Abstract
Plants can recruit parasitic wasps in response to egg deposition by herbivorous insects–a sophisticated indirect plant defense mechanism. Oviposition by the Large Cabbage White butterfly Pieris brassicae on Brussels sprout plants induces phytochemical changes that arrest the egg parasitoid Trichogramma brassicae. Here, we report the identification of an elicitor of such an oviposition-induced plant response. Eliciting activity was present in accessory gland secretions released by mated female butterflies during egg deposition. In contrast, gland secretions from virgin female butterflies were inactive. In the male ejaculate, P. brassicae females receive the anti-aphrodisiac benzyl cyanide (BC) that reduces the females' attractiveness for subsequent mating. We detected this pheromone in the accessory gland secretion released by mated female butterflies. When applied onto leaves, BC alone induced phytochemical changes that arrested females of the egg parasitoid. Microarray analyses revealed a similarity in induced plant responses that may explain the arrest of T. brassicae to egg-laden and BC-treated plants. Thus, a male-derived compound endangers the offspring of the butterfly by inducing plant defense. Recently, BC was shown to play a role in foraging behavior of T. brassicae, by acting as a cue to facilitate phoretic transport by mated female butterflies to oviposition sites. Our results suggest that the anti-aphrodisiac pheromone incurs fitness costs for the butterfly by both mediating phoretic behavior and inducing plant defense.
Keywords: egg parasitoid, elicitor, Trichogramma brassicae, Pieris brassicae, Brussels sprouts
Chemical signals play a crucial role in the interactions between herbivorous insects and parasitic wasps (1). To locate the tiny eggs of herbivorous host insects in an ‘ocean’ of plant biomass, egg parasitoids have been shown to employ chemical cues either induced in the plant by host egg deposition or from the adult host stage [i.e., infochemical detour, (2)], whereas only short-range cues emanate from the eggs themselves (3). Plants injured by feeding herbivores often start to release chemical cues that attract predators and parasitoids to effectively defend the plant by killing the herbivores (4, 5). This indirect plant defense may be triggered by compounds in the regurgitant of the herbivore, allowing the plant to discriminate between mechanical wounding and insect feeding (6–8). Plants can also respond before being damaged by insect feeding. Egg deposition by herbivorous insects induces volatiles attractive to egg parasitoids in tritrophic interactions associated with elm, pine, and bean plants (9). The elicitor of these oviposition-induced parasitoid attractants (synomones) was shown to be located in the oviduct secretion of the female herbivore, which is used to glue eggs onto leaves (10, 11).
Recent data revealed that egg deposition by Pieris brassicae on Brussels sprouts plants (Brassica oleracea var. gemmifera cv. Cyrus) induces chemical changes in the leaf surface that arrest the egg parasitoid Trichogramma brassicae (12). This arrestment was not observed immediately after egg deposition but became apparent locally at the egg-laden leaf three days after egg deposition when the host eggs are most suitable for parasitism (12). The chemical nature of the leaf surface modification is unknown. A recent molecular study analyzed the expression of genes of Arabidopsis plants in response to egg deposition by P. brassicae. Considerable oviposition-induced changes in expression levels of genes known to be involved in plant defense were detected three days after egg deposition (13).
The aim of this study was to investigate which egg-associated components induce the leaf surface changes exploited by T. brassicae and to confirm these induced changes in the plant at the molecular level. We focused on the secretion of the accessory reproductive gland (ARG) of female P. brassicae as the likely source of such components, because this secretion is released with the eggs onto the plant surface. Furthermore, we were interested in the possible role of male products transferred to the females during mating. The females receive a spermatophore that contains male ARG products in addition to sperm and nutrients (14). The male ARG components exert their effects at all reproductive phases of the mated female from the moment sperm is deposited in the reproductive tract to the time of egg deposition (14). In butterflies, anti-aphrodisiacs are transferred during copulation. These substances curtail courtship and decrease the likelihood of female remating (15–17). In P. brassicae, males transfer the anti-aphrodisiac benzyl cyanide (BC), a component of their own body odor, as well as their spermatophores to females (16). BC acts as a kairomone for the egg parasitoid T. brassicae by attracting it to mated P. brassicae females. The wasp subsequently uses the female as a transport vehicle to reach the oviposition site of the butterfly (18). We hypothesized that after egg deposition on Brussels sprouts plants, this anti-aphrodisiac is also involved in eliciting an induced plant response, which leads to chemical leaf surface modifications and arrestment of the egg parasitoid. Finally, to confirm oviposition- and BC-induced changes in Brussels sprouts, we investigated the transcriptional response to P. brassicae eggs in comparison to application of BC alone in these plants. For this purpose, we used a 70-mer oligonucleotide microarray representing the genome of Arabidopsis. This microarray minimizes cross-hybridization, recognizes related DNA sequences of B. oleracea, and shows intensity signals for 90% of the oligonucleotides present on the microarray (19), making it a valuable tool to study molecular changes in B. oleracea (20).
Results
Effect of Accessory Reproductive Glands.
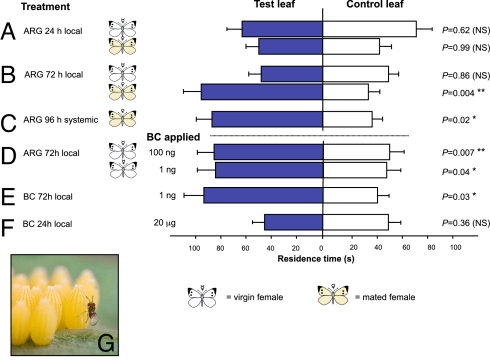
In two-choice bioassays, T. brassicae wasps contacted leaf squares cut from the vicinity of the leaf area treated with female ARG homogenate. These leaf squares are denoted here as “locally induced.” The wasps' responses to such squares depended on the time since application. Neither treatment by ARG homogenate obtained from mated females, nor homogenate from virgin females (P = 0.62 and P = 0.99, resp., Wilcoxon signed rank test; Fig. 1A) arrested the wasps when tested 24 h after treatment; however, 72 h after application, the wasps spent significantly more time on locally induced squares from leaves treated with ARG homogenate of mated females than on control leaf squares from plants treated with the solvent PBS only (P = 0.004, Wilcoxon signed rank test; Fig. 1B). In contrast, ARG homogenate from virgin butterfly females applied 72 h before the behavioral test did not elicit such an effect (P = 0.86, Wilcoxon signed rank test; Fig. 1B). A systemic effect of ARG homogenate from mated females was found 96 h after application. The wasps spent significantly more time on leaf disks from untreated leaves neighboring treated leaves than on control leaf surfaces (P = 0.02, Wilcoxon signed rank test; Fig. 1C).
