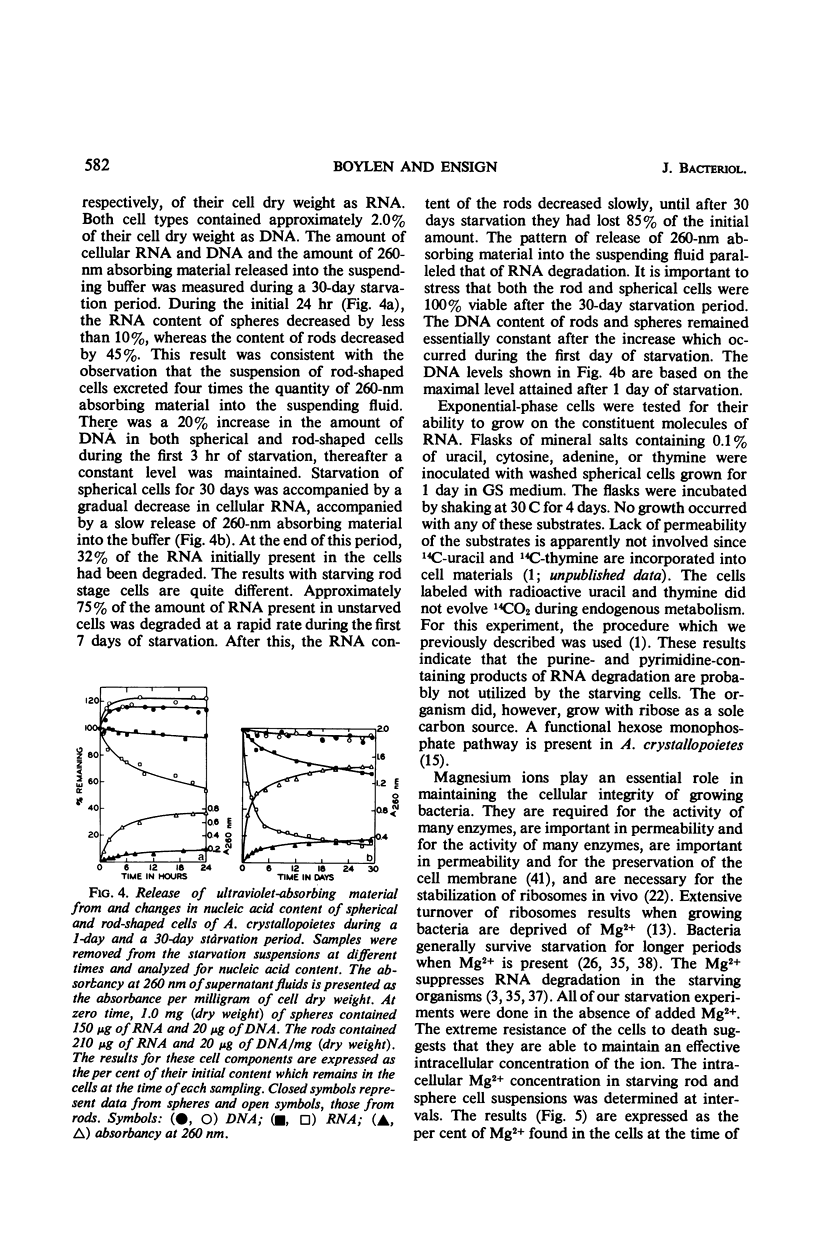
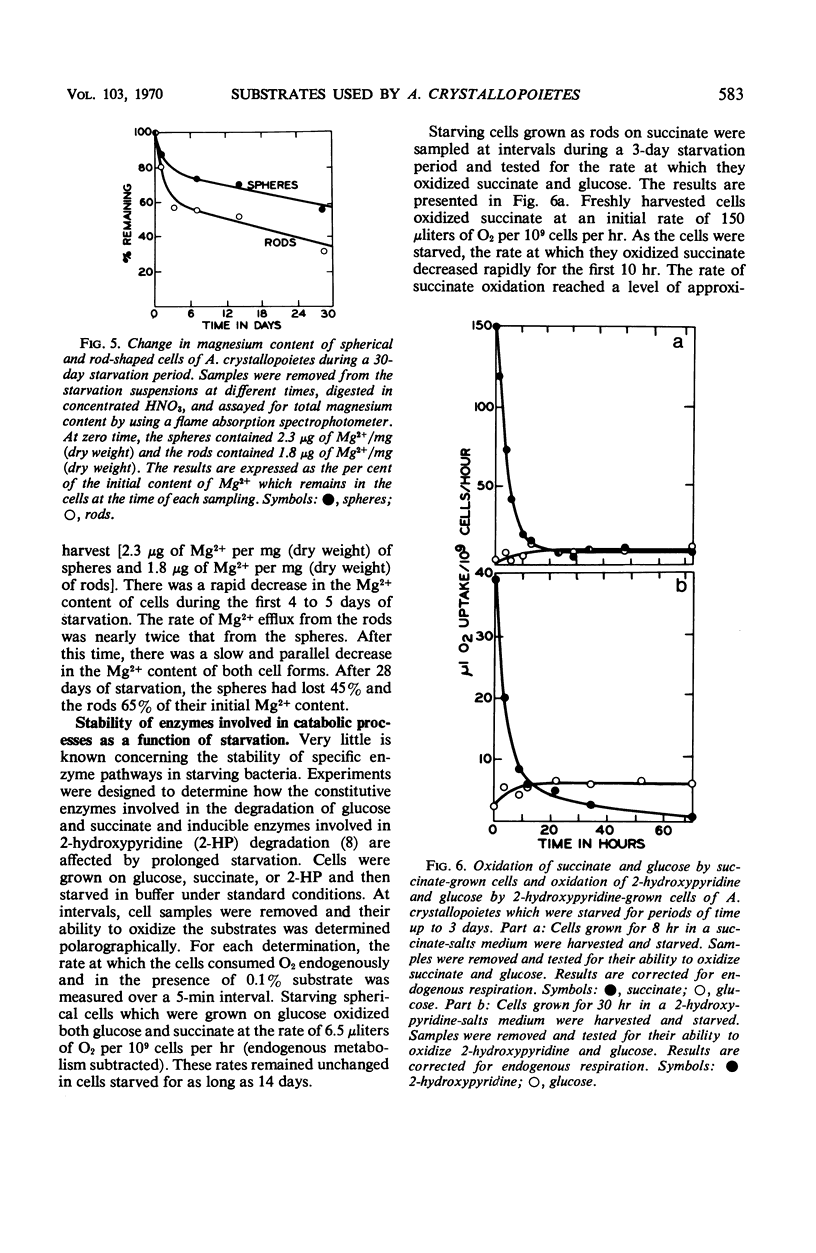
Abstract
Cells of Arthrobacter crystallopoietes, harvested during growth as spheres and as rods, were starved by shaking at 30 C in phosphate buffer for 30 days, during which time they maintained 100% viability. Changes in cellular components and the activity of specific enzyme pathways were monitored. A glycogen-like polysaccharide comprised 40% of the dry weight of growing spherical cells and 10% of the dry weight of rod cells. This material was utilized at approximately the same rate, on a percentage basis, during starvation of both cell forms. The rods degraded intracellular protein at approximately twice the rate of the spheres. At the end of 30 days, the rods had degraded 40% and the spheres 20% of their initial content of protein. Ribonucleic acid (RNA) was degraded significantly more rapidly in the rods. After 30 days starvation, 85 and 32% of the initial RNA of rods and spheres, respectively, had been depleted. Magnesium ion followed this same general pattern; the rods lost 65% and the spheres 45% of their initial content during 28 days of starvation. Deoxyribonucleic acid increased by 20% during the first few hours of starvation of both cell forms and then remained constant. The ability of glucose-, succinate-, and 2-hydroxypyridine (2-HP)-grown cells to oxidize glucose remained constant during 14 days of starvation. The ability of succinate-grown cells to oxidize succinate decreased rapidly during the first few hours of starvation to a rate which remained constant for 14 days. Cells adapted to growth on 2-HP completely lost their ability to oxidize this substrate after 3 days starvation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brdar B., Kos E., Drakulić M. Metabolism of nucleic acids and protein in starving bacteria. Nature. 1965 Oct 16;208(5007):303–304. doi: 10.1038/208303a0. [DOI] [PubMed] [Google Scholar]
