Abstract
β-arrestins critically regulate G protein-coupled receptors (GPCRs), also known as seven-transmembrane receptors (7TMRs), both by inhibiting classical G protein signaling and by initiating distinct β-arrestin-mediated signaling. The recent discovery of β-arrestin-biased ligands and receptor mutants has allowed characterization of these independent “G protein-mediated” and “β-arrestin-mediated” signaling mechanisms of 7TMRs. However, the molecular mechanisms underlying the dual functions of β-arrestins remain unclear. Here, using an intramolecular BRET (bioluminescence resonance energy transfer)-based biosensor of β-arrestin 2 and a combination of biased ligands and/or biased mutants of three different 7TMRs, we provide evidence that β-arrestin can adopt multiple “active” conformations. Surprisingly, phosphorylation-deficient mutants of the receptors are also capable of directing similar conformational changes in β-arrestin as is the wild-type receptor. This indicates that distinct receptor conformations induced and/or stabilized by different ligands can promote distinct and functionally specific conformations in β-arrestin even in the absence of receptor phosphorylation. Our data thus highlight another interesting aspect of 7TMR signaling—i.e., functionally specific receptor conformations can be translated to downstream effectors such as β-arrestins, thereby governing their functional specificity.
Keywords: 7TMRs, BRET, phosphorylation
G protein-coupled receptors (GPCRs), also known as seven-transmembrane receptors (7TMRs), are the largest family of cell-surface receptors that communicate extracellular stimuli to the cell interior (1). The classical view of GPCR signaling is that a ligand activates the receptor and an activated receptor then couples to and activates heterotrimeric G proteins, leading to generation of second messengers such as cAMP, DAG, and IP3. Subsequently, G protein-coupled receptor kinases (GRKs) phosphorylate the receptor and promote β-arrestin recruitment to the receptor. Binding of β-arrestins to the receptor sterically inhibits further G protein coupling and leads to receptor desensitization. In recent years, however, a number of additional functions of β-arrestins have been discovered, which include crucial roles in clathrin-mediated endocytosis of receptors and as signal transducers for a growing list of effector pathways such as MAP kinases and phosphatidylinositol 3-kinase (PI3-kinase) (2, 3). Thus, β-arrestins, while turning off G protein-dependent signaling, can simultaneously initiate parallel G protein-independent signaling pathways.
Recent studies have revealed that G protein-dependent and β-arrestin-dependent signaling pathways of 7TMRs are pharmacologically separable. Certain ligands are capable of selectively activating one of these two signaling pathways; such ligands are referred to as biased ligands and the phenomenon as “biased agonism” or “ligand directed signaling” (4, 5). β-arrestin-biased ligands are of particular interest because they offer the possibility to design an entirely novel class of therapeutic agents (6). Such ligands, in common with conventional antagonists, prevent agonist-activated G protein signaling. However, in contrast with the conventional antagonists, they simultaneously stimulate potentially beneficial effects of β-arrestin-mediated signaling.
The existence of independent G protein-mediated and β-arrestin-mediated signaling via 7TMRs requires that receptors adopt multiple “active” conformations or “ligand selective states.” Biochemical and biophysical data suggest that different ligands can indeed induce and/or stabilize subsets of the multiple active conformations of a receptor (7, 8). A major challenge now is to decipher the molecular mechanisms underlying biased agonism at 7TMRs, and to establish a link between different receptor conformations and selective functional outcomes of β-arrestin. One attractive hypothesis is that, analogous to the multiple active conformations in 7TMRs, β-arrestins might also adopt multiple “active” conformations, and depending on its conformation, β-arrestin can either activate different signaling effectors and/or desensitize G protein coupling. Accordingly, we set out to elucidate the conformational changes in β-arrestin in response to the activation of 7TMRs by β-arrestin-biased or unbiased ligands.
Results and Discussion
We used a recently described intramolecular bioluminescence resonance energy transfer (BRET)-based biosensor (9) of β-arrestin 2. In this biosensor, bioluminescent Renilla luciferase (Luc) and the yellow fluorescent protein (YFP) are fused at the N and the C termini, respectively, of β-arrestin 2. Structural changes in β-arrestin cause rearrangement of the two ends of the molecule (and therefore, changes in the distance and/or orientation of Luc and YFP relative to each other), altering BRET efficiency in such a way as to indicate conformational changes.
Distinct Conformational Changes in β-Arrestin upon Activation of AT1aR.