Fig. 1.
Arrestment of Trichogramma brassicae wasps on treated Brussels sprouts leaves. Mean residence time (± SE) spent by the wasps on two simultaneously offered leaf squares in a Petri dish. Leaf squares were treated with the following: (i) accessory reproductive gland (ARG) homogenate from either virgin or mated butterfly (Pieris brassicae) females and tested versus leaves treated with solvent only (control), (ii) ARG homogenate from virgin butterflies and added benzyl cyanide (BC) (test) and tested versus leaves treated with solvent only (control), or (iii) with BC (test) and tested versus leaves treated with solvent only (control). (A and B) ARG 24 h or 72 h local. Untreated leaf area, adjacent to a site on the same leaf that was treated (A) 24 h or (B) 72 h previously with ARG homogenate, was offered to the parasitoid. (C) ARG 96 h systemic. Untreated leaf area, adjacent to a leaf treated 96 h before with ARG homogenate, was offered. (D) ARG and BC 72 h local. Untreated leaf area, adjacent to a site on the same leaf that was treated 72 h before with 100 ng or 1 ng BC in ARG homogenate of virgin P. brassicae. (E) BC 72 h local. Untreated leaf area, adjacent to a site on the same leaf that was treated 72 h before with 1 ng BC. (F) BC 24 h local. Untreated leaf area, adjacent to a site on the same leaf that was treated 24 h before with 20 μg BC. (G) Female Trichogramma wasp of approx. 0.5 mm on P. brassicae eggs that were deposited on Brussels sprouts (credits: N.E.F., www.bugsinthepicture.com). In each treatment, 50 wasps were tested. Asterisks indicate significant differences between test and control within the same treatment. *, P < 0.05, **, P < 0.01; n.s., not significant (Wilcoxon's matched pairs signed rank test).
Amount of BC in Accessory Reproductive Glands.
To determine whether the amount of BC present in the ARG of mated females is sufficient to induce the observed arrestment, we quantified these levels by chemical analysis. When analyzing extracts of mated females of which the exact time of mating was unknown, we detected 1.4 ± 1.54 ng BC/μl dichloromethane/ARG. BC was not recorded in any of the ARG extracts of virgin females.
Effects of the Butterfly Anti-Aphrodisiac BC.
Locally induced leaf squares from leaves treated with ARG homogenate from virgin females supplemented with either 100 ng or 1 ng BC arrested the wasps 72 h after application (P = 0.007 and P = 0.04, resp., Wilcoxon signed rank test; Fig. 1 D and E, respectively). Locally induced leaf squares from plants treated with 1 ng BC only caused an arresting effect when tested 72 h after application (P = 0.03, Wilcoxon signed rank test; Fig. 1E). To test whether BC itself was responsible for wasp arrestment, we tested the wasps' response to squares from leaves onto which 20 μg BC had been applied 24 h before the test. This treatment did not result in arrestment of the wasps (P = 0.36, Wilcoxon signed rank test; Fig. 1F). Similar BC dosages had been used in bioassays to demonstrate its immediate kairomonal activity on the wasps (18) and its intraspecific anti-aphrodisiac activity among the butterflies (16).
Gene-Expression Changes in Response to Eggs and BC.
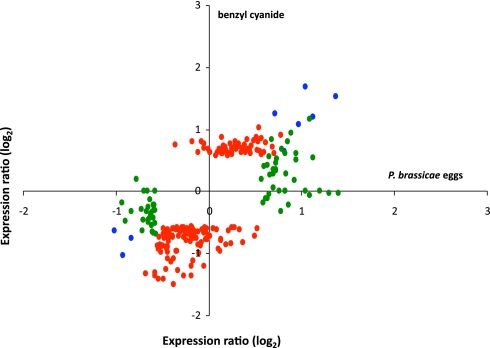
By using whole-genome Arabidopsis microarrays, we identified an induction of 42 genes and a repression of 32 genes in leaves analyzed 72 h after egg deposition by P. brassicae, compared to non-challenged control leaves. To investigate the role of BC in the transcriptional response to egg deposition, we compared the response to application of BC to that triggered by egg deposition. BC- treated leaves showed an induction of 61 genes and a repression of 103 genes 72 h after application. A direct comparison of gene expression ratios in leaves after egg deposition or BC application shows that there is a clear correspondence between the responses to these two treatments (Fig. 2).
Fig. 2.
Contribution of BC to P. brassicae egg-inducible gene expression. Relative changes in gene expression of Brussels sprouts are shown 72 h after P. brassicae egg deposition or treatment with the butterfly anti-aphrodisiac BC. Log2-transformed expression ratios after egg deposition are plotted against log2-transformed expression ratios after BC application. For the sake of clarity of presentation, only differentially regulated genes (log2-transformed expression ratios ≥1.5 or ≤0.67 and P < 0.05) have been plotted. Blue dots represent genes that are regulated by both egg deposition and BC application, green dots represent genes only regulated after egg deposition, and red dots represent genes only regulated after BC application.
Egg-Deposition Regulated Genes.
Among others, two genes involved in cell wall metabolism (EXPA15 and XTH6) were found to be induced in egg-laden leaves (Table 1). Genes repressed by P. brassicae eggs are mainly involved in protein metabolism or transport. Remarkably, two defense-related genes (receptor-like protein kinase and PED1) were repressed (Table 1).
Table 1.
