Abstract
Retrograde signaling by endocannabinoids (eCBs) mediates a widely expressed form of long-term depression at excitatory and inhibitory synapses (eCB-LTD), involving a reduction in neurotransmitter release. In the hippocampus, eCB-LTD occurs at interneuron (IN)-pyramidal cell (PC) synapses (I-LTD), and its induction requires a presynaptic reduction of cAMP/PKA signaling resulting from minutes of type 1 cannabinoid receptor (CB1R) activation. Although repetitive activity of glutamatergic synapses initiates the eCB mobilization required for I-LTD, it is unclear whether CB1R-containing GABAergic terminals are passive targets of eCBs or whether they actively contribute to induction. Here, we show that the minutes-long induction period for I-LTD may serve as a window to integrate associated spontaneous activity in the same IN receiving the retrograde eCB signal. Indeed, reducing spontaneous IN firing blocked I-LTD, which could be rescued with extra stimulation of inhibitory afferents. Moreover, cell pair recordings showed that a single IN expressed LTD onto a PC only if it was active during eCB signaling. Several methods of disrupting presynaptic Ca2+ dynamics all blocked I-LTD, strongly suggesting that IN spikes regulate I-LTD by raising Ca2+ at the nerve terminal. Finally, inhibiting the Ca2+-activated phosphatase, calcineurin, fully blocked I-LTD, but blocking another phosphatase did not. Our findings support a model where both CB1R signaling and IN activity shift the balance of kinase and phosphatase activity in the presynaptic terminal to induce I-LTD.
Keywords: CA1, CB1, hippocampus, inhibition, LTD
Synaptic plasticity is characterized as presynaptic or postsynaptic depending on whether neurotransmitter release or a target neuron's sensitivity to the transmitter is modified. Neural and behavioral adaptations likely involve both types of plasticity, yet our understanding of presynaptic mechanisms lags far behind what we know about the postsynaptic side. This discrepancy is particularly evident when considering synaptic learning rules. As a prime example, the associative properties of LTP and LTD at the Schaffer collateral-CA1 pyramidal cell (Sch-CA1) synapse result from coincidence detection by postsynaptic NMDA receptors (NMDARs), Ca2+ influx, and the subsequent activation of kinase and phosphatase networks (1). For most forms of presynaptic plasticity, it is unknown whether they possess associative properties, let alone the identity of the molecular pathways involved.
Perhaps the most prevalent form of long-term presynaptic plasticity is endocannabinoid-mediated LTD (eCB-LTD), occurring in multiple brain areas at both excitatory and inhibitory synapses (for a recent review, see ref. 2). In the hippocampus, brief repetitive stimulation of Schaffer collaterals activates group I metabotropic glutamate receptors (mGluR-I) on CA1 PCs, promoting eCB release. The retrograde release of eCBs targets type 1 cannabinoid receptors (CB1Rs) on presynaptic GABAergic terminals, inducing a heterosynaptic LTD at inhibitory synapses (I-LTD) (3–5). CB1Rs are Gαi-coupled receptors whose suppression of cAMP/protein kinase A (PKA) signaling is required for I-LTD induction (6). Although excitatory activity initiates I-LTD induction, it is unclear whether inhibitory terminals bearing the CB1R are simply passive players in this process or whether I-LTD has an intrinsic associative property. Given that I-LTD strongly influences excitability and plasticity in CA1 PCs (7), an active contribution from INs would add a new fine-tuning mechanism to the Sch-CA1 synapse.
The mechanisms of eCB production leading to eCB-LTD, including I-LTD, are relatively well characterized (2). However, it is unclear whether CB1R activation alone is sufficient to trigger this form of plasticity (8, 9), but see refs. 10 and 11. Interestingly, eCB-LTD induction requires several minutes of CB1R activation (3, 9), a temporal window that may allow the integration of a relatively slow coincident signal at the presynaptic terminal (12). Here, we show that, in addition to CB1R activation, IN firing is required for I-LTD, and a single IN can integrate both signals to express plasticity. Furthermore, we found that, in addition to IN activity, I-LTD requires a rise in presynaptic Ca2+ and activation of a Ca2+-sensitive phosphatase, calcineurin.
Results
IN Firing Is Required for I-LTD Induction.
We considered three potential sources of IN activity that might contribute to hippocampal I-LTD during the minutes of CB1R activation: extracellular test stimulation used to evoke inhibitory postsynaptic currents (evIPSCs) and monitor synaptic strength, the spontaneous IN firing that likely underlies the spontaneous IPSCs (sIPSCs), or the theta burst stimulation (TBS) used for I-LTD induction. We first evaluated whether periodic test stimulation is necessary for I-LTD induction. In contrast to other forms of presynaptic LTD (10, 13), stopping stimulation after TBS had no bearing on the expression of I-LTD [see supporting information (SI) Fig. S1]. To assess the role of spontaneous IN firing in I-LTD, we recorded IPSCs in the presence of a low dose of the voltage-gated sodium channel blocker Tetrodotoxin (TTX; 10 nM), an approach successfully used by others in the visual cortex (14). We found that this dose reduced spontaneous IN firing and sIPSCs while preserving GABA release evoked by extracellular stimulation (Fig. S2). I-LTD was blocked under these recording conditions, indicating that, of the three types of inhibitory activity described above, TBS and periodic test stimulation are insufficient, on their own, to support plasticity (Fig. 1A; TBS alone, 5.4 ± 2.8%, n = 4). Rather, I-LTD induction may require spontaneous inhibitory activity.
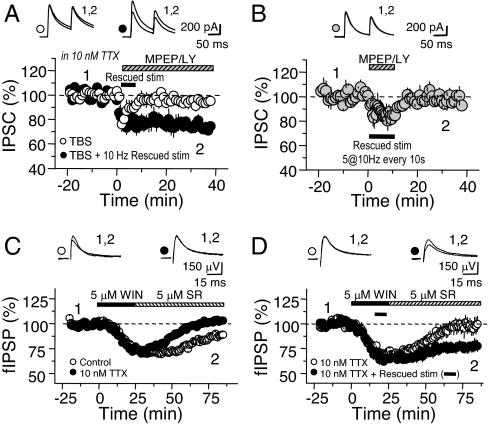
Fig. 1.