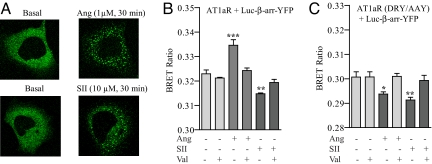
A well studied example of biased agonism exists for the AT1aR. Based on mutational analysis of the full agonist Angiotensin II (Ang), a β-arrestin-biased agonist termed [Sar1, Ile4, Ile8]-angiotensin II (SII) has been developed and characterized (10, 11). Stimulation of AT1aR with SII does not activate Gαq/11 signaling but promotes β-arrestin recruitment and stimulates ERK activation in an entirely β-arrestin 2-dependent manner (11). As shown in Fig. 1A, both Ang and SII stimulation resulted in a “class B” recruitment of the biosensor, i.e., strong and prolonged interaction of β-arrestin with the receptor, reflected by localization of β-arrestin in the endocytotic vesicles (12). This suggests that the β-arrestin biosensor is functional in terms of ligand-stimulated recruitment to the receptor. To investigate whether β-arrestin can adopt distinct conformations in response to receptor stimulation by an unbiased or biased ligand, we monitored the BRET signal upon stimulation of AT1aR by either Ang or SII. As reported earlier (9), stimulation of AT1aR with Ang resulted in an increase in the intramolecular BRET ratio indicating a conformational change upon β-arrestin recruitment to the receptor (Fig. 1B). However, more strikingly, stimulation of AT1aR with SII resulted in a decrease in the intramolecular BRET ratio (Fig. 1B). Pretreatment of cells with the angiotensin receptor blocker (ARB) valsartan blocks the Ang- and SII-induced BRET signal. Because BRET changes result from changes in the distance and/or orientation of the donor and acceptor molecules, an increase versus a decrease in BRET ratio must arise from different positional changes of the Luc and YFP (13). In other words, directionally opposite changes in the BRET ratio (i.e., increase with Ang and decrease with SII) indicate different conformations of β-arrestin 2. To correlate β-arrestin recruitment to the receptor and conformational change in β-arrestin, we monitored the dose dependency of Ang- and SII-mediated BRET change. As shown in supporting information (SI) Fig. S1 A and B, an EC50 of 2.9 ± 1.4 × 10−9 M for Ang and 2.5 ± 1.9 × 10−6 M for SII was observed. These EC50 values for the conformational change in β-arrestin correspond well to the Kd of the receptor for Ang and SII (≈1 nM for Ang and ≈300 nM for SII) (10).
Fig. 1.
Conformational changes in β-arrestin 2 upon stimulation of AT1aR measured by intramolecular BRET. HEK-293 cells overexpressing wild type or DRY/AAY mutant of AT1aR were transiently transfected with β-arrestin 2 “double brilliance biosensor” (Luc–β-arr–YFP). (A) Cells were stimulated with Ang (1 μM) or SII (30 μM) for 30 min, and translocation of β-arrestin 2 biosensor was followed in real time by confocal microscopy. A representative image from three independent experiments is shown. (B and C) Changes in intramolecular BRET ratio upon stimulation of wild-type AT1aR (B) and DRY/AAY mutant of AT1aR (C) with Ang II (100 nM, 10 min) and SII (10 μM, 5 min) with or without pretreatment with the AT1R antagonist (ARB) valsartan (50 μM, 10 min). Data are the mean ± SD of six to eight independent experiments, each performed in triplicates. *, P < 0.05; **, P < 0.01; ***, P < 0.001—between basal and stimulated condition as analyzed by one-way ANOVA with Bonferroni's post-test.
Based on the previous finding that SII initiates exclusively β-arrestin but not G protein signaling, it is tempting to speculate that the two conformations of β-arrestin are associated with different functions. For example, one conformation associated with G protein-mediated signaling presumably promotes the desensitization function of β-arrestin (possibly reflected by an increase in BRET ratio), and the second would be responsible for inducing β-arrestin signaling (possibly reflected by a decrease in BRET ratio). The BRET signal upon Ang stimulation represents the sum of the BRET changes for these two conformations, whereas SII stimulation would give rise to only the second type of conformation. To explore this hypothesis, we used a biased mutant of AT1aR (DRY/AAY) (11). Alanine substitution of the first two amino acids of a conserved DRY motif in the second intracellular loop of AT1aR completely uncouples the receptor from Gαq/11 without affecting its capacity to recruit β-arrestins, and activate β-arrestin-dependent ERK signaling (11). If distinct conformations of β-arrestin are indeed responsible for desensitization and signaling as we propose, stimulation of the DRY/AAY mutant of AT1aR with either Ang or SII should lead to a decrease in intramolecular BRET ratio. Indeed, as shown in Fig. 1C, both Ang stimulation and SII stimulation lead to a decrease in the BRET ratio, suggesting that they both induce the same conformation in β-arrestin that promotes β-arrestin-mediated signaling. Again, pretreatment of cells with valsartan blocks the Ang- and SII-induced BRET signal. The molecular basis for the inability of AT1aR (DRY/AAY) mutant to induce G protein-mediated signaling is not yet clear. It is likely that both Ang and SII induce a similar conformation of this mutant receptor that is then recognized by β-arrestin and in turn results in a similar conformation in β-arrestin.
