Abstract
Telomeres are nucleoprotein structures that cap the ends of chromosomes, protecting them from exonucleases and distinguishing them from double-stranded breaks. Their integrity is maintained by telomerase, an enzyme consisting of a reverse transcriptase, TERT and an RNA template, TERC, and other components, including the pseudouridine synthase, dyskerin, the product of the DKC1 gene. When telomeres become critically short, a p53-dependent pathway causing cell cycle arrest is induced that can lead to senescence, apoptosis, or, rarely to genomic instability and transformation. The same pathway is induced in response to DNA damage. DKC1 mutations in the disease dyskeratosis congenita are thought to act via this mechanism, causing growth defects in proliferative tissues through telomere shortening. Here, we show that pathogenic mutations in mouse Dkc1 cause a growth disadvantage and an enhanced DNA damage response in the context of telomeres of normal length. We show by genetic experiments that the growth disadvantage, detected by disparities in X-inactivation patterns in female heterozygotes, depends on telomerase. Hemizygous male mutant cells showed a strikingly enhanced DNA damage response via the ATM/p53 pathway after treatment with etoposide with a significant number of DNA damage foci colocalizing with telomeres in cytological preparations. We conclude that dyskerin mutations cause slow growth independently of telomere shortening and that this slow growth is the result of the induction of DNA damage. Thus, dyskerin interacts with telomerase and affects telomere maintenance independently of telomere length.
Keywords: dyskeratosis congenita, heterozygous, mosaic analysis, telomerase, αp53
Telomeres are specialized nucleoprotein complexes that cap the ends of linear chromosomes, protecting them from degradation and inappropriate DNA recombination (1). Human telomere DNA consists of hundreds of tandem TTTAGG repeats terminating in a single-stranded overhang of the G-rich strand that loops back to form the terminal t-loop (2). Telomeres are maintained by telomerase, a nucleoprotein complex that uses an RNA template and a reverse transcriptase to add telomere repeats onto the 3′ end of telomeric DNA (3). In the absence of telomerase, telomeres shorten with every round of replication (4) until they reach a critical length when cell cycle arrest is induced (5). Critically short telomeres activate the DNA damage pathway and the tumor suppressor p53, leading to cell cycle arrest or cell death (6).
X-linked dyskeratosis congenita (DC) is an inherited bone marrow failure disorder that is usually fatal (7). It is caused by mutations in the X-linked gene DKC1 encoding the 57-kDa nucleolar protein, dyskerin (8). Dyskerin is a pseudouridine synthase that is a component of box H/ACA ribonucleoprotein particles (RNPs) that function in the pseudouridylation of specific residues in nascent rRNA and snRNA molecules (9). In vertebrates, telomerase RNA contains a box H/ACA domain (10) and is associated with dyskerin (11) and the other three box H/ACA RNP proteins (12).
Dyskerin therefore plays an essential role in several distinct cellular processes, namely ribosome biogenesis, snRNA maturation, telomere maintenance, and possibly others that are so far elusive (9). A null mutation has never been described in humans, and in mice it is not compatible with life (13). The mutations identified in DC patients therefore only impair but do not destroy dyskerin function. To what extent each of the different dyskerin functions is affected and contributes to the pathogenesis of disease is controversial (14–16), although the finding of mutations in genes encoding other telomerase and telomere components in DC (17–19) and studies in DC cell lines showing impaired telomere maintenance (11, 16) emphasize the importance of the telomere maintenance pathway in disease pathology. Moreover, a universal feature of all forms of DC is the presence in the patients of very short telomeres (20). However, the X-linked form of DC is usually more severe, and some defects in ribosome biogenesis have been observed in mice and in mouse embryonic stem cells with targeted dyskerin mutations (14, 15) suggesting that functions of dyskerin other than the prevention of short telomeres may contribute to the disease.
To clarify the role of dyskerin in telomere maintenance with particular regard to the effect of the mutations causing DC, we have constructed a mouse strain with a dyskerin mutation that mimics a mutation found in a family with X-linked DC (21). Laboratory mice have long telomeres, such that several generations with complete absence of telomerase are required before a phenotype caused by short telomeres becomes apparent (22–24). We reasoned that any phenotype caused by a mutant dyskerin that depended on the presence of critically short telomeres would not be apparent in early generations of inbreeding of our mice, enabling us to differentiate a “short-telomere” phenotype from the phenotype caused by other functions of dyskerin. Our studies revealed a subtle but distinct phenotype in early generations of female mice heterozygous for a pathogenic mutation in Dkc1. We found a significant impairment of cell proliferation in cells expressing the mutant dyskerin, and we demonstrate that this impairment depended on the activation of a p53 DNA damage response. Despite normal telomere length, the proliferative impairment depended on telomerase activity, thus revealing an additional pathway whereby mutant dyskerin impairs cell proliferation in telomerase-expressing cells that is independent of telomere length.