List of selected genes differentially regulated after either Pieris brassicae oviposition or BC application
| Probe identification | Gene symbol | AGI code | Eggs | P value | BC | P value |
|---|---|---|---|---|---|---|
| Induced genes | ||||||
| Cell wall metabolism | ||||||
| Expansin 15 | EXPA15 | At2g03090 | 1.56 | 0.014 | 0.96 | 0.500 |
| Xyloglucan endotransglycosylase 6 | XTH6 | At5g65730 | 1.76 | 0.025 | 1.56 | 0.045 |
| Defense | ||||||
| Plant defensin 1.2b | PDF1.2b | At2g26020 | 1.22 | 0.481 | 1.66 | 0.049 |
| Tryptophan synthase, subunit 1 | TSA1 | At3g54640 | 0.97 | 0.946 | 1.62 | 0.044 |
| Protein metabolism/ metabolism | ||||||
| Eukaryotic translation initiation factor 3 subunit H1 | TIF3H1 | At1g10840 | 1.96 | 0.011 | 2.11 | 0.019 |
| Gamma-glutamyl hydrolase 2 | GGH2 | At1g78680 | 2.06 | 0.040 | 3.22 | 0.011 |
| Cyclophilin 5 | CYP5 | At2g29960 | 1.81 | 0.012 | 0.86 | |
| Ribosomal protein large subunit 16A | RPL16A | At2g42740 | 1.77 | 0.043 | † | |
| MMS 2 homolog 4 | MMZ4 | At3g52560 | 2.18 | 0.007 | 2.30 | 0.032 |
| Clp amino terminal domain-containing protein | At4g12060 | 1.72 | 0.032 | 0.80 | ||
| Aldehyde dehydrogenase 3F1 | ALDH3F1 | At4g36250 | 2.29 | 0.016 | † | |
| 14–3-3 protein GF14 λ | GRF6 | At5g10450 | 1.65 | 0.005 | 2.39 | 0.036 |
| Acetolactate synthase small subunit | At5g16290 | 1.65 | 0.021 | 1.28 | 0.791 | |
| Protein kinase | At5g50000 | 2.12 | 0.022 | 0.96 | * | |
| Transport | ||||||
| MATE efflux family protein | At1g73700 | 1.77 | 0.001 | 1.52 | 0.065 | |
| Pleitropic drug resistance 3 | PRD3 | At2g29940 | 1.65 | 0.001 | 1.37 | 0.031 |
| Metal transporter | At3g08650 | 1.92 | 0.030 | 1.43 | * | |
| Pleitropic drug resistance 1 | PDR1 | At3g16340 | 1.62 | 0.011 | 1.27 | 0.480 |
| Other processes | ||||||
| 50S ribosomal protein L21 | RPL21 | At1g35680 | 1.85 | 0.014 | 1.91 | * |
| Dehydrin | At1g54410 | 1.77 | 0.035 | 1.00 | 0.999 | |
| Delta 7-sterol-C5-desaturase | At3g02590 | 1.57 | 0.044 | 1.48 | 0.382 | |
| Telomerase activator 1 | TAC1 | At3g09290 | 2.13 | 0.017 | 2.24 | 0.097 |
| Cardiolipin synthase | CLS | At4g04870 | 2.59 | 0.021 | 2.89 | 0.012 |
| Chlorina 42 | CHLI1 | At4g18480 | 1.78 | 0.019 | 1.22 | 0.518 |
| Prenylcysteine alpha-carboxyl methyltransferase | STE14B | At5g08335 | 2.63 | 0.043 | 0.98 | * |
| hydrolase, α/β fold | At5g16120 | 2.46 | 0.002 | 0.98 | * | |
| Repressed genes | ||||||
| Cell structure metabolism | ||||||
| GDSL-motif lipase/hydrolase | At1g29660 | 1.26 | 0.246 | 0.66 | 0.041 | |
| Kinesin 13A | At3g16630 | 0.77 | 0.082 | 0.66 | 0.041 | |
| Proline-rich extensin-like protein | At4g08370 | 1.08 | 0.802 | 0.53 | 0.033 | |
| Proline-rich extensin-like protein | At4g08400 | 1.09 | 0.751 | 0.58 | 0.004 | |
| Phosphatidate cytidylyltransferase | At4g26770 | 0.74 | 0.497 | 0.57 | 0.003 | |
| BCL-2-associated athanogene 1 | BAG1 | At5g52060 | 0.77 | 0.474 | 0.66 | 0.009 |
| Defense | ||||||
| Peroxisome defective 1 | PED1 | At2g33150 | 0.56 | 0.029 | 0.80 | 0.249 |
| receptor-like protein kinase | At3g45860 | 0.61 | 0.030 | 0.64 | 0.099 | |
| Protein metabolism / metabolism | ||||||
| Ribosomal protein L4 | RPL4 | At1g07320 | 0.67 | 0.002 | 0.81 | * |
| Cysteine proteinase | At1g29110 | 0.63 | 0.035 | 0.84 | 0.123 | |
| RNA binding protein | At5g12280 | 0.63 | 0.007 | 0.73 | 0.203 | |
| Protein kinase | At5g41260 | 0.61 | 0.038 | 0.73 | * | |
| Transport | ||||||
| Amino acid permease | At5g01240 | 0.54 | 0.025 | 0.72 | 0.405 | |
| Mitochondrial substrate carrier family protein | At5g15640 | 0.52 | 0.015 | 0.89 | 0.441 | |
| Copper-binding family protein | At5g24580 | 0.66 | 0.035 | 0.86 | 0.069 | |
| Other processes | ||||||
| bZIP transcription factor | At1g19490 | 0.63 | 0.048 | † | ||
| One-helix protein 2 | OHP2 | At1g34000 | 0.67 | 0.012 | 0.82 | 0.194 |
| Quinolinate phophoribosyltransferase | QPT | At2g01350 | 0.53 | 0.004 | 0.49 | 0.021 |
| Hydrolase | At2g27500 | 0.66 | 0.040 | 0.81 | 0.042 | |
| Cytochrome P450 | CYP705A21 | At3g20120 | 0.65 | 0.041 | 0.69 | 0.036 |
| Phospholipase C 1 | PLC1 | At4g38530 | 0.67 | 0.010 | † | |
Relative changes in gene expression 72 h after egg deposition or BC application in Brussels sprout plants. Mean expression ratios are calculated from three biologically independent replicates. Only genes for which information about their possible function in Arabidopsis is present are shown.