The data obtained with the AT1aR (DRY/AAY) mutant taken together with the data on wild-type AT1aR support the hypothesis that an increase in BRET ratio corresponds with a conformation of β-arrestin that can govern both desensitization and signaling function of β-arrestin, while a decrease in BRET represents a signaling conformation.
To rule out the possibility that changes in BRET ratio are due to intermolecular interaction between β-arrestin molecules brought together through dimerization (14) or aggregation at the plasma membrane, we coexpressed Luc–β-arr and β-arr–YFP under conditions that lead to comparable levels of fluorescence and luminescence as obtained in Luc–β-arr–YFP-expressing cells. As shown in Fig. S1C, upon coexpression of Luc–β-arr and β-arr–YFP, no statistically significant changes in BRET ratio were observed after Ang or SII stimulation, suggesting that ligand-induced changes in the intramolecular BRET signal result from conformational changes in β-arrestin and are not due to dimerization or aggregation at the plasma membrane.
Distinct Conformational Changes in β-Arrestin upon Activation of Wild-Type β2AR and a β-Arrestin-Biased Mutant, β2ARTYY.
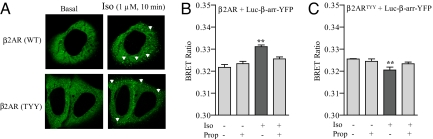
To investigate whether the occurrence of distinct conformations of β-arrestin is a general consequence of biased agonism at 7TMRs, we next asked whether similar patterns could be observed for other 7TMRs. The β2AR is another highly studied 7TMR that, similar to AT1aR, also exhibits both G protein-dependent and β-arrestin-dependent signaling. Although there are some indications of β-arrestin-biased ligands for β2AR (15, 16), their weak efficacy for β-arrestin recruitment and signaling limits their usefulness in our BRET assay system. However, based on evolutionary trace analysis, a mutant β2AR (T68F-Y132G-Y219A, referred to as β2ARTYY) has been designed (17) that, similar to the DRY/AAY mutant of AT1aR, does not couple to G proteins but exhibits only β-arrestin-mediated signaling (17). As shown in Fig. 2A, HEK-293 cells stably expressing either β2AR or β2ARTYY receptors exhibited plasma membrane translocation of the β-arrestin biosensor upon receptor stimulation in a “class A” pattern, i.e., transient interaction of β-arrestin with the receptor, reflected by rapid concentration of β-arrestin at the plasma membrane (12). To monitor the conformational changes in β-arrestin, we compared the changes in intramolecular BRET ratio upon isoproterenol stimulation of β2AR or β2ARTYY. Consistent with a previous report (9), stimulation of wild-type β2AR leads to an increase in the BRET ratio, indicating a conformational change in β-arrestin upon recruitment to the receptor (Fig. 2B). In contrast, isoproterenol stimulation of β2ARTYY resulted in a decrease in the intramolecular BRET ratio (Fig. 2C), similar to the data obtained for the DRY/AAY mutant of AT1aR upon Ang stimulation. For both β2AR and β2ARTYY, pretreatment of cells with propranolol, a β-adrenergic receptor antagonist, blocks the isoproterenol-induced BRET signal. We also analyzed the dose dependency of isoproterenol-induced conformational changes in β-arrestin for both the wild-type β2AR and the β2ARTYY. As shown in Fig. S2 A and B, an EC50 of 1 ± 1.8 × 10−7 M was observed for the wild-type β2AR and 6.7 ± 5.0 × 10−8 M for the β2ARTYY mutant. Neither isoproterenol nor propranolol induce any change in the intermolecular BRET signal upon coexpression of Luc–β-arr and β-arr–YFP, thus ruling out the possibility that the BRET signal arises from dimerization of β-arrestin (Fig. S2C).
Fig. 2.