I-LTD induction requires both CB1R activation and spontaneous activity of interneurons in the hippocampus. (A) Reducing spontaneous IPSCs with a low dose of TTX (10 nM) blocked I-LTD induced by TBS even while maintaining .067 Hz of test stimulation after TBS. The loss of I-LTD in 10 nM TTX could be rescued by bursts of afferent activity with extracellular stimulation. mGluR-I antagonists (4 μM MPEP and 100 μM LY367385) were washed in at the time of I-LTD induction. (B) Ten minutes of rescue stimulation alone (as in A) does not induce I-LTD. (C) Application of the CB1R agonist WIN (5 μM, 25 min) chased with the CB1R antagonist SR 141716 (5 μM) induced a lasting depression of fIPSPs under normal recording conditions. Similar to I-LTD, this depression was abolished in 10 nM TTX. (D) In experiments where two independent pathways were being stimulated, long-term depression of fIPSPs, blocked by 10 nM TTX in one pathway, could be rescued in the other pathway by burst stimulation of afferents (as in A, indicated by solid line, applied during final 10 min in WIN). Averaged sample traces taken at times indicated by numbers are shown above each panel.
If TTX blocked I-LTD by reducing spontaneous inhibitory activity, supplying afferent activity during the critical period of induction, right after TBS, should rescue I-LTD. In the presence of 10 nM TTX, bursts of activity (five pulses at 10 Hz), delivered every 10 s in the 10 min after TBS fully rescued I-LTD (Fig. 1A; TBS + 10 Hz bursts, 23.7 ± 3.4%, n = 5; P < 0.01 vs. TBS alone). We perfused Group I mGluR antagonists immediately after TBS (4 μM MPEP and 100 μM LY367385) to ensure that rescue stimulation did not exert its effect through mGluR-mediated eCB release. As shown in Fig. 1B, 10 min of rescue stimulation did not trigger any plasticity on its own (Fig. 1B; 1.8 ± 3.2%, n = 4; P = 0.61 vs. baseline, one sample t test).
These results suggest that I-LTD relies on both CB1R activation and the firing of inhibitory afferents. We next tested whether these factors were sufficient for I-LTD by recording extracellular field IPSPs (fIPSPs), a noninvasive technique well suited to the long-term recordings required for effective washin and washout of the lipophilic CB1R agonist WIN55,212–2 (WIN) (6). fIPSPs were recorded in stratum pyramidale of CA1, where glutamatergic inputs (and, hence, eCB release) are unlikely to be recruited by extracellular stimulation, yet whose inhibitory inputs are as susceptible to I-LTD as those in stratum radiatum (3, 8). A 25-min application of 5 μM WIN persistently depressed fIPSPs, lasting 1 h after washout with a CB1R antagonist, SR141716 (5 μM). Similar to TBS-induced I-LTD, the WIN-induced I-LTD was completely abolished in the presence of 10 nM TTX (Fig. 1C; Control, 15.7 ± 3.0%, n = 7 slices, vs. 10 nM TTX, −1.9 ± 1.1%, n = 4 slices; P < 0.01). We once again attempted to rescue the effects of 10 nM TTX with afferent stimulation, this time using two independent inhibitory inputs in the same slice. Whereas 10 nM TTX still blocked the WIN-induced I-LTD on one pathway, stimulating pyramidal layer inhibitory afferents on the second pathway, during the final 10 min of WIN application, induced robust I-LTD (Fig. 1D; 10 nM TTX, 2.2 ± 7.0%, vs. 10 nM TTX + 10-Hz bursts, 23.5 ± 4.4%, n = 4 slices; P < 0.05). Taken together, these results strongly suggest that spontaneous IN firing plays a critical role in the induction of I-LTD.
Although useful, TTX and extracellular stimulation are indirect ways to control IN activity. So far, our results could be explained by the activity-dependent release of a neuromodulator that may facilitate I-LTD induction. We therefore performed paired recordings between single INs and CA1 PCs, permitting direct control of IN activity by injecting depolarizing or hyperpolarizing currents. Because only a fraction of INs form CB1R-positive synapses (15), we tested cell pairs for depolarization-induced suppression of inhibition (DSI), a form of short-term plasticity expressed only by CB1R-containing synapses (16). Consistent with previous reports (17, 18), we observed DSI in ≈20% of cell pairs. The actual sites of inhibitory synaptic contact are highly variable, making it difficult to predict which Schaffer collateral must be stimulated to produce eCB release close to the relevant IN-CA1 synapses. To circumvent this problem, we made use of our previous findings that transient mGluR-I activation (i.e., with the agonist DHPG) stimulates eCB release and induces a form of chemical LTD that entirely mimics TBS-induced I-LTD (3, 8). Furthermore, soma- and dendrite-targeting inhibitory inputs express an equivalent magnitude of DHPG-induced I-LTD (3, 8), allowing us to study INs independent of the subcellular localization of their axonal terminals.
After detecting a unitary IPSC (uIPSC), testing for DSI, and obtaining a stable baseline, 50 μM DHPG was applied to the slice for 10 min. During this time, the presynaptic IN was either fired (Fig. 2A) or held silent, including test stimulation (Fig. 2B). DHPG was washed out with 4 μM MPEP and 100 μM LY367385. In some cases, INs were successfully reconstructed after filling with biocytin (see Fig. S3). In DSI-positive cell pairs, pairing DHPG with IN firing triggered an LTD associated with a dramatic reduction of the average uIPSC amplitude and an increased number of transmission failures (Fig. 2A). Conversely, silencing the IN during DHPG application completely prevented LTD and had no effect on transmission failures (Fig. 2B).
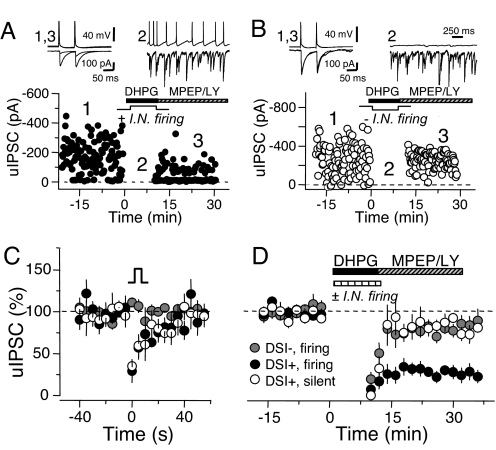
Fig. 2.