*70-mer oligonucleotide hybridized only in one replicate.
†70-mer oligonucleotide did not hybridize in any of the three replicates. AGI, Arabidopsis Genome Initiative.
BC-Regulated Genes.
Six of the genes induced after BC treatment were also induced after egg deposition (Fig. 2). These genes are involved in general or protein metabolism (TIF3H1, GGH2, MMZ4, and GRF6), cell wall metabolism (XTH6), and phospholipid synthesis in mitochondria (CLS) (Table 1). In contrast to egg deposition, application of BC resulted in the induction of two defense-related genes (PDF1.2b and TSA1, Table 1). When looking at repressed genes, only three genes were repressed by both egg deposition and BC application. One of these genes is involved in nicotinamide adenine dinucleotide (NAD) synthesis, whereas no reports were found on the possible function of the other repressed gene. Genes that were repressed only after BC application consisted mostly of genes involved in protein metabolism, transport, or transcription. Remarkably, genes involved in apoptosis (BAG1), cell wall metabolism (two genes encoding proline-rich extensin-like proteins), lipid metabolism (a gene encoding a GDSL-motif lipase/hydrolase), and trichome morphogenesis (Kinesin 13A) were found to be repressed after BC application.
Discussion
Our results show that the application of ARG secretion of mated females induced a local plant response arresting the egg parasitoid after 72 h but not after 24 h. This finding is in agreement with the fact that egg deposition by the butterfly also induced this plant response only after 72 h (12). In contrast, ARG secretion of virgin females did not induce such a plant response, suggesting that mating changes the composition of the ARG secretion. We further demonstrated that the male anti-aphrodisiac BC induces the plant response that leads to arrestment of T. brassicae at a dose of 1 ng. Additionally, we provided evidence for an induced plant response upon egg deposition and BC application at the level of gene transcription. Thus, a single compound that the female received from the male during mating induces a plant defense in response to egg deposition.
A few studies have addressed the identity of the elicitor of oviposition-induced plant responses (2). The chemical structure of the elicitor of a direct plant defense response to egg deposition is known from a bruchid beetle (Callosobruchus maculatus). Here, the so-called bruchins, esters of long-chain diols, elicit plant responses directly detrimental to the eggs (21). Besides BC, only one other compound has been suggested to elicit an indirect plant defense. Several tests indicated that a small unidentified protein in the oviduct secretion of the pine sawfly Diprion pini elicits the release of pine volatiles attracting the eulophid egg parasitoid Chrysonotomyia ruforum (11). Oviduct secretion of the elm leaf beetle Xanthogaleruca luteola elicits a plant response in elm leaves that results in the release of volatiles attracting egg parasitoids (22); however, the chemical identity of this elicitor remains unknown.
The chemical nature of a few elicitors of feeding-induced plant defense responses are known: fatty acid-amino acid conjugates, including volicitin, of several lepidopterans (6, 7, 23–26); peptides derived by proteolysis from chloroplastic ATP synthase of herbivore-damaged plants (27, 28); disulfo-oxy fatty acids of grasshopper regurgitant, named caeliferins (29); and the enzyme β-glucosidase from Pieris brassicae oral secretions (8). Application of these elicitors onto plant wounds results in the release of volatiles that repel ovipositing herbivores (30) or attract natural enemies of herbivores (31). The mechanisms by which plants respond to elicitors from herbivores have not been studied extensively. A study on volicitin in the regurgitant of Spodoptera exigua showed that the initiation of the response of maize plants to feeding damage was mediated by a protein-ligand interaction in plasma membrane fractions (32). In cowpea, perception of Spodoptera frugiperda herbivory is mediated by fragments of ATP synthase that induce a targeted defense mechanism (27). Interestingly, herbivore saliva is not only able to elicit plant defense but is also able to suppress it (33–35). Aphid saliva was demonstrated to contain proteins with calcium-binding properties, which thereby prevent sieve tube occlusion, the normal plant defense response to injury of sieve tubes (34). Elicitors isolated from plant pathogens and their interactions with the plant surface have been studied more intensively than herbivore-released elicitors. Receptor-mediated activation of ion channels or direct interaction with lipid bilayers leading to pore formation appear to be possible mechanisms of pathogen elicitor action (36), but these can also be regulated by insect-derived elicitors (37).
Wounding of plant tissue is considered essential for functioning of the elicitor in the oviduct secretion of the pine sawfly and the elm leaf beetle (10, 11). Whereas pine sawflies damage the pine needle with their ovipositor and apply the oviduct secretion into the needle's wound, the elm leaf beetle just nibbles on the leaf epidermis before egg deposition (22). Except for some slight scratches in the wax layer, no wounding of the leaf tissue is visible after P. brassicae has laid eggs (38, N.E. Fatouros, personal observations). Thus, BC seems to pass through the waxy plant epicuticle and initiates a response to egg deposition in the leaf.