Conformational changes in β-arrestin 2 upon stimulation of β2AR measured by intramolecular BRET. HEK-293 cells overexpressing β2AR or β2ARTYY mutant were transiently transfected with β-arrestin 2 double brilliance biosensor (Luc–β-arr–YFP). (A) Cells were stimulated with isoproterenol (1 μM, 10 min), and translocation of β-arrestin 2 biosensor was visualized by confocal microscopy. Images are representative of four independent experiments. (B and C) Changes in intramolecular BRET ratio upon stimulation of wild-type β2AR (B) and β2ARTYY mutant (C) by isoproterenol (1 μM, 10 min) with or without pretreatment with β2AR antagonist propranolol (10 μM, 10 min). Data are mean ± SD of seven independent experiments, each performed at least in triplicates. *, P < 0.05; **, P < 0.01—between basal and stimulated condition as analyzed by one-way ANOVA with Bonferroni's post-test.
Conformational Changes in β-Arrestin Are Directly Correlated with the Efficacies of β2AR Ligands.
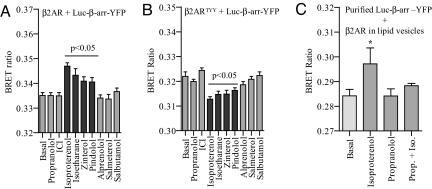
To correlate the efficacy of a given ligand at its cognate receptor with the conformational change in β-arrestin, we investigated seven additional ligands for the β2AR (in addition to isoproterenol and propranolol) with varying efficacies in terms of cAMP response and β-arrestin recruitment (18) in this BRET assay. We monitored the changes in BRET ratio upon stimulation of both the wild-type β2AR and β2ARTYY. As shown in Fig. 3 A and B, the conformational changes in β-arrestin induced by these ligands compare well with their efficacies. For example, Zinterol, Pindolol, and Isoetharine, which are partial agonists on β2AR but weaker than isoproterenol, give qualitatively similar but quantitatively weaker responses on both wild-type β2AR and β2ARTYY. Similarly, ICI, which is an inverse agonist, leads to no detectable change in BRET ratio. In addition, very weak partial agonists did not show any detectable change in BRET ratio. These data thus establish that the efficacy of ligands is directly correlated with the conformational change in β-arrestin.
Fig. 3.
Effect of different β2AR ligands with varying efficacies on the conformational changes in β-arrestin. HEK-293 cells overexpressing β2AR (A) or β2ARTYY (B) mutant were transiently transfected with β-arrestin 2 double brilliance biosensor (Luc–β-arr–YFP). Forty-eight hours after transfection, cells were stimulated with receptor-saturating concentration of different ligands for 10 min, and changes in intramolecular BRET ratio were monitored. Data are mean ± SD of four independent experiments, each performed in triplicates. P < 0.05 between basal and stimulated condition as analyzed by one-way ANOVA with Bonferroni's post-test. (C) Conformational change in β-arrestin upon direct interaction with β2AR. Purified Luc–β-arr–YFP was incubated with β2AR reconstituted in lipid vesicles with isoproterenol (1 μM), with propranolol (10 μM), or preincubated with propranolol followed by addition of isoproterenol (n = 4, two independent purifications of Luc–β-arr–YFP).
Direct Interaction Between Isolated β2AR and β-Arrestin Biosensor Leads to a Conformational Change in β-Arrestin.
To examine whether the conformational changes in β-arrestin result from a direct interaction with the receptor or represent a subsequent event in downstream signaling such as β-arrestin binding to other interaction partners, we performed in vitro BRET experiments with defined components (i.e., purified β-arrestin biosensor and purified β2AR reconstituted in lipid vesicles). As shown in Fig. 3C, incubation of β-arrestin biosensor with purified β2AR in the presence of isoproterenol results in an increase in BRET ratio. The increase in BRET ratio is abolished by preincubation of vesicles with the β2AR antagonist propranolol. These data thus suggest that the conformational change in β-arrestin, at least for the unbiased ligand isoproterenol, results from a direct interaction of β-arrestin with the receptor.
Distinct Conformational Changes in β-Arrestin upon Activation of PTH1R.