A single interneuron integrates eCB signaling with its own firing to express eCB-mediated LTD. (A and B) Individual experiments where unitary IPSCs (uIPSCs) were recorded from synaptically connected INs and PCs. In each case, transient eCB release was triggered with a 10-min bath application of the mGluR-I agonist DHPG (50 μM). In DSI+ cell pairs in CA1, DHPG application is coupled with either spontaneous IN firing (A) or silence (B) by depolarizing or hyperpolarizing the presynaptic IN. DHPG was chased with group I mGluR antagonists (4 μM MPEP and 100 μM LY367385) to ensure washout. Each representative trace shows IN membrane potential above the paired PC current for indicated time points. Average traces taken before and after DHPG application are shown (overlaid) at the top left of each panel; individual traces taken during DHPG application are shown at the top right. (C and D) Cell pairs were classified as DSI+ or DSI−, and the presynaptic IN was either fired or held silent during a 10-min DHPG application: (DSI+, silent, open circles; DSI+, firing, filled circles; DSI−, firing, gray circles). (C) The two groups of DSI+ INs showed a similar transient depression in response to PC depolarization, unlike DSI− INs. (D) Coupling DHPG application with IN firing in DSI+ cell pairs (DSI+, firing) yielded a robust depression lasting at least 35 min after DHPG washout. In contrast, silencing the IN of DSI+ cell pairs during DHPG (DSI+, silent) produced significantly less LTD. The residual depression observed in DSI+, silent cell pairs was indistinguishable from that measured in DSI−, firing cell pairs, a group not expected to express eCB-LTD.
The analysis of these experiments was performed as follows (Fig. 2C). Cell pairs were first divided into those showing DSI (DSI+) and those not showing DSI (DSI−). DSI+ cell pairs were further divided into either “DSI+, firing” or “DSI+, silent,” depending on the firing rate of the presynaptic IN during DHPG application. The DSI− group served as a control for CB1R-independent effects of the induction protocol and for potential uIPSC rundown (19). For this reason, INs in this group were fired during DHPG application (DSI−, firing; DSI: −11.1 ± 4.5%, n = 7). Both groups of DSI+ cell pairs showed a similar degree of DSI and, therefore, similar sensitivity to the short-term effects of eCBs (Fig. 2A; DSI+, firing: 70.7 ± 1.8%, n = 7 vs. DSI+, silent: 63.2 ± 6.9%, n = 6; P = 0.63). Yet, DHPG application produced a robust LTD of uIPSCs when INs were active, but not when INs were silent (Fig. 2B; DSI+, firing, 72.5 ± 7.5% vs. 17.1 ± 7.1%; P < 0.001). The small, residual depression observed in the DSI+, silent group is likely nonspecific and CB1R-independent because the DSI−, firing cell pairs showed a virtually identical endpoint (19.9 ± 6.3%, n = 7; P = 0.78 vs. DSI+, silent). In terms of sIPSC frequency (recorded from the pyramidal cell), all three groups shared similar starting points, as well as DHPG-induced increases, indicating comparable levels of mGluR-I-induced activity, and presumably eCB release, across groups (Fig. S4A). Current injection into INs of DSI+, firing and DSI−, firing groups was adjusted to yield firing frequencies in the range observed during in vivo recordings (20) (Fig. S4B). Finally, analysis of transmission failure rate and paired-pulse ratio, two measures of neurotransmitter release probability, showed that the DHPG-induced LTD of uIPSCs, like TBS-induced I-LTD, is expressed presynaptically as a long-lasting reduction of GABA release (Fig. S4 C and D). Altogether, we cannot account for the loss of LTD in DSI+ cell pair groups with any factor other than the firing status of the presynaptic IN. These cell pair recordings directly demonstrate that DSI+ INs regulate eCB-LTD through their own firing.
I-LTD Requires Presynaptic Ca2+.
IN firing may shape I-LTD by raising Ca2+ at the terminal. Fortunately, buffering postsynaptic Ca2+ does not change the magnitude of I-LTD (3)(also see Fig. S5A), allowing us to target presynaptic Ca2+ homeostasis through manipulations of the perfused bath solution. Our first strategy was simply to reduce the Ca2+ driving force by perfusing a Ca2+-free external solution during the induction period (see Methods). Lowered extracellular Ca2+ could compromise the ability of a TBS to drive eCB production, so we used DHPG to directly stimulate mGluRs-I. Co-application of 50 μM DHPG with a Ca2+-free extracellular recording solution failed to induce I-LTD, whereas, after recovery, a subsequent application of DHPG to the same cell in normal extracellular Ca2+ successfully triggered I-LTD (Fig. 3B; DHPG first application, 5.1 ± 2.7%, P = 0.10; DHPG second application, 25.1 ± 4.6%, n = 4, P = 0.01).
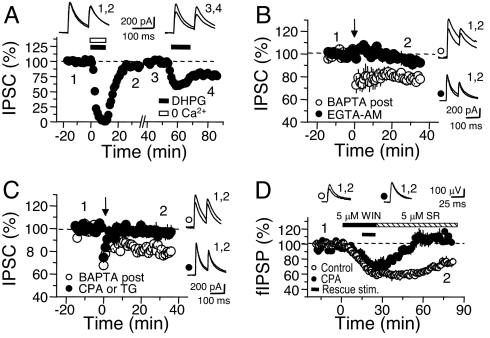
Fig. 3.
eCB-mediated LTD requires presynaptic, but not postsynaptic Ca2+. (A) DHPG does not induce I-LTD when coapplied with a Ca2+-free (0 Ca2+) recording solution. In the same cell, a subsequent DHPG application under normal recording conditions successfully triggers I-LTD. (B) Pretreating slices with 100 μM EGTA-AM and washing it out before TBS significantly attenuated I-LTD, compared with I-LTD induced with 20 mM BAPTA added to the recording pipette. (C) Depleting intracellular Ca2+ stores with either 30 μM bath-applied CPA or 2 μM TG prevents I-LTD induction. (D) Incubating slices with CPA also blocks the persistent depression of fIPSPs produced by WIN paired with extracellular burst stimulation (protocol as in Fig. 1D).