Transcriptional analyses provided molecular evidence confirming the oviposition- and BC-induced changes in the plant. We showed that the presence of P. brassicae eggs triggers a transcriptional response in Brussels sprouts plants that correlates well with the response to BC. Several of these differentially expressed genes may be involved in leaf surface changes that arrest T. brassicae. Genes encoding an expansin and a xyloglucan endotransglucosylase (XTH) were induced after egg deposition. Expansins and XTHs have been shown to play a role in cell wall strengthening (39, 40). The regulation of these cell wall modification genes may indicate leaf surface remodeling at the site of oviposition. Genes encoding expansins or XTHs were also differentially regulated in Arabidopsis after oviposition by P. brassicae (13). In contrast to the response triggered in Arabidopsis after egg deposition by P. brassicae (13), we did not identify a hypersensitive response (HR) in Brussels sprouts. The absence of a necrotic zone, which has been observed in Arabidopsis leaves, at the site of egg deposition in Brussels sprouts leaves is in accordance with the lack of a HR in our study. Part of the plant response to egg deposition can be triggered by treating plants with the male anti-aphrodisiac BC. Interestingly, a gene involved in the apoptosis, which is a characteristic of HRs, was suppressed by BC in Brussels sprouts, indicating a role for this compound in suppressing this direct plant defense mechanism. BC application repressed several genes that may be responsible for leaf surface changes, but these genes were not differentially expressed after egg deposition. Two defense-related genes were repressed in egg-laden leaves, but these changes did not occur after BC application. Additionally, two other defense-related genes were induced after BC application but not after egg deposition. This result suggests that other components from the eggs or transferred with the eggs may suppress the expression of certain defense-related genes, thereby influencing the plant defense response. Another explanation may result from differences in the dose of BC between egg deposition and pure BC application.
This study represents a first step in elucidating the transcriptional responses underlying the plant response that results in arrestment of T. brassicae on leaves of Brussels sprouts with three-day-old eggs of P. brassicae. The use of microarrays based on the Arabidopsis genome, however, limits this study in that differentially expressed genes without sufficient homology between Arabidopsis and Brussels sprouts will not be detected. Single gene analyses or functional studies using knock-out plants are needed to further understand the molecular response of the plant to egg deposition by P. brassicae.
The anti-aphrodisiac BC is not the only component transferred from male P. brassicae to females. In Pieris spp., the male ejaculate has at least three effects: fertilizing the eggs of the mating partner, increasing the period during which females are refractory to mating, and increasing female fecundity and longevity through nutrients (41, 42). We cannot exclude that additional components in the male ejaculate also play a role in the eliciting activity of mated female ARG secretion. The difference in transcriptional responses after egg deposition and BC treatment also indicates that other components are involved in the response of the plant.
The use of host-induced plant cues is likely adaptive for egg parasitoid individuals, which are searching for host eggs in the vegetation, compared with those transported through phoresy on host butterflies. After egg deposition, the time period during which BC per se can be perceived is limited because of its volatility and possibly rapid penetration into the epicuticular wax layer of the plant.
This report shows that males contribute to elicitation of an oviposition-induced defensive plant response. Male sexual signals are usually very conspicuous, because they enhance reproductive success and indicate high mate quality. These signals are, therefore, assumed to be energetically costly (43). Anti-aphrodisiacs may be viewed as honest but costly male-derived signals that ensure the male's genetic investment. We, therefore, expect the direct and indirect use of anti-aphrodisiacs to be a common host location strategy for egg parasitoids. As Trichogramma spp. are significant mortality factors for P. brassicae (44), these parasitoids may severely constrain the evolution of the butterflies' intraspecific sexual communication system in areas of high parasitoid density. Local variation in parasitoid-mediated selection on the sexual signal may lead to host population divergence and eventually speciation (43).
Materials and Methods
Plants and Insects.
Brussels sprouts plants (Brassica oleracea L. var. gemmifera cv. Cyrus) were grown in a greenhouse (18 ± 5°C, 50–70% rh, L16:D8). Pieris brassicae were reared on Brussels sprouts plants in a climate room (21 ± 1°C, 50–70% rh, L16:D8). Virgin males and females were obtained by sexing in the pupal phase and subsequently keeping them separately for 2–4 days until the two sexes were combined in one cage. As soon as a butterfly couple was observed to mate, it was isolated to obtain females and males mating for the first time. Trichogramma brassicae Bezdenko (Hymenoptera: Trichogrammatidae; strain Y175) was reared in eggs of P. brassicae for several generations (25 ± 1°C, 50–70% rh, L16:D8). For the rearing, 1–3-day-old P. brassicae eggs on leaves were used. Only mated, 2–5-day-old, oviposition-experienced female wasps were used for the experiments. An oviposition experience was given for a period of 18 h before the experiment with <3-day-old P. brassicae eggs deposited on Brussels sprouts leaves.
Preparation of ARG Extracts.
To obtain samples for the bioassays, ARGs were dissected from three gravid or virgin P. brassicae females (4–10 days old) in PBS (PBS, pH 7.2), transferred to a vial with 100 μl PBS, and subsequently homogenized. Then, 100 μl PBS were added. The homogenate was centrifuged, and 100 μl of the supernatant were applied with a brush onto the edge of a cabbage leaf as described below (equivalents 1.5 glands per leaf). For chemical analysis, one ARG of either a mated (n = 10) or a virgin (n = 5) P. brassicae female (4–10 days old) were dissected and transferred to a vial containing 50 μl dichloromethane (DCM) with phenyl cyanide (>98% purity, Sigma–Aldrich) as an internal standard (0.5 ng/μl).
Chemical Analysis of ARG Extracts.
ARG extracts were analyzed by coupled gas chromatography–mass spectrometry (GC-MS) by using a gas chromatograph (5890 series II, Hewlett-Packard) equipped with a 30-m Zebron ZB-5ms column (0.25 mm i.d., 0.25-μm film thickness; Phenomenex, Torrance, USA) and a mass-selective detector (model 5972A, Hewlett-Packard). A 5-m Guardian™ column (deactivated fused silica tubing without stationary phase; Phenomenex, Torrance, USA) was built directly into the analytical column. The GC was programmed at an initial temperature of 45°C for 1 min with a ramp of 10°C/min to 150°C and then with a ramp of 30°C to 280°C (3.5 min hold). The sample volume (1 μl) was injected in splitless mode. The injection port and interface temperature were 250°C and 290°C, respectively, and the helium inlet pressure was controlled with an electronic pressure control to achieve a constant column flow of 1.0 μl/min. The solvent delay was set to 4 min. The ionization potential was set at 70 eV, and scanning was performed from 45 to 150 atomic mass units with a scan rate of 5.5 scans s−1. Identification of BC and the internal standard phenyl cyanide was based on the injection of authentic reference standards (>98% purity, Sigma–Aldrich). Quantification of BC was based on comparison with the internal standard. A calibration series of BC and phenyl cyanide, injected from a concentration of 0.05–50 ng/μl DCM, showed very similar linear response factors for both compounds within this concentration range (data not shown).