Results
Dkc1Δ15 Causes Telomere Shortening in ES Cells.
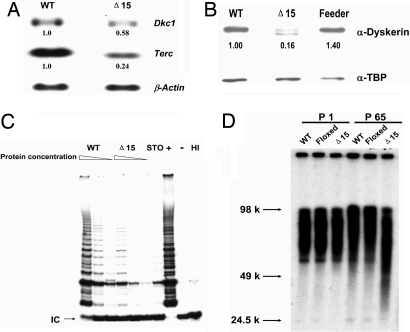
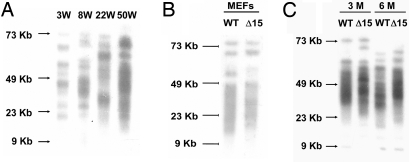
We chose to copy, in mouse, a deletion mutation that has been found in a family with X-linked DC. In humans this is a 2-kb deletion removing the last exon (exon 15) of the DKC1 gene (21). In humans, the deleted gene codes for a truncated protein lacking the C-terminal 22 aa, whereas the deletion of exon 15 in mice (Dkc1Δ15) lacks the C-terminal 21 aa. The Cre-loxP system was used to delete exon 15 in the X-linked Dkc1 gene in ES cells and mice [see Materials and Methods and supporting information (SI) Materials and Methods]. The ES cells used are male and therefore have only one X chromosome, and after homologous recombination and Cre-mediated loxP-dependent DNA deletion, they express only the mutant dyskerin. We first determined the effect of Dkc1Δ15 on the expression of dyskerin and on telomerase activity and telomere length. Northern and Western blotting (Fig. 1) showed that the expression of Dkc1Δ15 at the mRNA level is reduced compared with wild type (WT) and that at the protein level the amount of the truncated dyskerin was reduced compared with WT dyskerin, suggesting that in ES cells the Dkc1Δ15 mRNA and the truncated dyskerin protein may be relatively unstable. In mutant ES cells, the amount of Terc RNA was reduced to 25% of that in WT ES cells, and the telomerase activity, measured by the TRAP assay, was lower in mutant compared with WT cells. To test whether the decrease in telomerase activity would affect the telomere length of the ES cells, we measured telomeres by Southern blotting before and after 65 passages, which corresponded to ≈250 population doublings. As shown in Fig. 1D, the telomere length in mutant cells was significantly shorter than that in either WT cells or in ES cells carrying the floxed Dkc1 allele (Dkc1Floxed15). We conclude that the Dkc1Δ15 deletion leads directly to defects in telomere maintenance caused by decreased levels of Terc RNA. These findings indicate that the Dkc1Δ15 mutation impairs the maintenance of telomere length.
Fig. 1.
Decreased levels of Terc RNA and accelerated telomere shortening in Dkc1Δ15 ES cells. (A) Decreased levels of Terc RNA in Dkc1Δ15 ES cells as assessed by Northern blot analysis. Ten micrograms of total RNA from WT and Dkc1Δ15 (Δ15) ES cells were loaded in each lane and hybridized sequentially with probes for Terc, Dkc1, and β-actin. (B) Western blot of nuclear extracts from WT and Dkc1Δ15 ES cells. Expression of dyskerin protein was detected by anti-dyskerin antibody. Anti-TBP was used as loading control. Lane Δ15 shows two bands, the lower representing the truncated dyskerin protein, and the upper derived from contaminating feeder cells. (C) Decreased telomerase activity in Dkc1Δ15 ES cells. Different amounts of proteins representing consecutive one in three dilutions were subjected to the TRAP assay. HI indicates samples treated with heat before the experimental reaction. The IC represents the 36-bp internal control for PCR amplification. STO represents the feeder cells. (D) Accelerated telomere shortening in Dkc1Δ15 ES cells. WT, Dkc1Floxed15 (Floxed), and Dkc1Δ15 ES cells were passaged 65 times. Telomeric terminal restriction fragments were analyzed by in-gel hybridization with a 32P-labeled (CCCTAA)4 probe. The sizes of molecular weight markers are shown on the left.