Lowering extracellular Ca2+ so significantly may have had unintended effects on I-LTD induction. So, in a second approach, we used the membrane-permeable Ca2+ chelator, EGTA-AM, to attenuate the presynaptic Ca2+ rise associated with repetitive IN firing (21). Slices were treated with 100 μM EGTA-AM for 30 min and then replaced into normal extracellular solution just before collecting a baseline for I-LTD. TBS, applied between 15 and 25 min after EGTA-AM washout, produced significantly less I-LTD compared with interleaved experiments in which PCs were loaded with 20 mM BAPTA (Fig. 3B; EGTA-AM, 5.9 ± 3.7%, n = 10 vs. BAPTA post, 21.4 ± 2.6%, n = 5, P < 0.05). EGTA-AM may have interfered with I-LTD induction by reducing IN excitability. To address this possibility, we monitored spontaneous IN firing with extracellular recordings under the same conditions used to measure I-LTD; 100 μM EGTA-AM had no effect on the spontaneous firing of individual INs, although over the same time period (separate experiments), EGTA-AM had depressed evIPSCs to a steady-state (Fig. S5B). Alternately, EGTA-AM may have interfered with I-LTD induction by reducing glutamate-driven eCB release or occluded I-LTD by reducing GABAergic release. However, we previously reported that adding low doses of Cd2+ or reducing the extracellular Ca2+/Mg2+ ratio, both manipulations reducing synaptic transmission to a similar extent as with EGTA-AM, rendered normal I-LTD (6, 7).
In a third approach, we interfered with presynaptic Ca2+ by incubating slices in either cyclopiazonic acid (CPA) or thapsigargin (TG), two blockers of the smooth endoplasmic reticulum Ca2+ ATPase (SERCA). Presynaptic Ca2+ stores are known to regulate neurotransmitter release at several central synapses, especially in response to repetitive activity, and they mediate several forms of synaptic plasticity (for a recent review, see ref. 22). Slices were incubated in either 30 μM CPA or 2 μM TG for 30–60 min, and experiments were performed in the continuous presence of the same compound to fully deplete presynaptic Ca2+ stores (23). Both of these treatments blocked TBS-induced I-LTD (Fig. 3D; CPA or TG, 3.9 ± 3.0%, n = 8 vs. BAPTA post, 19.8 ± 1.4%, n = 6; P < 0.001). The SERCA blockers did not significantly change the average frequency or amplitude distribution of sIPSCs (Fig. S5C), arguing against a simple reduction of IN activity as the mechanism of I-LTD blockade. In both sets of experiments (Fig. 3 B and C), the drug treatments may have masked I-LTD by modifying the CB1R's efficacy. However, slices treated with a mixture of all three compounds (100 μM EGTA-AM, 30 μM CPA, and 2 μM TG) showed a similar sensitivity to the CB1R agonist, WIN (Fig. S6). Finally, the SERCA blockers may have disrupted eCB production in an unexpected way. To short-cut eCB production during induction, we triggered I-LTD by direct activation of CB1Rs with WIN (as shown in Fig. 1D). We found that WIN failed to induce I-LTD in slices incubated in CPA (Fig. 3D; control: 75.2 ± 2.8%, n = 5; CPA, 107.1 ± 5.5%, n = 7; P = 0.0002), supporting the notion that blocking presynaptic Ca2+ stores interferes with I-LTD induction. Altogether, these four sets of experiments strongly suggest that presynaptic Ca2+ has an essential, dynamic function in I-LTD.
I-LTD Requires Calcineurin.
We recently found that CB1R triggers I-LTD by inhibiting presynaptic cAMP/PKA signaling. This down-regulation, in turn, targets the vesicle release machinery, in particular RIM1α (6), a protein involved in several forms of PKA-dependent plasticity (24). In this model, CB1R activation shifts the balance of kinase/phosphatase activity, implying the involvement of a serine/threonine phosphatase to complement PKA activity. Of the known phosphatases, relatively few act at serine/threonine residues (25). Among these phosphatases, calcineurin (CaN, or protein phosphatase 2B/PP2B) is highly expressed in neurons, is activated by Ca2+, and has a well described function in postsynaptic forms of synaptic plasticity (26, 27).
We hypothesized that IN firing raises presynaptic Ca2+ and activates CaN to consolidate I-LTD. A critical prediction of this hypothesis is that blocking CaN should prevent I-LTD even if inhibitory afferents are sufficiently active. We therefore tested I-LTD in two different CaN inhibitors, using rescue stimulation after the TBS (as in Fig. 1A). Even with this induction protocol, incubating slices for 1–2 h in either 50 μM FK506 or 25 μM Cyclosporin A (CyA), both potent, selective blockers of CaN, completely blocked I-LTD (Fig. 4A; FK506, 4.9 ± 2.0%, n = 7 vs. solvent, 22.4 ± 3.5%, n = 5, P < 0.01) (Fig. S7A; CyA, −1.7 ± 5.5%, n = 6, vs. solvent, 21.7 ± 4.5%, n = 6, P < 0.01). To rule out a postsynaptic role for CaN in I-LTD, I-LTD also was tested with 50 μM FK506 delivered directly to the postsynaptic cell via the recording pipette. We verified that this procedure could reliably inhibit postsynaptic CaN by assaying LTD of AMPA receptors in CA1 PCs (E-LTD), a form of plasticity known to depend on this phosphatase (28). Blocking postsynaptic CaN fully blocked E-LTD (Fig. S7B), but did not affect I-LTD (Fig. 4A; FK506 post, 23.3 ± 1.3%, n = 5 vs. solvent; P = 0.84), strongly suggesting that the requirement for CaN in I-LTD is presynaptic. Finally, we examined whether the blockade of CaN can affect evoked GABA release. However, bath application of 25 μM CyA had no effect on basal transmission (Fig. S7C), suggesting that CaN alone is unable to increase evoked GABA release.