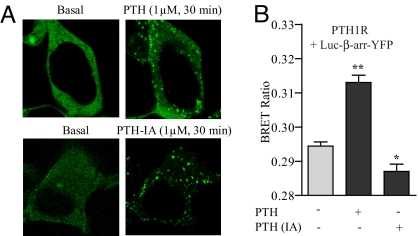
To further confirm the generality of distinct β-arrestin conformations in response to stimulation of the receptor with an unbiased or biased ligand, we next studied the parathyroid hormone receptor type 1 (PTH1R). We have previously reported that stimulation of PTH1R with PTH-(1–34) leads to both G protein-dependent and β-arrestin-dependent signaling, whereas stimulation with the PTH analogue [D-Trp12,Tyr34]PTH-(7–34), which is an inverse agonist (PTH-IA), results in only β-arrestin signaling (19). As shown in Fig. 4A, both PTH-(1–34) and PTH-IA stimulation resulted in “class B” recruitment of β-arrestin biosensor. To monitor the conformational changes in β-arrestin, we compared the changes in BRET ratio upon stimulation of PTH1R with either PTH or PTH-IA. As shown in Fig. 4B, stimulation of PTH1R with PTH resulted in an increase in BRET ratio, whereas PTH-IA resulted in a decrease. These data are analogous to those obtained for AT1aR upon Ang and SII stimulation and further confirm that β-arrestin adopts distinct conformations depending on the context of receptor stimulation. An EC50 of 1.0 ± 1.4 × 10−8 for PTH and 1.1 ± 1.9 × 10−8 for PTH-IA was calculated for the conformational change in β-arrestin (Fig. S3 A and B), which corresponds well with the affinity of these ligands for PTH1R. Stimulation of mock transfected (with pcDNA3.1) HEK-293 cells by PTH-(1–34) or PTH-IA did not induce any statistically significant change in BRET ratio (Fig. S3C). In addition, similar to the AT1aR and the β2AR systems, the changes in intramolecular BRET are not the result of β-arrestin dimerization because no detectable changes in intermolecular BRET ratio were observed upon stimulation of cells coexpressing Luc–β-arr and β-arr–YFP (Fig. S3D).
Fig. 4.
Conformational changes in β-arrestin upon stimulation of PTH1R measured by intramolecular BRET. HEK-293 cells overexpressing human PTH1R were transiently transfected with either β-arrestin double brilliance biosensor (Luc–β-arr–YFP). (A) Cells were stimulated with PTH (1–34) (1 μM, 30 min) and PTH-IA (10 μM, 30 min), and translocation of β-arrestin 2 biosensor was visualized by confocal microscopy. Images are representative of three independent experiments. (B) Changes in intramolecular BRET ratio upon stimulation of PTH1R by PTH (1–34) (100 nM, 10 min) and PTH-IA (1 μM, 10 min). Data are mean ± SD of six independent experiments, each performed at least in triplicates. *, P < 0.05; **, P < 0.01—between basal and stimulated condition as analyzed by one-way ANOVA with Bonferroni's post-test.
Taken together, the intramolecular BRET data obtained for these three different 7TMRs, using either biased ligands or biased receptor mutants, display a common pattern (i.e., increase for an unbiased ligand and decrease for a β-arrestin-biased ligand or a β-arrestin-biased receptor mutant), and thereby establish that functionally specific conformations adopted by β arrestin are likely to be a general feature for the 7TMRs (Fig. S4). However, mapping of finer structural details underlying these conformational changes in β-arrestin will require additional technologies. It is also plausible that these distinct conformations of β-arrestin direct the initial signaling versus internalization events of 7TMRs, although it remains to be established.
Conformational Changes in β-Arrestin Are Independent of Receptor Phosphorylation.
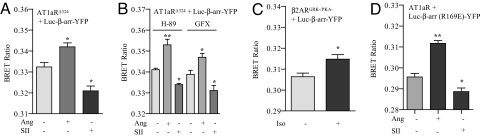
Activation-dependent conformational rearrangements in visual arrestin and β-arrestins appear to depend on the phosphorylation state of rhodopsin and of a peptide derived from the C terminus of the vasopressin receptor (V2R-pp), respectively (20–22). Therefore, we set out to determine the role of receptor phosphorylation in inducing the conformational changes in β-arrestin observed with the Luc–β-arr–YFP biosensor. We used a truncation mutant of AT1aR lacking the complete C terminus and, therefore, lacking all known GRK and PKC phosphorylation sites (AT1aR324). Stimulation of this truncation mutant by Ang or SII does not result in any detectable phosphorylation (23). This mutant, however, recruits β-arrestin, although in a weaker “class A” pattern (compared with “class B” pattern for the wild-type AT1aR), and exhibits a level of β-arrestin-dependent ERK signaling comparable