Dkc1Δ15 Affects the Levels of Specific Box H/ACA RNAs and Delays rRNA Processing in ES Cells.
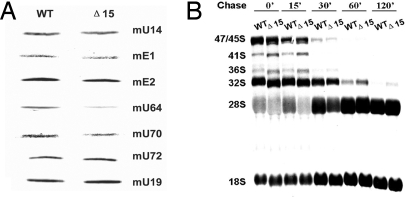
To investigate whether Dkc1Δ15 can affect the accumulation of H/ACA RNAs other than Terc, we probed Northern blots of RNA from mutant and WT ES cells for a set of box H/ACA RNAs. We found that in the Dkc1Δ15 ES cells, the expression of mU64 was significantly decreased whereas the others we tested showed no difference (Fig. 2A). Because dyskerin may affect the maturation of pre-rRNA through altered accumulation of specific box H/ACA RNAs, altered pseudouridylation activity, or rRNA splicing, we examined the rate of rRNA processing by [3H]uridine labeling and [3H-methyl]methionine pulse–chase labeling experiments. Labeling RNA with [3H]uridine did not show any appreciable differences in the accumulation of mature 18S and 28S rRNA (date not shown). Using the more sensitive [3H-methyl]methionine pulse–chase labeling experiments, however, we found that in mutant cells maturation of 18S and 28S in Dkc1Δ15 ES cells was slower than WT ES cells, whereas the accumulation of labeled 41S, 36S, and 32S precursors is slightly higher than in WT ES cells (Fig. 2B). These results indicate rRNA maturation in mutant cells is slightly delayed compared with WT cells.
Fig. 2.
Altered H/ACA RNA levels and delayed rRNA processing in Dkc1Δ15 ES cells. (A) Decreased levels of specific H/ACA RNA Dkc1Δ15 ES cells as assessed by Northern blot analysis. Five micrograms of total RNA from WT and Dkc1Δ15 ES cells was loaded in each lane and hybridized with 32P-labeled mouse H/ACA RNA probes. The C/D box RNA mU14 was used as a loading control. (B) Pulse–chase labeling experiments of rRNA isolated from WT and Dkc1Δ15 ES cells. Cells were labeled with l-[3H-methyl]methionine for 30 min and then chased in nonradioactive medium for the times shown. The RNA was separated on a 1.25% agarose gel, transferred to a nylon filter, and exposed to x-ray film.
Competitive Proliferative Disadvantage of Mutant Dyskerin Cells in Female Mice Heterozygous for Dkc1Δ15.
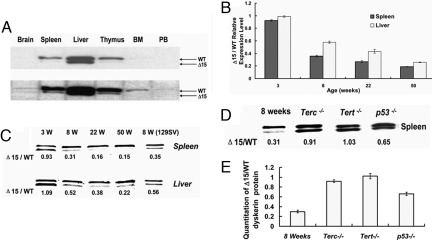
Examination of first-generation Dkc1Δ15 male mice showed no overt phenotype (data not shown). Male and female mice were born with the expected Mendelian ratios, and male mutant mice showed no signs of bone marrow failure, nail dystrophy, skin pigmentation problems, or other features characteristic of human DC. Interestingly however, in 3-month-old female heterozygous mice, we found that in spleen, thymus, bone marrow, and peripheral blood, the expression of truncated dyskerin protein was much lower than WT dyskerin, whereas in liver and brain, these two types of dyskerin were almost equal (Fig. 3A). The most likely explanation for this finding was that in these mice, after random X inactivation has taken place in early embryogenesis, cells in which the X chromosome carrying the WT Dkc1 allele remains active have a growth advantage over those in which the mutant allele is active.
Fig. 3.
The increased expression of WT dyskerin in aging Dkc1+/Δ15 heterozygous female mice represents the proliferative disadvantage of cells expressing the mutant (Δ15) dyskerin protein. (A) Western blot analysis of WT and mutant (Δ15) dyskerin in various tissues in 3-month-old heterozygous female mice. (Lower) Longer exposure of the film. (B) Expression levels of WT and Dkc1Δ15 mRNAs in Dkc1+/Δ15 heterozygous female mice. RNA from spleen and liver tissues from mice of different ages was assessed by real-time PCR. (C) Expression level of WT and mutant dyskerin in spleen and liver in Dkc1+/Δ15 heterozygous female mice. The last lane, 129SV, shows that the proliferative disadvantage of the mutant dyskerin cells persisted in the 129SV genetic background. (D) Mutant and WT cells proliferate similarly in a telomerase-null genetic background, whereas the proliferative defect is partially rescued in p53-deficient mice. A representative Western blot of protein from the spleen of female Dkc1+/Δ15 heterozygous mice with the genetic backgrounds indicated is shown. The ratio of mutant to WT dyskerin is indicated below. (E) Ratio of mutant to WT dyskerin protein as determined from four (Dkc1Δ15/+, Dkc1Δ15/+ Terc−/−, Dkc1Δ15/+ Tert−/−) and three (Dkc1Δ15/+ p53−/−) animals.