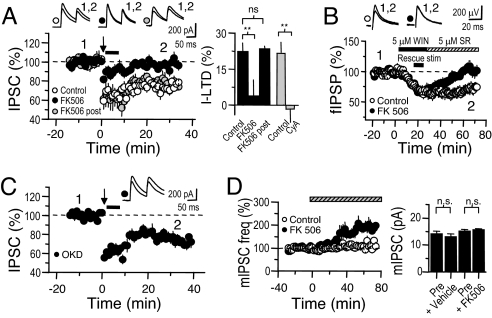
Fig. 4.
I-LTD requires activation of a presynaptic Ca2+-dependent phosphatase. (A) In slices pretreated for 1–2 h with the CaN blocker FK506 (50 μM), TBS (arrow) failed to induce I-LTD even with rescue stimulation after TBS (bar), as in Fig. 1. This protocol produced normal I-LTD both with FK506 in the postsynaptic pipette (FK post) and in the solvent alone. Summary bar graph at right for I-LTD in FK506 (bath applied and in postsynaptic pipette), as well as in CyA, compared with appropriate solvent controls for each drug. Time course for I-LTD in CyA shown in Fig. S7A. (B) Similarly, WIN paired with bursts of extracellular stimulation fails to persistently depress fIPSPs in the presence of FK506 (protocol as in Fig. 1D). (C) Okadaic acid (1 μM), which inhibits PP1/2A, had no effect on the magnitude of I-LTD. (D) (Left) mIPSC frequency is significantly increased when the CaN blocker FK506 (50 μM) is washed in. (Right) Average mIPSC amplitude remained constant over this time period. Vehicle treatment did not affect either mIPSC frequency or amplitude (see SI Methods).
Because I-LTD induction is a multistep process, treating slices with these inhibitors may have blocked I-LTD induction upstream of the inhibitory terminal. However, incubation in a mixture of protein phosphatase inhibitors (PPIs; same doses as in Fig. 4) had no effect on slices' sensitivity to WIN (Fig. S6), and CaN inhibitors do not affect DSI (17), another index of CB1R sensitivity that we independently confirmed (Fig. S7D). Furthermore, available evidence indicates that PPIs do not depress glutamatergic transmission (25) and do not interfere with mGluR-I function per se (29, 30). To verify that factors upstream of CB1R cannot account for the CaN inhibitors' block of I-LTD, we tested whether WIN, coupled with rescue stimulation, could trigger I-LTD in the presence of a CaN inhibitor, FK506. Despite producing identical acute effects, we observed no WIN-induced I-LTD in slices incubated with FK506 (Fig. 4B; control: 71.4 ± 7.5%, n = 6; FK506, 105.6 ± 5.92, n = 5; P = 0.0073).
We considered the possibility that any phosphatase could potentially cooperate with CB1R signaling to induce I-LTD. That is, CaN may only be involved to the extent that it is responsible for some fraction of constitutive dephosphorylation. In this case, blocking another serine/threonine phosphatase not regulated by Ca2+ should be equally effective in blocking I-LTD. We performed the same experiment as in Fig. 4A, now using 1 μM okadaic acid (OKD), a potent inhibitor of PP1/2A. In contrast to CaN blockers, OKD did not affect I-LTD induction (Fig. 4C, I-LTD: 23.9 ± 3.7%, n = 6; P = 0.77 vs. solvent control for FK506). We confirmed that this dose of OKD effectively blocks PP1/2A by once again testing E-LTD, which, in addition to its sensitivity to CaN inhibitors, also requires PP1/2A (Fig. S7B) (28). Finally, if CaN regulates I-LTD, it should interact with GABAergic release machinery. We applied a CaN inhibitor, FK506 (50 μM), while recording miniature IPSCs (mIPSCs) from a CA1 PC. Over the course of 90 min, FK506 produced a slow, but significant enhancement of mIPSC frequency without affecting average mIPSC amplitude (Fig. 4D; frequency, pre- 5.4 ± 0.9 Hz vs. post-FK506, 10.6 ± 0.7 Hz, n = 4, P < 0.01; amplitude, pre- 15.2 ± 0.5 pA vs. post-FK506, 15.7 ± 0.4 pA, n = 4, P = 0.67). Similar results were obtained with CyA (see SI Methods). The selective enhancement of mIPSC frequency suggests that CaN is present and functional at GABAergic terminals in CA1 (but see ref. 31) and that CaN can interact with GABAergic release machinery, downstream calcium influx. Together, our results show that constitutive phosphatase activity does not consolidate I-LTD, but, rather, IN firing regulates a presynaptic Ca2+-activated phosphatase (CaN) that complements CB1R signaling to induce I-LTD.
Discussion
Our major finding is that INs actively regulate I-LTD induction at their synapses with CA1 PCs. We propose that IN firing and CB1R activation converge through their complementary effects on CaN and PKA, respectively. Together, these signals change the phosphorylation state of a release machinery component, such as RIM1α, resulting in the expression of I-LTD. The ability of a synapse to integrate multiple signals (associativity) is a critical computational ability most often understood in terms of coincidence detection at excitatory synapses mainly via NMDAR. However, I-LTD occurs at inhibitory synapses, is NMDAR-independent, and its induction requires heterosynaptic retrograde signaling by eCBs.