- Burleigh I. G., Dawes E. A. Studies on the endogenous metabolism and senescence of starved Sarcina lutea. Biochem J. 1967 Jan;102(1):236–250. doi: 10.1042/bj1020236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWES E. A., RIBBONS D. W. SOME ASPECTS OF THE ENDOGENOUS METABOLISM OF BACTERIA. Bacteriol Rev. 1964 Jun;28:126–149. doi: 10.1128/br.28.2.126-149.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWES E. A., RIBBONS D. W. STUDIES ON THE ENDOGENOUS METABOLISM OF ESCHERICHIA COLI. Biochem J. 1965 May;95:332–343. doi: 10.1042/bj0950332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWES E. A., RIBBONS D. W. The endogenous metabolism of microorganisms. Annu Rev Microbiol. 1962;16:241–264. doi: 10.1146/annurev.mi.16.100162.001325. [DOI] [PubMed] [Google Scholar]
- ENSIGN J. C., RITTENBERG S. C. A CRYSTALLINE PIGMENT PRODUCED FROM 2-HYDROXYPYRIDINE BY ARTHROBACTER CRYSTALLOPOIETES N.SP. Arch Mikrobiol. 1963 Dec 10;47:137–153. doi: 10.1007/BF00422519. [DOI] [PubMed] [Google Scholar]
- Ensign J. C. Long-term starvation survival of rod and spherical cells of Arthrobacter crystallopoietes. J Bacteriol. 1970 Sep;103(3):569–577. doi: 10.1128/jb.103.3.569-577.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronlund A. F., Campbell J. J. Enzymatic Degradation of Ribosomes During Endogenous Respiration of Pseudomonas aeruginosa. J Bacteriol. 1965 Jul;90(1):1–7. doi: 10.1128/jb.90.1.1-7.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLDEN J. T. Degradation of intracellular nucleic acid and leakage of fragments by Lactobacillus arabinosus. Biochim Biophys Acta. 1958 Sep;29(3):667–668. doi: 10.1016/0006-3002(58)90041-6. [DOI] [PubMed] [Google Scholar]