to the wild-type receptor (3). As shown in Fig. 5A, stimulation of AT1aRΔ324 with Ang and SII lead to similar conformational changes in β-arrestin as observed for wild-type receptor. Even after pretreatment of cells with either H-89 or GFX (to inhibit PKA- and PKC-mediated phosphorylation of the receptor, respectively), the conformational changes in β-arrestin are still apparent (Fig. 5B). These data suggest that absence of receptor phosphorylation does not affect the conformational changes in β-arrestin as detected by the Luc–β-arr–YFP biosensor. However, it is important to note that the AT1aR324 may still contain some GRK phosphorylation sites in the intracellular loops. Indeed, sites for GRK phosphorylation are reported to be present in the third intracellular loops of the α-2 adrenergic receptor and others (24). To examine whether phosphorylation-independent conformational changes in β-arrestin exist for other receptors, we next used a β2AR mutant that lacks both GRK and PKA phosphorylation sites (β2ARGRK-/PKA-). This mutant receptor does not exhibit β-arrestin-mediated ERK signaling when tested at endogenous levels of β-arrestin (18), and β-arrestin recruitment to this mutant receptor could not be detected by confocal microscopy and cross-linking studies (18). However, a FRET-based approach revealed weak recruitment of β-arrestin to β2ARGRK-/PKA- upon high expression levels of exogenous β-arrestin (25). Therefore, in this experiment, the Luc–β-arr–YFP biosensor was expressed at an ≈4- to 5-fold higher level (as measured by luminescence and fluorescence levels) compared with the expression level for wild-type β2AR. As shown in Fig. 5C, stimulation of HEK-293 cells expressing β2ARGRK-/PKA- and high levels of the Luc–β-arr–YFP biosensor leads to similar conformational changes in β-arrestin as observed for the wild-type β2AR. Thus, taken together the data obtained for AT1aRΔ324 and β2ARGRK-/PKA- suggest that the conformational changes in Luc–β-arr–YFP biosensor upon stimulation of receptors with biased or unbiased ligands are independent of receptor phosphorylation.
Fig. 5.
Receptor phosphorylation independent conformational changes in β-arrestin 2. (A) HEK-293 cells were cotransfected with AT1aRΔ324 and Luc–β-arr–YFP, and stimulated with Ang II (100 nM, 10 min) and SII (10 μM, 5 min), and changes in intramolecular BRET ratio were measured. (B) Cells expressing AT1aRΔ324 mutant and Luc–β-arr–YFP were pretreated with H-89 (10 μM, 10 min) or GFX (10 μM, 10 min), and stimulated with Ang II (100 nM, 10 min) and SII (10 μM, 5 min), and changes in intramolecular BRET ratio were measured. (C) HEK-293 cells cotransfected with β2ARGRK-/PKA- mutant and Luc–β-arr–YFP were stimulated by isoproterenol (1 μM, 10 min), and changes in the intramolecular BRET ratio were measured. (D) HEK-293 cells overexpressing AT1aR were transfected with Luc–β-arr (R169E)-YFP and stimulated with Ang II (100 nM, 10 min) and SII (10 μM, 5 min), and changes in intramolecular BRET ratio were measured. Data are mean ± SD of four to six independent experiments, each performed at least in triplicates. *, P < 0.05; **, P < 0.01—between basal and stimulated condition as analyzed by one-way ANOVA with Bonferroni's post-test (A, B, and D) or paired t test (C).
To further investigate this intriguing finding, we monitored the conformational change in a phosphorylation-independent mutant of β-arrestin—i.e., β-arrestin (R169E). This mutant exists in a constitutively active conformation, presumably due to a disrupted polar core, and was originally reported to bind to the receptors irrespective of receptor phosphorylation (20). However, more rigorous analysis has shown that the R169E mutant of β-arrestin 1 is only partially independent of receptor phosphorylation (25). As shown in Fig. 5D, the conformational changes in Luc–β-arr (R169E)–YFP were essentially similar to the wild-type β-arrestin biosensor, in response to both the unbiased ligand Ang and the biased ligand SII. Along the same line, the conformational change in the Luc–β-arr (R169E)–YFP were similar to the wild-type β-arrestin biosensor upon stimulation of β2AR (Fig. S5A) and PTH1R (Fig. S5B). These data agree with the conclusions drawn from AT1aRΔ324 and β2ARGRK-/PKA- that the conformational changes in Luc–β-arr–YFP are not influenced by receptor phosphorylation.