Mechanistically, I-LTD resembles two other forms of presynaptically expressed LTD, one at mossy fiber to CA3 pyramidal cell synapses, the other, an eCB-mediated LTD, at dorsal corticostriatal synapses. Like I-LTD, these two are NMDAR-independent, and induction reportedly has a dual requirement for both presynaptic activity and presynaptic metabotropic signaling: Group II mGluRs at mossy fiber synapses (32) and CB1Rs at corticostriatal ones (33). Although all three forms of LTD require minutes of metabotropic stimulation (3, 9, 34), it appears that this factor alone is insufficient for induction (8, 9, 32). Both mossy fiber and striatal LTD are blocked by transiently halting test stimulation for some minutes during the application of the agonists for their respective metabotropic receptors (10, 13). Because these studies were entirely performed with bulk afferent simulation, the role of evoked test stimuli, critical for consolidating these forms of LTD, could either be to (i) supply activity at the test synapse, or (ii) release a cofactor for induction (e.g., a heterosynaptic neuromodulator). This latter concern is not trivial, especially in light of recent findings that eCB-dependent striatal LTD may involve a complex interplay between dopamine receptors and cholinergic INs, in addition to events at the glutamatergic corticostriatal synapse (35, 36). Our IN-PC pair experiments provide direct experimental evidence that association of CB1Rs and presynaptic activity at the same synapse is necessary to induce I-LTD. We cannot discard, however, the possibility (albeit small) that processes taking place outside of the pre- or postsynaptic neurons also could contribute to I-LTD.
eCB-LTD induced by spike timing protocols has been reported to require presynaptic activity (37–39). In contrast, for I-LTD, the postsynaptic CA1 pyramidal cell need never even fire a spike during the induction period (3). Rather, a single bout of high-frequency stimulation of Schaffer collaterals can result in several minutes of eCB release (40), setting the stage for the slow integration of presynaptic activity we describe here. Unlike I-LTD, spike timing eCB-LTD, at least in the neocortex (37, 39), appears to share a requirement for the activation of presynaptic NMDARs. The involvement of a presynaptic NMDAR strongly suggests (but does not demonstrate) that presynaptic Ca2+ influx is required during induction, consistent with studies of mossy fiber LTD (13) and corticostriatal LTD (10), which also have found a role for presynaptic Ca2+. However, the actual mechanism whereby Ca2+ influx and metabotropic signaling converge has not been addressed previously. Our present results strongly suggest that CaN, a Ca2+-activated phosphatase involved in both exocytosis and endocytosis of neurotransmitters (25, 41, 42) and in several forms of long-term plasticity (26, 27), complements CB1R activation at the presynaptic terminal.
Thus, the prolonged eCB release occurring after a high-frequency stimulus creates a temporal window where the CB1R-containing terminals are especially sensitive to incoming action potentials (APs). This mechanism could account for an unusual feature of I-LTD, where minutes of CB1R activation are required after the induction stimulus (3). This form of associative plasticity integrates two signals over minutes, whereas other well studied forms of coincidence detection operate on the scale of milliseconds (1, 43). This type of associativity may limit the impact of widespread eCB release. Indeed, our previous observations showed that eCBs produced during I-LTD induction are effective over a 10-μm radius (7) where dozens of inhibitory inputs may synapse (44). The presynaptic activity requirement for the induction of I-LTD may contribute to select inhibitory inputs expressing this form of plasticity.
During some behavioral states, it seems that the two conditions for generating I-LTD, CB1R activation and IN firing, can be independently regulated. In vivo recordings of cholecystokinin (CCK)-containing INs (which almost exclusively express the CB1R) show that, during intermittent bursts of CA1 activity known as sharp-wave ripples (SWR), these INs react in an episode-dependent manner, sometimes laying silent and at other times firing (20). This complex pattern is consistent with independent regulatory mechanisms governing SWRs, which may result in eCB release, and CCK+ IN firing, which may be controlled by subcortical afferents. Projections from septal nuclei, dorsal raphe nuclei, and the ventral tegmental area are all known to target CCK+ INs (45). Identifying the relevant factors that modulate the spontaneous activity of these INs is a crucial next step to understanding the role of I-LTD in CA1 plasticity.
Methods
Experiments were performed on Sprague–Dawley rats (P17–P25; Charles River Laboratories). Transverse hippocampal slices were prepared as described previously (3). Animals were killed by decapitation in accordance with institutional regulations. Then 400-μm-thick slices were cut on a vibratome (Dosaka) in ice-cold extracellular solution containing 215 mM sucrose, 2.5 mM KCl, 20 mM glucose, 26 mM NaHCO3, 1.6 mM NaH2PO4, 1 mM CaCl2, 4 mM MgSO4, and 4 mM MgCl2. The cutting medium was gradually switched to the recording solution (ACSF) that contained 124 mM NaCl, 2.5 mM KCl, 10 mM glucose, 26 mM NaHCO3, 1 mM NaH2PO4, 2.5 mM CaCl2, and 1.3 mM MgSO4. The 0 Ca2+ solution (Fig. 3A) contained 0 mM CaCl2, 6 mM MgSO4, and 0.1 mM EGTA. Slices were kept at room temperature for at least 1.5 h before recording. Cutting and recording solutions were saturated with 95% O2 and 5% CO2 (pH 7.4). Experiments were performed at 25.0 ± 0.1°C.
CA1 PCs were blind-patched and recorded in whole-cell voltage clamp mode. For recording all IPSCs, hippocampal slices were completely submerged and continuously superfused at a flow rate of 2 ml/min with ACSF containing NMDA and AMPA/Kainate receptor antagonists (25 μM d-APV and 10 μM NBQX). sIPSCs and evIPSCs were recorded at +10 mV with an internal solution containing 123 mM cesium gluconate, 1 mM CaCl2, 10 mM EGTA, 10 mM HEPES, 10 mM glucose, 5 mM ATP, and 0.4 mM GTP (pH 7.2; 280–290 mOsm). For some experiments (Fig. 3 B and C and Fig. S5), 20 mM BAPTA was added to this internal solution. uIPSCs were recorded at −60 mV with an internal solution containing CsCl instead of CsGluconate (Fig. 2 and Fig. S4). Series resistance (Rs, typically 8–15 MΩ) was continuously monitored throughout each experiment, with a −5-mV, 80-ms command pulse delivered before each stimulus pair; cells with >10% change in Rs were excluded from analysis.
For evIPSCs, paired, monopolar, square-pulse stimuli (200-μs pulse width, 100-ms interval) were delivered through a patch-type pipette filled with ACSF and placed in the middle third of s. radiatum. Stimulation pipette tips were broken to ≈20 μm, and we adjusted the stimulus intensity to evoke IPSCs of comparable amplitudes across experiments (0.3–1.3 nA). evIPSCs were monitored every 15–20 s. The 3- to 5-s epochs were concurrently obtained for sIPSC analysis. I-LTD was induced after a stable baseline either by a TBS consisting of a series of 10 bursts of five stimuli (100 Hz within the burst, 200-ms interburst interval, repeated four times, 5 s apart) or by a 10-min application of 50 μM DHPG. I-LTD magnitude was quantified by averaging IPSC amplitudes for 10 min before and 20 min after TBS or DHPG.