- Jacobson A., Gillespie D. Metabolic events occurring during recovery from prolonged glucose starvation in Escherichia coli. J Bacteriol. 1968 Mar;95(3):1030–1039. doi: 10.1128/jb.95.3.1030-1039.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennell D., Kotoulas A. Magnesium starvation of Aerobacter aerogenes. I. Changes in nucleic acid composition. J Bacteriol. 1967 Jan;93(1):334–344. doi: 10.1128/jb.93.1.334-344.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krulwich T. A., Ensign J. C. Alteration of glucose metabolism of Arthrobacter crystallopoietes by compounds which induce sphere to rod morphogenesis. J Bacteriol. 1969 Feb;97(2):526–534. doi: 10.1128/jb.97.2.526-534.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARK C., LARK K. G. EVIDENCE FOR TWO DISTINCT ASPECTS OF THE MECHANISM REGULATING CHROMOSOME REPLICATION IN ESCHERICHIA COLI. J Mol Biol. 1964 Oct;10:120–136. doi: 10.1016/s0022-2836(64)80032-2. [DOI] [PubMed] [Google Scholar]
- LAW J. H., SLEPECKY R. A. Assay of poly-beta-hydroxybutyric acid. J Bacteriol. 1961 Jul;82:33–36. doi: 10.1128/jb.82.1.33-36.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., LEINER K. Y., WU M. L., FARR A. L. The quantitative histochemistry of brain. I. Chemical methods. J Biol Chem. 1954 Mar;207(1):1–17. [PubMed] [Google Scholar]
- Lusk J. E., Williams R. J., Kennedy E. P. Magnesium and the growth of Escherichia coli. J Biol Chem. 1968 May 25;243(10):2618–2624. [PubMed] [Google Scholar]
- Mulder E. G., Zevenhuizen L. P. Coryneform bacteria of the Arthrobacter type and their reserve material. Arch Mikrobiol. 1967;59(1):345–354. doi: 10.1007/BF00406348. [DOI] [PubMed] [Google Scholar]
- POSTGATE J. R., HUNTER J. R. The survival of starved bacteria. J Gen Microbiol. 1962 Oct;29:233–263. doi: 10.1099/00221287-29-2-233. [DOI] [PubMed] [Google Scholar]
- SIERRA G., GIBBONS N. E. Role and oxidation pathway of poly-beta-hydroxybutyric acid in Micrococcus halodenitrificans. Can J Microbiol. 1962 Apr;8:255–269. doi: 10.1139/m62-032. [DOI] [PubMed] [Google Scholar]
- STRANGE R. E. Induced enzyme synthesis in aqueous suspensions of starved stationary phase Aerobacter aerogenes. Nature. 1961 Sep 23;191:1272–1273. doi: 10.1038/1911272a0. [DOI] [PubMed] [Google Scholar]