To assess whether the decreased expression of the mutant dyskerin compared with WT is indeed caused by a proliferative growth disadvantage rather than by altered stability of the mutant protein, we next compared the level of mutant and WT Dkc1 mRNA expression. The levels of mutant and WT Dkc1 mRNAs, as determined by using quantitative PCR, showed decreases reflecting those seen at the protein level (Fig. 3B). The correlation between protein and mRNA levels suggests that there is no major difference in protein stability in adult mouse tissues, as had been suggested by the expression of Dkc1Δ15 in ES cells.
Because X inactivation in embryogenesis is a random process, with 50% of cells inactivating the maternal X and 50% inactivating the paternal X, we next wished to know whether the growth advantage was effective before or after birth and how it developed in different tissues during aging. Fig. 3C shows that in the spleen cells of 3-week-old mice both proteins are approximately equally present, whereas by the age of 8 weeks significantly more WT protein was present. At 22 and 50 weeks only minimal amounts of the truncated mutant dyskerin were detected (16% and 12%). The growth advantage was also evident in liver but to a lesser extent than in spleen (Fig. 3C). Again, the age-related changes in protein levels were reflected in the mRNA levels (Fig. 3B), further confirming that the decreased level of mutant dyskerin is caused by a competitive growth disadvantage rather than by decreased protein stability.
These experiments were performed in mice that were backcrossed for at least four generations into a C57BL/6 background (n ≥4). The ES cells used for homologous recombination were derived from 129SV mice. To exclude the possibility that the observed proliferative disadvantage was caused by 129SV sequences segregating with mutant dyskerin we bred the mutant dyskerin into a 129SV background. The analysis of mutant and WT dyskerin expression revealed a similar growth disadvantage of the mutant cells compared with WT as seen in the C57BL6 n ≥4 background (Fig. 3B), thus excluding strain differences as the cause of this phenomenon.
We conclude that cells expressing WT dyskerin have a growth advantage over those that express mutant dyskerin and that this advantage is greater in spleen, thymus, and bone marrow than in brain or liver.
The Growth Advantage of WT Dyskerin Cells Depends on Telomerase.
According to our initial hypothesis, any effects seen in early generations should not be mediated through short telomeres because no effects of telomere shortening are apparent for several generations of laboratory mice in the complete absence of telomerase activity (22–24). We therefore expected that the observed growth disadvantage of dyskerin mutant cells was caused by an impairment of a dyskerin function other than telomere maintenance. To confirm this assumption, we bred our Dkc1Δ15 mice with both Terc−/− and Tert−/− mice to create female mice that were heterozygous for the Dkc1Δ15 deletion and null for either Terc RNA or TERT. The Terc− and Tert− alleles used in this breeding were always maintained in the first generation of inbreeding (G1), i.e., they should not have undergone significant telomere shortening (22, 24). We then examined these mice for the expression pattern of the mutant and WT dyskerin that we had observed in the Dkc1Δ15/+ heterozygotes. To our surprise, at the age of 8 weeks an equal expression of mutant and WT dyskerin protein was present in Dkc1Δ15/+Terc−/− and in Dkc1Δ15/+Tert−/− mouse tissues (see Fig. 3 D and E), indicating that the growth advantage of the WT dyskerin cell is mediated by the interaction of dyskerin with the active telomerase and that in the absence of active telomerase both the mutant and WT cells grow equally.
The Proliferative Growth Advantage of WT Dyskerin Cells Depends on p53.
It is known that dysfunctional telomeres cause growth arrest and cell senescence by activating a pathway that depends on p53 (25, 26). We thus wished to determine whether a p53-dependent mechanism was also responsible for the proliferative disadvantage conferred by the mutant dyskerin in our female heterozygous mice. We therefore bred our mice with mice containing a deletion of the p53 gene to produce Dkc1Δ15/+ p53−/− female mice. In such mice at 8 weeks of age the ratio of mutant dyskerin to WT dyskerin was 0.65 whereas in female Dkc1Δ15/+ p53+/+ mice of the same age the ratio was 0.31 (Fig. 3 D and E), suggesting that lack of p53 partially rescues the proliferative disadvantage of dyskerin mutant cells, indicating that the proliferative defect is, at least partially, mediated by a pathway involving p53.