For IN-PC cell pair recordings, IN somata spanning the inner half of s. radiatum of CA1 were morphologically identified, patched under visual guidance, and recorded in current clamp mode (without current injection, unless otherwise stated) with an internal solution containing 135 mM KMeSO3, 5 mM KCl, 1 mM CaCl2, 5 mM EGTA-Na, 10 mM HEPES, 10 mM glucose, 5 mM ATP, and 0.4 mM GTP (pH 7.2; 280–290 mOsm). Resting membrane potential ranged from −55 to −75 mV, and input resistance (Ri) from 150 to 500 MΩ. uIPSC amplitude was tested with a pair of APs evoked by a 2-ms square command pulse (0.8–1.5 nA), separated by 75 ms. CA1 PCs were recorded as described above. For further cell pair recording details, see SI Methods.
Extracellular field EPSPs and IPSPs were recorded as described previously (6). See SI Methods for details of extracellular spike recordings. Whole-cell recordings were performed with a MultiClamp 700A amplifier (Axon Instruments) whose output signals were filtered at 3 kHz. Data were digitized at 5 kHz and analyzed online by using customized software for IgorPro (Wavemetrics). Results are reported as mean ± SEM. All statistical comparisons were made with independently run control groups unless otherwise noted. Statistical analyses were performed by using a two-tailed, independent sample Student's t test, except for sIPSC amplitude and interevent interval distributions (Kolmogorov–Smirnov test). For information about chemicals used in this study, see SI Methods.
Supplementary Material
Acknowledgments.
We thank Chiayu Chiu for valuable technical assistance and Alexander Harris for helpful comments. This work was supported by National Institutes of Health/National Institute on Drug Abuse grants, a National Institutes of Health training grant (to B.D.H.), The Pew Scholars Program in the Biomedical Sciences, and the National Alliance for Research on Schizophrenia and Depression.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. R.C.M. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0711880105/DCSupplemental.
References
- 1.Malenka RC, Bear MF. LTP and LTD: An embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 2.Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci. 2006;29:37–75. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- 3.Chevaleyre V, Castillo PE. Heterosynaptic LTD of hippocampal GABAergic synapses: A novel role of endocannabinoids in regulating excitability. Neuron. 2003;38:461–472. doi: 10.1016/s0896-6273(03)00235-6. [DOI] [PubMed] [Google Scholar]
- 4.Kang-Park MH, Wilson WA, Kuhn CM, Moore SD, Swartzwelder HS. Differential sensitivity of GABAA receptor-mediated IPSCs to cannabinoids in hippocampal slices from adolescent and adult rats. J Neurophysiol. 2007;98:1223–1230. doi: 10.1152/jn.00091.2007. [DOI] [PubMed] [Google Scholar]
- 5.Zhu PJ, Lovinger DM. Persistent synaptic activity produces long-lasting enhancement of endocannabinoid modulation and alters long-term synaptic plasticity. J Neurophysiol. 2007;97:4386–4389. doi: 10.1152/jn.01228.2006. [DOI] [PubMed] [Google Scholar]
- 6.Chevaleyre V, et al. Endocannabinoid-mediated long-term plasticity requires cAMP/PKA signaling and RIM1alpha. Neuron. 2007;54:801–812. doi: 10.1016/j.neuron.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chevaleyre V, Castillo PE. Endocannabinoid-mediated metaplasticity in the hippocampus. Neuron. 2004;43:871–881. doi: 10.1016/j.neuron.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 8.Edwards DA, Kim J, Alger BE. Multiple mechanisms of endocannabinoid response initiation in hippocampus. J Neurophysiol. 2006;95:67–75. doi: 10.1152/jn.00813.2005. [DOI] [PubMed] [Google Scholar]
- 9.Ronesi J, Gerdeman GL, Lovinger DM. Disruption of endocannabinoid release and striatal long-term depression by postsynaptic blockade of endocannabinoid membrane transport. J Neurosci. 2004;24:1673–1679. doi: 10.1523/JNEUROSCI.5214-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singla S, Kreitzer AC, Malenka RC. Mechanisms for synapse specificity during striatal long-term depression. J Neurosci. 2007;27:5260–5264. doi: 10.1523/JNEUROSCI.0018-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kreitzer AC, Malenka RC. Dopamine modulation of state-dependent endocannabinoid release and long-term depression in the striatum. J Neurosci. 2005;25:10537–10545. doi: 10.1523/JNEUROSCI.2959-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duguid I, Sjostrom PJ. Novel presynaptic mechanisms for coincidence detection in synaptic plasticity. Curr Opin Neurobiol. 2006;16:312–322. doi: 10.1016/j.conb.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Tzounopoulos T, Janz R, Sudhof TC, Nicoll RA, Malenka RC. A role for cAMP in long-term depression at hippocampal mossy fiber synapses. Neuron. 1998;21:837–845. doi: 10.1016/s0896-6273(00)80599-1. [DOI] [PubMed] [Google Scholar]
- 14.Liu HN, et al. Presynaptic activity and Ca2+ entry are required for the maintenance of NMDA receptor-independent LTP at visual cortical excitatory synapses. J Neurophysiol. 2004;92:1077–1087. doi: 10.1152/jn.00602.2003. [DOI] [PubMed] [Google Scholar]
- 15.Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- 16.Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- 17.Wilson RI, Kunos G, Nicoll RA. Presynaptic specificity of endocannabinoid signaling in the hippocampus. Neuron. 2001;31:453–462. doi: 10.1016/s0896-6273(01)00372-5. [DOI] [PubMed] [Google Scholar]