- STRANGE R. E., WADE H. E., NESS A. G. The catabolism of proteins and nucleic acids in starved Aerobacter aerogenes. Biochem J. 1963 Feb;86:197–203. doi: 10.1042/bj0860197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobek J. M., Charba J. F., Foust W. N. Endogenous metabolism of Azotobacter agilis. J Bacteriol. 1966 Sep;92(3):687–695. doi: 10.1128/jb.92.3.687-695.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes J. L., Parson W. L. Role of poly-beta-hydroxybutyrate in survival of Sphaerotilus discophorus during starvation. Can J Microbiol. 1968 Jul;14(7):785–789. doi: 10.1139/m68-130. [DOI] [PubMed] [Google Scholar]
- Strange R. E. Stability of beta-galactosidase in starved Escherichia coli. Nature. 1966 Jan 22;209(5021):428–429. doi: 10.1038/209428a0. [DOI] [PubMed] [Google Scholar]
- Thomas T. D., Batt R. D. Degradation of cell constituents by starved Streptococcus lactis in relation to survival. J Gen Microbiol. 1969 Nov;58(3):347–362. doi: 10.1099/00221287-58-3-347. [DOI] [PubMed] [Google Scholar]
- Thomas T. D., Batt R. D. Survival of Streptococcus lactis in starvation conditions. J Gen Microbiol. 1968 Mar;50(3):367–382. doi: 10.1099/00221287-50-3-367. [DOI] [PubMed] [Google Scholar]
- Willetts N. S. Intracellular protein breakdown in non-growing cells of Escherichia coli. Biochem J. 1967 May;103(2):453–461. doi: 10.1042/bj1030453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zevenhuizen L. P. Formation and function of the glycogen-like polysaccharide of Arthrobacter. Antonie Van Leeuwenhoek. 1966;32(4):356–372. doi: 10.1007/BF02097485. [DOI] [PubMed] [Google Scholar]
- van Houte J., Jansen H. M. Role of glycogen in survival of Streptococcus mitis. J Bacteriol. 1970 Mar;101(3):1083–1085. doi: 10.1128/jb.101.3.1083-1085.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]