Dkc1Δ15 MEFs Accumulate More DNA Damage Foci After Etoposide Treatment.
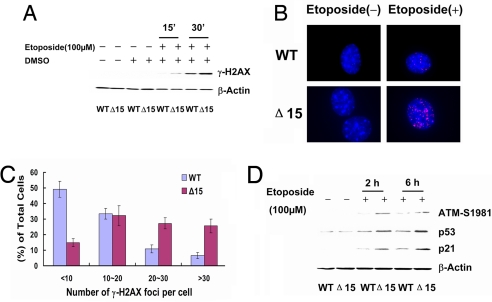
The classical p53 pathway whereby dysfunctional telomeres lead to growth arrest and cell senescence is the ATM-p53/p21-dependent DNA damage checkpoint response, which is also activated in the cellular response to DNA damage (25, 26). The pathway involves the formation of DNA damage foci containing γ-H2AX and leads to phosphorylation of ATM and proceeds via p53, p21 and Cdk2 to cause arrest in the G1 phase of the cell cycle. We therefore asked whether this pathway was activated in cells with the dyskerin mutation Dkc1Δ15. We thus grew male mouse embryo fibroblasts (MEFs) expressing either the mutant or the WT dyskerin gene. We treated both types of cells with 100 μM etoposide, a topoisomerase inhibitor known to induce double-stranded breaks in DNA. Using an antibody for γ-H2AX and Western blotting we found that, as expected, etoposide treatment caused a rapid induction of γ-H2AX in both mutant and WT dyskerin cells. However, the level of γ-H2AX expression was much higher in dyskerin mutant cells than in cells expressing the WT dyskerin (Fig. 4A). Next, we investigated the presence of γ-H2AX foci before and after treatment with etoposide. Without treatment, both WT and mutant MEFs showed very few γ-H2AX foci. After treatment with 5 μM etoposide, the number of γ-H2AX foci per cell increased in both cell types, but in dyskerin mutant MEFs it was much higher than in WT MEFs. Approximately 50% WT MEFs had <10 foci per cell, whereas >60% dyskerin mutant MEFs had >20 foci (Fig. 4 B and C). The increased number of γ-H2AX foci prompted us to investigate further whether the ATM-p53/p21-dependent DNA damage checkpoint response was activated differentially in mutant MEFs after etoposide treatment. The results (Fig. 4D) showed that expression levels of phosphorylated ATM-S1981, p53, and p21 in dyskerin mutant MEFs were all significantly higher than in WT MEFs after etoposide treatment. These results indicate that dyskerin mutant MEFs are hypersensitive to DNA damage, having an enhanced DNA damage response compared with WT cells.
Fig. 4.
Etoposide treatment induces an enhanced ATM-p53/p21-dependent DNA damage response in Dkc1Δ15 MEFs. (A) WT and Dkc1Δ15 (Δ15) MEFs were treated with 100 μM etoposide. After 15 and 30 min, the cells were collected and analyzed by Western blotting with an anti-γ-H2AX antibody. The solvent, DMSO, was used as a control. (B) Immunofluorescence staining of γ-H2AX (red), DNA was counterstained with DAPI (blue). MEFs were plated onto coverslips and treated with 5 μM etoposide for 30 min. (C) Quantititation of γ-H2AX foci between WT and Dkc1Δ15 (Δ15) MEFs after treatment with 5 μM etoposide for 30 min. More than 200 cells were analyzed. (D) Western blot analysis of ATM-S1981P, p53, and p21 expression level of MEFs after treatment with 100 μM etoposide. β-Actin was used as a loading control.
Proliferative Impairment and Increased DNA Damage Response Are Not Associated with Short Telomeres in Dkc1Δ15 Mice and MEFs.
Growth impairment caused by telomerase defects is thought to take place because telomeres become critically short and the short telomeres induce cell cycle arrest (5, 25–27). To verify that the proliferative growth disadvantage and increased DNA damage response was not caused by short telomeres, we performed telomere measurements in dyskerin mutant mice and dyskerin mutant MEFs. Fig. 5A shows that telomere lengths of spleen cells from Dkc1Δ15/+ females did not significantly shorten with age. Similarly (Fig. 5B), we found that the MEFs used in the etoposide assay have a telomere length similar to control MEFs. Telomere lengths from Dkc1Δ15 and WT spleen cells showed no significant difference (Fig. 5C).