- 18.Glickfeld LL, Scanziani M. Distinct timing in the activity of cannabinoid-sensitive and cannabinoid-insensitive basket cells. Nat Neurosci. 2006;9:807–815. doi: 10.1038/nn1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diana MA, Marty A. Characterization of depolarization-induced suppression of inhibition using paired interneuron–Purkinje cell recordings. J Neurosci. 2003;23:5906–5918. doi: 10.1523/JNEUROSCI.23-13-05906.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klausberger T, et al. Complementary roles of cholecystokinin- and parvalbumin-expressing GABAergic neurons in hippocampal network oscillations. J Neurosci. 2005;25:9782–9793. doi: 10.1523/JNEUROSCI.3269-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hefft S, Jonas P. Asynchronous GABA release generates long-lasting inhibition at a hippocampal interneuron-principal neuron synapse. Nat Neurosci. 2005;8:1319–1328. doi: 10.1038/nn1542. [DOI] [PubMed] [Google Scholar]
- 22.Collin T, Marty A, Llano I. Presynaptic calcium stores and synaptic transmission. Curr Opin Neurobiol. 2005;15:275–281. doi: 10.1016/j.conb.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Galante M, Marty A. Presynaptic ryanodine-sensitive calcium stores contribute to evoked neurotransmitter release at the basket cell-Purkinje cell synapse. J Neurosci. 2003;23:11229–11234. doi: 10.1523/JNEUROSCI.23-35-11229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaeser PS, Sudhof TC. RIM function in short- and long-term synaptic plasticity. Biochem Soc Trans. 2005;33:1345–1349. doi: 10.1042/BST0331345. [DOI] [PubMed] [Google Scholar]
- 25.Sim AT, Baldwin ML, Rostas JA, Holst J, Ludowyke RI. The role of serine/threonine protein phosphatases in exocytosis. Biochem J. 2003;373:641–659. doi: 10.1042/BJ20030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mansuy IM. Calcineurin in memory and bidirectional plasticity. Biochem Biophys Res Commun. 2003;311:1195–1208. doi: 10.1016/j.bbrc.2003.10.046. [DOI] [PubMed] [Google Scholar]
- 27.Winder DG, Sweatt JD. Roles of serine/threonine phosphatases in hippocampal synaptic plasticity. Nat Rev Neurosci. 2001;2:461–474. doi: 10.1038/35081514. [DOI] [PubMed] [Google Scholar]
- 28.Mulkey RM, Herron CE, Malenka RC. An essential role for protein phosphatases in hippocampal long-term depression. Science. 1993;261:1051–1055. doi: 10.1126/science.8394601. [DOI] [PubMed] [Google Scholar]
- 29.Li ST, et al. Calcineurin plays different roles in group II metabotropic glutamate receptor- and NMDA receptor-dependent long-term depression. J Neurosci. 2002;22:5034–5041. doi: 10.1523/JNEUROSCI.22-12-05034.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oliet SH, Malenka RC, Nicoll RA. Two distinct forms of long-term depression coexist in CA1 hippocampal pyramidal cells. Neuron. 1997;18:969–982. doi: 10.1016/s0896-6273(00)80336-0. [DOI] [PubMed] [Google Scholar]
- 31.Sik A, Hajos N, Gulacsi A, Mody I, Freund TF. The absence of a major Ca2+ signaling pathway in GABAergic neurons of the hippocampus. Proc Natl Acad Sci USA. 1998;95:3245–3250. doi: 10.1073/pnas.95.6.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yokoi M, et al. Impairment of hippocampal mossy fiber LTD in mice lacking mGluR2. Science. 1996;273:645–647. doi: 10.1126/science.273.5275.645. [DOI] [PubMed] [Google Scholar]
- 33.Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi K, Manabe T, Takahashi T. Presynaptic long-term depression at the hippocampal mossy fiber-CA3 synapse. Science. 1996;273:648–650. doi: 10.1126/science.273.5275.648. [DOI] [PubMed] [Google Scholar]
- 35.Wang Z, et al. Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron. 2006;50:443–452. doi: 10.1016/j.neuron.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 36.Centonze D, Gubellini P, Pisani A, Bernardi G, Calabresi P. Dopamine, acetylcholine and nitric oxide systems interact to induce corticostriatal synaptic plasticity. Rev Neurosci. 2003;14:207–216. doi: 10.1515/revneuro.2003.14.3.207. [DOI] [PubMed] [Google Scholar]
- 37.Sjostrom PJ, Turrigiano GG, Nelson SB. Neocortical LTD via coincident activation of presynaptic NMDA and cannabinoid receptors. Neuron. 2003;39:641–654. doi: 10.1016/s0896-6273(03)00476-8. [DOI] [PubMed] [Google Scholar]
- 38.Tzounopoulos T, Rubio ME, Keen JE, Trussell LO. Coactivation of pre- and postsynaptic signaling mechanisms determines cell-specific spike-timing-dependent plasticity. Neuron. 2007;54:291–301. doi: 10.1016/j.neuron.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bender VA, Bender KJ, Brasier DJ, Feldman DE. Two coincidence detectors for spike timing-dependent plasticity in somatosensory cortex. J Neurosci. 2006;26:4166–4177. doi: 10.1523/JNEUROSCI.0176-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- 41.Liu JP, Sim AT, Robinson PJ. Calcineurin inhibition of dynamin I GTPase activity coupled to nerve terminal depolarization. Science. 1994;265:970–973. doi: 10.1126/science.8052858. [DOI] [PubMed] [Google Scholar]
- 42.Cousin MA, Robinson PJ. The dephosphins: Dephosphorylation by calcineurin triggers synaptic vesicle endocytosis. Trends Neurosci. 2001;24:659–665. doi: 10.1016/s0166-2236(00)01930-5. [DOI] [PubMed] [Google Scholar]
- 43.Dan Y, Poo MM. Spike timing-dependent plasticity of neural circuits. Neuron. 2004;44:23–30. doi: 10.1016/j.neuron.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 44.Megias M, Emri Z, Freund TF, Gulyas AI. Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience. 2001;102:527–540. doi: 10.1016/s0306-4522(00)00496-6. [DOI] [PubMed] [Google Scholar]
- 45.Freund TF. Interneuron diversity series: Rhythm and mood in perisomatic inhibition. Trends Neurosci. 2003;26:489–495. doi: 10.1016/S0166-2236(03)00227-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.