As mentioned earlier, binding of a phosphopeptide corresponding to the C terminus of rhodopsin or V2R leads to a conformational rearrangement in visual arrestin or β-arrestins, respectively. It is plausible that the conformational rearrangement reported by limited proteolysis and this biosensor in live cells represent different subsets of β-arrestin conformations. To assess this possibility, we performed in vitro BRET experiments with purified Luc–β-arr–YFP. As shown in Fig. S5C, incubation of purified Luc–β-arr–YFP with the V2Rpp leads to no detectable change in BRET ratio. This observation agrees with our notion that the limited proteolysis assay and the Luc–β-arr–YFP report qualitatively different conformational changes in β-arrestin. The importance of receptor phosphorylation, especially the role of individual GRK isoforms, in β-arrestin-mediated regulation of 7TMRs has been intensively studied; however, a general pattern has not emerged (3, 26). Obviously, further studies such as mapping of receptor phosphorylation sites for specific GRKs, are necessary to obtain a definitive answer for the role of GRK phosphorylation in β-arrestin-mediated 7TMR regulation.
The data presented here suggest that discrete conformations of 7TMRs induced and/or stabilized by specific ligands, even in the absence of receptor phosphorylation, can promote distinct and functionally specific conformations in β-arrestins. These observations further extend our current understanding of activation-dependent conformational changes in arrestins as previously studied by limited proteolysis experiments for visual arrestin (20) and β-arrestins (21, 22). It seems likely that the conformational changes in β-arrestins observed in vitro by using limited proteolysis of purified proteins and the conformational changes reported here are qualitatively different and reflect different subsets of the repertoire of conformations that β-arrestins can adopt. Furthermore, it is also plausible that the β-arrestin conformations detected by the Luc–β-arr–YFP biosensor specifically represent those conformations that are essentially insensitive to receptor phosphorylation, and that there are additional conformations of β-arrestin that are indeed governed by receptor phosphorylation. Our data underscore the conformational complexity of β-arrestins and provide a possible mechanism underlying the biased agonism at 7TMRs—i.e., stimulation of 7TMRs by unbiased vs. biased ligands promotes distinct conformations of β-arrestins associated with specific functional outcomes. This also emphasizes the fact that fine tuning of 7TMR signaling and regulation is far more complicated than originally thought. Future challenges include understanding the molecular nature of β-arrestin conformational changes and determining whether there are any receptor-type-dependent selectivities in the repertoire of these conformations.
In conclusion, our data provide definitive evidence that β-arrestin can adopt multiple “active” conformations. Moreover, this finding also directly demonstrates that functionally specific receptor conformations can indeed be translated to downstream effectors such as β-arrestins thereby governing their functional specificity.
Materials and Methods
HEK-293 cells were grown in Eagle's MEM and transfected with FuGENE 6, using a standard protocol. BRET assays were performed as described in ref. 9. For a detailed description of experimental procedures, please refer to SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank Donna Addison and Elizabeth Hall for secretarial assistance and Drs. Ali Salahpour and Bernard Masri for technical help in the BRET experiments and for critical comments. A.K.S. thanks Dr. Chris Nelson for designing the schematic diagram shown in Fig. S4. This work was supported in part by National Institutes of Health Grants HL16037 and HL70631 (to R.J.L.). R.J.L. is an Investigator with the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804246105/DCSupplemental.
References
- 1.Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2007;9:639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 2.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by β-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 3.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. β-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 4.Kenakin T. Inverse, protean, and ligand-selective agonism: Matters of receptor conformation. FASEB J. 2001;15:598–611. doi: 10.1096/fj.00-0438rev. [DOI] [PubMed] [Google Scholar]
- 5.Kenakin T. Ligand-selective receptor conformations revisited: The promise and the problem. Trends Pharmacol Sci. 2003;24:346–354. doi: 10.1016/S0165-6147(03)00167-6. [DOI] [PubMed] [Google Scholar]