Fig. 5.
Similar telomere lengths in WT and Dkc1Δ15 cells. (A) Telomere length in spleen cells from heterozygous Dkc1Δ15/+ mice of different ages. (B) Telomere length of male WT and male Dkc1Δ15 MEFs. (C) Telomere length in spleen cells from male WT and Dkc1Δ15 mice of different ages.
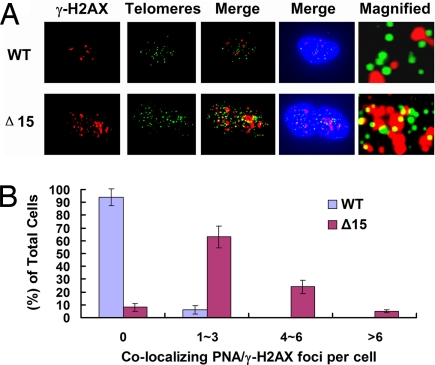
DNA Damage Foci Colocalize with Telomeres in Dkc1Δ15 MEFs.
The DNA damage response foci that accumulate in cells undergoing a short-telomere-induced DNA damage response are enriched at the telomeres (25, 26, 28). To investigate whether the foci of the increased DNA damage response we found in Dkc1Δ15 mutant cells were similarly localized, we used fluorescence in situ hybridization (FISH) detection of telomeres using a telomere-specific peptide nucleic acid (PNA) probe in combination with immunological detection of γ-H2AX foci. The results (Fig. 6) showed that in WT MEFs essentially no γ-H2AX foci localized to telomeres, whereas in Dkc1Δ15 MEFs ≈3∼4 γ-H2AX foci per cell, ≈10–15% of all γ-H2AX foci localized to telomeres. This result suggests that the elevated DNA damage response seen in the dyskerin mutant MEFs may be caused by the presence in these cells of a structural defect at the telomeres.
Fig. 6.
Localization of γ-H2AX foci to telomeres in Dkc1Δ15 (Δ15) MEFs. (A) Colocalization of γ-H2AX foci (Alexa Fluor 568, red) and telomeres by telomere PNA-FISH (FITC, green) in WT and Dkc1Δ15 MEFs after treatment with 5 μM etoposide for 30 min. DNA was counterstained with DAPI (blue). Magnified views of merged images showed details of the colocalization. (B) Quantitation of colocalization of signals from (A) of γ-H2AX foci and telomeres. More than 100 cells were analyzed.
Discussion
Mouse ES cells with the Dkc1Δ15 mutation showed decreased accumulation of TERC, lower telomerase activity, and more rapid telomere shortening compared with WT ES cells. They were only mildly impaired in ribosomal RNA synthesis in agreement with our previous findings with other mutations (15) (Figs. 1 and 2). To determine the in vivo consequences of the mutation, we generated mice expressing the Dkc1Δ15 truncated dyskerin protein. Female heterozygous mice and male mice hemizygous for Dkc1Δ15 did not show an appreciable phenotype. However, the truncated dyskerin protein produced by the Dkc1Δ15 allele gave us a cellular marker for the mutant dyskerin cells and thus enabled us to perform mosaic analysis in female Dkc1Δ15/+ heterozygous mice. Because one of the X chromosomes is inactivated at random in early embryonic development, the representation of cells with the maternal or paternal X chromosome active in adult tissues reflects the relative rate of proliferation of the two types of cells during development providing a very powerful tool to identify proliferative or survival differences of cells in a tissue. The only difference between the two types of cells is the expression, in this case, of a truncated dyskerin gene. Using this genetic tool, we found that cells expressing the truncated dyskerin protein had a proliferative disadvantage compared with those expressing WT dyskerin (Fig. 3). This proliferative disadvantage was already apparent in the first generation of female mice at the age of 8 weeks and was greater in rapidly dividing tissues, such as bone marrow, spleen, and thymus, than in slower dividing tissues, such as liver and brain. To our surprise, and against our predictions, this phenotype depended on an active telomerase complex (both TERT and Terc RNA have to be present) but was independent of telomere length. Thus, although dyskerin interacts with multiple H/ACA RNAs involved in ribosome biogenesis, mRNA splicing and possibly other processes, the only defects caused by the Dkc1Δ15 mutation that impact on growth were mediated through the interaction of dyskerin with telomerase. Moreover, only very subtle alterations in ribosome biogenesis were detected in our mutant cells or indeed in cells from DC patients (16). Together, these results underline the importance of the interaction of dyskerin with telomerase in the pathogenic effects of dyskerin mutations. The involvement of dyskerin in activity-dependent effects of telomerase is in keeping with the presence of dyskerin in a purified active telomerase complex from human cells (29).