Bioassay.
A wasp was released in a small glass Petri dish (5.5 cm diameter) between a test and a control 1.5-cm2 leaf square directly cut from the plants. The total duration of time spent on the leaves was observed for a period of 300 s using The Observer software 4.0 (Noldus Information Technology). A detailed description of the bioassay methods is given elsewhere (12). Two leaves of corresponding size and position from the test and control plants were used. A total of 50 wasps per treatment were tested, and five plants per treatment were used. A maximum of 10 wasps per experimental day were tested. Leaf squares were renewed and changed after every third wasp tested. All bioassays were analyzed using Wilcoxon signed rank test (45).
Plant Treatments.
For the bioassays, an ARG sample was applied onto the edge of a Brussels sprout leaf in a stretch of 2 cm on the lower leaf side. Control plants were treated with PBS in the same way. After treatment, plants were kept for 24, 72, or 96 h in a climate chamber (21 ± 1°C, 50–70% rh, L16:D8). Fatouros et al. (12) showed that eggs of P. brassicae induce a change in the leaf surface close to the eggs following 72 h after oviposition, but not following only 24 h after oviposition. Subsequently, to test local effects, leaf squares 1–5 cm away from the treated leaf section were tested against leaf squares 1–5 cm away from the PBS-treated section of the control plant in a two-choice bioassay after 24 or 72 h. To test for a possible systemic effect, leaf squares were taken from an untreated leaf (systemic leaf) of an ARG-treated plant 96 h after treatment. A leaf right above the treated leaf was used as a systemic leaf. Control leaf squares were cut from a systemic leaf of a plant located right above a leaf treated with PBS. Low amounts of benzyl cyanide (BC, 210 ng and 2.1 ng) were diluted in 10 μl hexane and added to 200 μl of ARG homogenate of virgin females in PBS. A volume of 100 μl of this mixture was applied onto a leaf as described above. Thus, 100 ng or 1 ng BC plus gland homogenate (equivalent 1.5 ARGs) were used per leaf. Test leaf squares were cut from the plant 72 h after application of the mixture. Control leaf squares were obtained from leaves treated with PBS and hexane. To examine whether pure BC induces a local plant response arresting the wasps 72 h after application, 100 μl of a 0.01 ng BC/μl methanol solution were applied as described above. We chose methanol as a solvent, because pure BC does not dissolve in PBS. A control plant was treated with 100 μl methanol only. Leaf squares from an untreated part of the test leaf were tested against leaf squares from the solvent-treated part of the control leaf in the two-choice bioassay. To determine whether BC per se arrests the wasps 24 h after application, 20 μg BC was applied per leaf as described above.
For transcriptional analysis, test plants were placed for a period of about 1 h into a cage with more than 100 P. brassicae adults to allow egg deposition onto the plants. After exposure to the butterflies, the treated plants were transferred to a climate chamber for another 72 h (see above). Control plants were never in contact with P. brassicae or any other insect; however, they were grown and kept under the same abiotic conditions as treated plants. To examine whether BC induces changes in the plant's transcriptome 72 h after application, 100 μl of a 0.01-ng BC/μl methanol solution were applied as described above. Control plants were treated with 100 μl methanol. Leaf discs of ≈2 cm in diameter were collected 1–5 cm away from the treated part or egg clutches and were immediately frozen in liquid nitrogen for later RNA extraction. As controls, an equal number of leaf disks from a control plant was collected. For each microarray, one leaf disk/plant from 10 individual plants was collected. Each treatment was repeated three times.
Microarray Experiments and Data Analysis.
Total RNA was isolated from leaf disks by using TRIzol reagent (Invitrogen) followed by a purification step (RNeasy clean up, Qiagen). Four μg of total RNA were linearly amplified by using the MessageAmp II aRNA kit (Ambion). Cy3 and Cy5 mono-reactive dyes (Amersham) were coupled to the amplified RNA (aRNA) in coupling buffer (provided with the MessageAmp II aRNA kit, Ambion) for 1 h at room temperature. Material from control samples was labeled with Cy3, and material from treated samples was labeled with Cy5. Labeling of aRNA was monitored by measuring the Cy3 and Cy5 fluorescence emissions using a Nanodrop spectrophotometer (BioRad). The oligonucleotide array elements were immobilized as described on the manufacturer's web site (see above). For hybridization, 100 pmol of the Cy3-labeled sample and 50 pmol of the Cy5-labeled sample were combined in 2× SSC, 0.08% SDS, and 4.8 μl Liquid Block (Amersham) in a final volume of 80 μl. The solution was incubated at 65 °C for 5 min and applied to the microarray under a lifterslip (Gerhard Menzel). The microarray was placed in a hybridization chamber (Genetix) and incubated at 50°C for 12 h. The microarray was washed once for 5 min in 2× SSC/0.5% SDS at 50°C, followed by a wash for 5 min in 0.5× SSC at room temperature. A final wash for 5 min in 0.05× SSC at room temperature was performed. The microarray was immediately dried by centrifugation for 4 min at 200 rpm and scanned with a ScanArray Express HT Scanner (PerkinElmer). Median fluorescent intensities for each dye and for each gene were determined by using the ScanArray Express program (PerkinElmer). Median background fluorescence around each spot was subtracted, and spots with adjusted intensities lower than half the background were manually raised to half the background to avoid extreme expression ratios. When signal intensities were less than half the background for both dyes, the spot was excluded from the analysis. Spots with an aberrant shape or spots located in a smear of fluorescence were also excluded from the analysis. To avoid spatial bias, Lowess (Locfit) normalization was carried out within each slide by using TIGR MIDAS version 2.18. Normalized signal intensities were used to calculate expression ratios. Statistical analyses were carried out by using TIGR-MEV version 3.0.3. A one-class Student's t test on log2-transformed expression ratios was conducted for each experimental condition. Transcription rates of genes with a log2-transformed expression ratio of ≥ 0.57 or ≤ −0.57, and P values of < 0.05 were considered significantly different. We used the names of Arabidopsis homologs to identify Brassica oleracea genes and examined the potential function of differentially regulated genes according to gene ontology (GO) terms from The Arabidopsis Information Resource [http://www.arabidopsis.org; (46)].