- 6.Violin JD, Lefkowitz RJ. β-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci. 2007;28:416–422. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Swaminath G, et al. Probing the β2 adrenoceptor binding site with catechol reveals differences in binding and activation by agonists and partial agonists. J Biol Chem. 2005;280:22165–22171. doi: 10.1074/jbc.M502352200. [DOI] [PubMed] [Google Scholar]
- 8.Vilardaga JP, Steinmeyer R, Harms GS, Lohse MJ. Molecular basis of inverse agonism in a G protein-coupled receptor. Nat Chem Biol. 2005;1:25–28. doi: 10.1038/nchembio705. [DOI] [PubMed] [Google Scholar]
- 9.Charest PG, Terrillon S, Bouvier M. Monitoring agonist-promoted conformational changes of β-arrestin in living cells by intramolecular BRET. EMBO Rep. 2005;6:334–340. doi: 10.1038/sj.embor.7400373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holloway AC, et al. Side-chain substitutions within angiotensin II reveal different requirements for signaling, internalization, and phosphorylation of type 1A angiotensin receptors. Mol Pharmacol. 2002;61:768–777. doi: 10.1124/mol.61.4.768. [DOI] [PubMed] [Google Scholar]
- 11.Wei H, et al. Independent β-arrestin 2 and G protein-mediated pathways for angiotensin II activation of extracellular signal-regulated kinases 1 and 2. Proc Natl Acad Sci USA. 2003;100:10782–10787. doi: 10.1073/pnas.1834556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oakley RH, et al. Differential affinities of visual arrestin, β arrestin 1, and β arrestin 2 for G protein-coupled receptors delineate two major classes of receptors. J Biol Chem. 2000;275:17201–17210. doi: 10.1074/jbc.M910348199. [DOI] [PubMed] [Google Scholar]
- 13.Andrews DL, Demidov AA. Resonance Energy Transfer. Chichester, UK: Wiley; 1999. [Google Scholar]
- 14.Storez H, et al. Homo- and hetero-oligomerization of β-arrestins in living cells. J Biol Chem. 2005;280:40210–40215. doi: 10.1074/jbc.M508001200. [DOI] [PubMed] [Google Scholar]
- 15.Azzi M, et al. β-arrestin-mediated activation of MAPK by inverse agonists reveals distinct active conformations for G protein-coupled receptors. Proc Natl Acad Sci USA. 2003;100:11406–11411. doi: 10.1073/pnas.1936664100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wisler J, et al. A unique mechanism of β-blocker action: Carvedilol stimulates β-arrestin signaling. Proc Natl Acad Sci USA. 2007;104:16657–16662. doi: 10.1073/pnas.0707936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shenoy SK, et al. β-arrestin-dependent, G protein-independent ERK1/2 activation by the β2 adrenergic receptor. J Biol Chem. 2006;281:1261–1273. doi: 10.1074/jbc.M506576200. [DOI] [PubMed] [Google Scholar]
- 18.Drake M, et al. β-arrestin-biased agonism at the β2-adrenergic receptor. J Biol Chem. 2007;283:5669–5676. doi: 10.1074/jbc.M708118200. [DOI] [PubMed] [Google Scholar]
- 19.Gesty-Palmer D, et al. Distinct β-arrestin- and G protein-dependent pathways for parathyroid hormone receptor-stimulated ERK1/2 activation. J Biol Chem. 2006;281:10856–10864. doi: 10.1074/jbc.M513380200. [DOI] [PubMed] [Google Scholar]
- 20.Gurevich VV, Gurevich EV. Arrestins: Ubiquitous regulators of cellular signaling pathways. Trends Pharmacol Sci. 2004;25:205–236. doi: 10.1186/gb-2006-7-9-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nobles KN, et al. The active conformation of β-arrestin 1: Direct evidence for the phosphate sensor in the N-domain and conformational differences in the active states of β-arrestins1 and -2. J Biol Chem. 2007;282:21370–21381. doi: 10.1074/jbc.M611483200. [DOI] [PubMed] [Google Scholar]
- 22.Xiao K, Shenoy SK, Nobles K, Lefkowitz RJ. Activation-dependent conformational changes in β-arrestin 2. J Biol Chem. 2004;279:55744–55753. doi: 10.1074/jbc.M409785200. [DOI] [PubMed] [Google Scholar]
- 23.Thomas WG, Qian H, Chang CS, Karnik S. Agonist-induced phosphorylation of the angiotensin II (AT(1A)) receptor requires generation of a conformation that is distinct from the inositol phosphate-signaling state. J Biol Chem. 2004;275:2893–2900. doi: 10.1074/jbc.275.4.2893. [DOI] [PubMed] [Google Scholar]
- 24.Liggett SB, et al. Sites in the third intracellular loop of the alpha 2A-adrenergic receptor confer short term agonist-promoted desensitization. Evidence for a receptor kinase-mediated mechanism. J Biol Chem. 1992;267:4740–4746. [PubMed] [Google Scholar]
- 25.Violin JD, Ren XR, Lefkowitz RJ. G-protein-coupled receptor kinase specificity for β-arrestin recruitment to the β2-adrenergic receptor revealed by fluorescence resonance energy transfer. J Biol Chem. 2006;281:20577–20588. doi: 10.1074/jbc.M513605200. [DOI] [PubMed] [Google Scholar]
- 26.Reiter E, Lefkowitz RJ. GRKs and β-arrestins: Roles in receptor silencing, trafficking and signaling. Trends Endocrinol Metab. 2006;17:159–165. doi: 10.1016/j.tem.2006.03.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.