Further analysis revealed that dyskerin mutant cells have an enhanced DNA damage response via the ATM-p53 pathway with increased DNA damage foci formation at the telomeres. These results led us to reevaluate the current thinking about the role of dyskerin in telomere maintenance and in the disease dyskeratosis congenita. It was thought that dyskerin acts as a part of the active telomerase complex preventing the telomeres from becoming excessively short and that it is the DNA damage response triggered by the short telomeres that leads to replicative cell senescence or cell death (27). Here, we demonstrate that dyskerin additionally participates in the maintenance of telomere integrity that is independent of telomere length and that a mutant dyskerin impairs cell proliferation through an increased DNA damage response and activation of p53 before the telomeres are critically short. The exact mechanism whereby dyskerin maintains telomere integrity is not clear. However, our results indicate that the pathway depends on the presence of active telomerase (TERT and Terc RNA). Whether the defect is mediated solely through impaired telomerase activity or is specific for mutant dyskerin is not clear. Short telomeres in telomerase-deficient bone marrow progenitor cells in late generation (≥G3) Terc-deficient mice confer a competitive disadvantage in bone marrow transplant experiments (30). However, competitive transplantation experiments with early generation telomerase-deficient bone marrow cells, with normal telomere length, have not been performed. Interestingly, a protective function of telomerase allowing cell proliferation without the net lengthening of telomeres has been postulated (31) in hTERT-transfected fibroblasts. Similarly, Meier et al. (28) observed weak DNA damage foci at telomeres in normal proliferating human fibroblasts. These foci were absent from TERT-immortalized fibroblasts, suggesting that low telomerase activity can lead to telomere uncapping and senescence in proliferating cells. Other telomere length-independent effects of telomerase have been reviewed (32). Here, we demonstrate in our dyskerin mutant mice that this telomerase-dependent but telomere length-independent DNA damage response leads to significant proliferative impairment in vivo, highlighting the biological importance of this pathway.
Interestingly, in human female carriers of pathogenic DKC1 mutations, hematopoietic cells express almost exclusively the WT gene, caused by a proliferative disadvantage of the mutant cells after random X inactivation (33). Originally, it had been thought that this proliferative disadvantage is the result of the short telomeres of the mutant cells, but our finding in mice suggests that a telomere length-independent DNA damage pathway might be responsible for this finding. Because the effect depends on telomerase activity, we expect that in humans this pathway would mainly affect telomerase-expressing cells, i.e., stem cells and their immediate progeny. This expectation would imply that in patients with DC the stem cell compartment is impaired before telomeres are excessively short. Thus, our results imply that two concurrent pathways contribute synergistically to the proliferative defect and depletion of stem cells in individuals with X-linked DC. Initially in dividing cells, a defect in telomere integrity activates a DNA damage response and impairs the proliferation in telomerase-expressing stem and progenitor cells, leading to an increased recruitment of stem cells into cell cycle. Second, insufficient telomerase activity leads to an accelerated telomere shortening and excessively short telomeres that additionally triggers replicative senescence and cell death. In mice the proliferative impairment caused by early DNA damage seems to be rather subtle compared with the more severe proliferative impairment caused by excessively short telomeres in later generation telomerase-deficient mice. Because humans have much shorter telomeres it will be more difficult to separate the DNA damage caused from impaired vs. short telomeres. However, human cells, and in particular hematopoietic progenitor cells, are much more sensitive to an altered DNA damage response (for review, see ref. 34). Thus, the increased DNA damage response before the telomeres are excessively short might significantly impact the onset and progression of disease in patients with X-linked DC.
In summary, the analysis of our dyskerin mutant mice revealed impaired cell proliferation of dyskerin mutant cells caused by an unanticipated DNA damage response. Moreover, the genetic system we have developed exploits the unique sensitivity of X-inactivation-generated mosaic analysis to provide a powerful assay system for the effects of telomerase disruption on mammalian development and physiology.
Materials and Methods
Unless stated otherwise, all molecular biology procedures were carried out by standard methods. Detailed methods are supplied in the SI Materials and Methods.
Generation of Dkc1Δ15 ES Cells and Mice.