Acknowledgments.
The authors thank David Galbraith from the University of Arizona (http://www.ag.arizona.edu/microarray) for supplying microarrays; Francel Verstappen for help with the chemical analysis; Leo Koopman, Frans van Aggelen, and André Gidding for culturing the insects; and the experimental farm of Wageningen University (Unifarm) for growing the Brussels sprout plants. The authors acknowledge funding from the German Research Foundation grants DFG Hi 416/15-1, Hi 416/15-2 (to M.H.) and FA 824/1–11 (to N.E.F.) and the Netherlands Organization for Scientific Research NWO/ALW VENI grant 863.05.020 (to M.E.H.) and NWO/ALW VICI 865.03.002 (to R.M. and M.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Vinson SB. Host selection by insect parasitoids. Ann Rev Entomol. 1976;21:109–133. [Google Scholar]
- 2.Vet LEM, Dicke M. Ecology of infochemical use by natural enemies in a tritrophic context. Ann Rev Entomol. 1992;37:141–172. [Google Scholar]
- 3.Fatouros NE, Dicke M, Mumm R, Meiners T, Hilker M. Foraging behavior of egg parasitoids exploiting chemical information. Behav Ecol. 2008;19:677–689. [Google Scholar]
- 4.Turlings TCJ, Tumlinson JH, Lewis WJ. Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science. 1990;250:1251–1253. doi: 10.1126/science.250.4985.1251. [DOI] [PubMed] [Google Scholar]
- 5.Takabayashi J, Dicke M. Plant-carnivore mutualism through herbivore-induced carnivore attractants. Trends Plants Sci. 1996;1:109–113. [Google Scholar]
- 6.Alborn HT, et al. An elicitor of plant volatiles from beet armyworm oral secretion. Science. 1997;276:945–949. [Google Scholar]
- 7.Halitschke R, Schittko U, Pohnert G, Boland W, Baldwin IT. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Spingidae) and its natural host Nicotinia attenuata. III Fatty acid-amino acid conjugates in herbivore oral secretions are necessary and sufficient for herbivore-specific plant responses. Plant Physiol. 2001;125:711–717. doi: 10.1104/pp.125.2.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mattiacci L, Dicke M, Posthumus MA. beta-Glucosidase: An elicitor of the herbivore-induced plant odor that attracts host-searching parasitic wasps. Proc Natl Acad Sci USA. 1995;92:2036–2040. doi: 10.1073/pnas.92.6.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hilker M, Meiners T. Early herbivore alert: Insect eggs induce plant defense. J Chem Ecol. 2006;32:1379–1397. doi: 10.1007/s10886-006-9057-4. [DOI] [PubMed] [Google Scholar]
- 10.Meiners T, Hilker M. Induction of plant synomones by oviposition of a phytophagous insect. J Chem Ecol. 2000;26:221–232. [Google Scholar]
- 11.Hilker M, Stein C, Schröder R, Varama M, Mumm R. Insect egg deposition induces defence responses in Pinus sylvestris: Characterisation of the elicitor. J Exp Biol. 2005;208:1849–1854. doi: 10.1242/jeb.01578. [DOI] [PubMed] [Google Scholar]
- 12.Fatouros NE, et al. Oviposition-induced plant cues: Do they arrest Trichogramma wasps during host location? Entomol Exp Appl. 2005;115:207–215. [Google Scholar]
- 13.Little D, Gouhier-Darimont C, Bruessow F, Reymond P. Oviposition by pierid butterflies triggers defense responses in Arabidopsis. Plant Physiol. 2007;143:784–800. doi: 10.1104/pp.106.090837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gillott C. Male accessory gland secretions: Modulators of female reproductive physiology and behavior. Ann Rev Entomol. 2003;48:163–184. doi: 10.1146/annurev.ento.48.091801.112657. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert LE. Postmating female odor in Heliconius butterflies: A male-contributed antiaphrodisiac? Science. 1976;193:419–420. doi: 10.1126/science.935877. [DOI] [PubMed] [Google Scholar]
- 16.Andersson J, Borg-Karlson A-K, Wiklund C. Antiaphrodisiacs in Pierid butterflies: A theme with variation! J Chem Ecol. 2003;29:1489–1499. doi: 10.1023/a:1024277823101. [DOI] [PubMed] [Google Scholar]
- 17.Andersson J, Borg-Karlson A-K, Wiklund C. Sexual cooperation and conflict in butterflies: A male-transferred anti-aphrodisiac reduces harassment of recently mated females. Proc R Soc Lond B. 2000;267:1271–1275. doi: 10.1098/rspb.2000.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fatouros NE, Huigens ME, van Loon JJA, Dicke M, Hilker M. Chemical communication-Butterfly anti-aphrodisiac lures parasitic wasps. Nature. 2005;433:704. doi: 10.1038/433704a. [DOI] [PubMed] [Google Scholar]
- 19.Lee HS, et al. Sensitivity of 70-mer oligonucleotides and cDNAs for microarray analysis of gene expression in Arabidopsis and its related species. Plant Biotech J. 2004;2:45–57. doi: 10.1046/j.1467-7652.2003.00048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broekgaarden C, et al. Genotypic variation in genome-wide transcription profiles induced by insect feeding: Brassica oleracea–Pieris rapae interactions. BMC Genomics. 2007;8:239. doi: 10.1186/1471-2164-8-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doss RP, et al. Bruchins: Insect-derived plant regulators that stimulate neoplasm formation. Proc Natl Acad Sci USA. 2000;97:6218–6223. doi: 10.1073/pnas.110054697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meiners T, Hilker M. Host location in Oomyzus gallerucae (Hymenoptera: Eulophidae), an egg parasitoid of the elm leaf beetle Xanthogaleruca luteola (Coleoptera: Chrysomelidae) Oecologia. 1997;112:87–93. doi: 10.1007/s004420050287. [DOI] [PubMed] [Google Scholar]
- 23.Alborn HT, Jones HT, Stenhagen JG, Tumlinson JH. Identification and synthesis of volaticin and related components from beet armyworm oral secretions. J Chem Ecol. 2000;26:203–220. [Google Scholar]
- 24.Mori N, Alborn HT, Teal PEA, Tumlinson JH. Enzymatic decomposition of elicitors and plant volatiles in Heliothis virescens and Helicoverpa zea. J Insect Physiol. 2001;47:749–757. doi: 10.1016/s0022-1910(00)00171-2. [DOI] [PubMed] [Google Scholar]
- 25.Pohnert G, Jung V, Haukioja E, Lempa K, Boland W. New fatty acid amides from regurgitant of lepidopteran (Noctuidae, Geometridae) caterpillars. Tetrahedron. 1999;55:11275–11280. [Google Scholar]
- 26.Alborn HT, Brennan MM, Tumlinson JH. Differential activity and degradation of plant volatile elicitors in regurgitant of tobacco hornworm (Manduca sexta) larvae. J Chem Ecol. 2003;29:1357–1372. doi: 10.1023/a:1024209302628. [DOI] [PubMed] [Google Scholar]
- 27.Schmelz EA, et al. Fragments of ATP synthase mediate plant perception of insect attack. Proc Natl Acad Sci USA. 2006;103:8894–8899. doi: 10.1073/pnas.0602328103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmelz EA, LeClere S, Carroll MJ, Alborn HT, Teal PEA. Cowpea chloroplastic ATP synthase is the source of multiple plant defense elicitors during insect herbivory. Plant Physiol. 2007;144:793–805. doi: 10.1104/pp.107.097154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alborn HT, et al . Disulfooxy fatty acids from the American bird grasshopper Schistocerca americana, elicitors of plant volatiles. Proc Natl Acad Sci USA. 2007;104:12976–12981. doi: 10.1073/pnas.0705947104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Moraes CM, Meschner MC, Tumlinson JH. Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature. 2001;410:577–580. doi: 10.1038/35069058. [DOI] [PubMed] [Google Scholar]
- 31.Dicke M, van Loon JJA. Multitrophic effects of herbivore-induced plant volatiles in an evolutionary context. Entomol Exp Appl. 2000;97:237–249. [Google Scholar]
- 32.Truitt CL, Wei HX, Pare PW. A plasma membrane protein from Zea mays binds with the herbivore elicitor volicitin. Plant Cell. 2004;16:523–532. doi: 10.1105/tpc.017723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Musser RO, et al. Evidence that the caterpillar salivary enzyme glucose oxidase provides herbivore offense in solanaceous plants. Arch Insect Biochem. 2005;58:128–137. doi: 10.1002/arch.20039. [DOI] [PubMed] [Google Scholar]
- 34.Will T, Tjallingi F, Thönnessen A, van Bel AJE. Molecular sabotage of plant defense by aphid saliva. Proc Natl Acad Sci USA. 2007;104:10536–10541. doi: 10.1073/pnas.0703535104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Musser RO, et al. Caterpillar saliva beats plant defences. Nature. 2002;416:599–600. doi: 10.1038/416599a. [DOI] [PubMed] [Google Scholar]
- 36.Klüsener B, Weiler EW. Pore-forming properties of elicitors of plant defense reactions and cellulolytic enzymes. FEBS Lett. 1999;459:263–266. doi: 10.1016/s0014-5793(99)01261-2. [DOI] [PubMed] [Google Scholar]
- 37.Maffei ME, Mithöfer A, Boland W. Before gene expression: Early events in plant-insect interaction. Trends Plants Sci. 2007;12:310–316. doi: 10.1016/j.tplants.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Bruessow F, Reymond P. Oviposition-induced changes in Arabidopsis genome expression. Plant Signal & Behav. 2007;2:e1–e3. doi: 10.4161/psb.2.3.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cosgrove DJ. Growth of the plant cell wall. Nature Reviews. 2005;6:850–861. doi: 10.1038/nrm1746. [DOI] [PubMed] [Google Scholar]
- 40.Campbell P, Braam J. Xyloglucan endotransglycosylases: Diversity of genes, enzymes, and potential wall-modifying functions. Trends Plants Sci. 1999;4:361–366. doi: 10.1016/s1360-1385(99)01468-5. [DOI] [PubMed] [Google Scholar]
- 41.Wiklund C, Kaitala A, Lindfors V, Abenius J. Polyandry and its effect on female reproduction in the green-veined white butterfly (Pieris napi L. ) Behav Ecol Sociobiol. 1993;33:25–33. [Google Scholar]
- 42.Bissoondath CJ, Wiklund C. Male butterfly investment in successive ejaculates in relation to mating system. Behav Ecol Sociobiol. 1996;39:285–292. [Google Scholar]
- 43.Champion de Crespigny FE, Hosken DJ. Sexual selection: Signals to die for. Curr Biol. 2007;17:R853–R855. doi: 10.1016/j.cub.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 44.van Heiningen TG, Pak GA, Hassan SA, van Lenteren JC. Four year's results of experimental releases of Trichogramma egg parasites against lepidopteran pests in cabbage. Med Fac Landbouw Rijksuniv Gent. 1985;50:379–388. [Google Scholar]
- 45.Sokal RR, Rohlf JF. The principles and practice of statistics in biological research. San Francisco: W.H. Freeman and Company; 1969. Biometry; pp. 399–400. [Google Scholar]
- 46.Berardini TZ, et al. Functional annotation of the Arabidopsis genome using controlled vocabularies. Plant Physiol. 2004;135:745–755. doi: 10.1104/pp.104.040071. [DOI] [PMC free article] [PubMed] [Google Scholar]