The Dkc1Floxed15 targeting construct was made by standard subcloning methods as described in ref. 15 (see Fig. S1). A stop codon was introduced into exon 14 by using the QuikChange kit (Stratagene). ES cells (RW-4, Siteman Cancer Center ES cell core at Washington University School of Medicine) were transfected with the Dkc1Floxed15 construct and selected with Geneticin (Sigma). To generate Dkc1Δ15 ES cells, Dkc1Floxed15 cells were transiently transfected with a plasmid expressing Cre, and the resulting clones were selected for loss of neomycin resistance. ES cells were cultured on mitomycin C-treated STO cell (CRL-2225; American Type Culture Collection) feeder layers in ES culture medium (DMEM, 17% ES qualified FCS) supplemented with 200 mM l-glutamine, 10 mM Hepes, 10 mM nonessential amino acids, 1000 units/ml leukemia inhibitory factor, and 0.14 mM β-mercaptoethanol. Dkc1Δ15 mice were derived from Dkc1Floxed15 ES cells by breeding with mice carrying the EIIA Cre transgene as described in SI Materials and Methods.
Measurement of Telomere Length.
ES cells or spleen cells were embedded in agarose plugs by using a CHEF agarose plug kit according to the manufacturer's instructions (Bio-Rad). DNA embedded in the plug was extracted, digested with MboI, and electrophoresed through a 1% agarose gel for 20 h at 6 V/cm, 1- to 6-s switch time by using a CHEF DR-III pulse-field system (Bio-Rad). A [γ-32P]ATP-labeled (CCCTAA)4 probe was used in the in-gel hybridization procedure (35).
Immunofluorescence and Immuno-FISH.
Immunofluorescence for γ-H2AX was performed by using a rabbit anti-γ-H2AX-S139 (Abcam) with a standard technique. Immuno-FISH for telomeric DNA was performed by using the protocol as described in ref. 36. The cells were examined at a magnification of ×1,000 with a fluorescence microscope (Nikon). FITC, Texas red, Alexa Fluor 568, and DAPI images were overlapped by using ISIS FISH imaging software (Metasystems).
Antibodies.
The sources of antibodies were as follows: anti-γ-H2AX-S139 (ab2893; Abcam), anti-p53 (ab26; Abcam), anti-p21 (ab7960; Abcam), and anti-ATM-phospho-S1981 (ab2888; Abcam). Anti-dyskerin was as described in ref. 15, anti-β-actin was used as total protein loading control (ab20272; Abcam), and anti-TATA box-binding protein (TBP) was used as nuclear loading control (ab818; Abcam).
Mice.
Terc−/− (22), Tert−/− (24), p53−/− (37), and EIIA-Cre mice (38) were obtained from R. DePinho (Dana-Farber Cancer Institute, Boston), L. Harrington (University of Toronto, Toronto), The Jackson Laboratory, and H. Westphal (National Institutes of Health, Bethesda, MD), respectively, and maintained in a C57BL6 background.
Establishment of Primary MEFs.
Dkc1Δ15 and control WT male MEFs were prepared from a cross of Dkc1Δ15/+ females with WT male mice. Primary MEFs were isolated from 13.5-day mouse embryos and harvested for analysis after two to three passages. Cells were cultured in DMEM supplemented with 10% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin and maintained at 37°C in a humidified atmosphere of 3% O2 and 10% CO2.
Supplementary Material
Acknowledgments.
We thank the Murine Embryonic Stem Cell Core at the Siteman Cancer Center for assistance with ES cell propagation and transfection. This work was supported by National Cancer Institute Grant R01 CA106995 (to P.J.M.) and RFA-HL-04-008 (to M.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803559105/DCSupplemental.
References
- 1.de Lange T. Shelterin: The protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- 2.de Lange T. T-loops and the origin of telomeres. Nat Rev Mol Cell Biol. 2004;5:323–329. doi: 10.1038/nrm1359. [DOI] [PubMed] [Google Scholar]
- 3.Greider CW, Blackburn EH. The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell. 1987;51:887–898. doi: 10.1016/0092-8674(87)90576-9. [DOI] [PubMed] [Google Scholar]
- 4.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 5.Wright WE, Shay JW. The two-stage mechanism controlling cellular senescence and immortalization. Exp Gerontol. 1992;27:383–389. doi: 10.1016/0531-5565(92)90069-c. [DOI] [PubMed] [Google Scholar]
- 6.Shay JW, Pereira-Smith OM, Wright WE. A role for both RB and p53 in the regulation of human cellular senescence. Exp Cell Res. 1991;196:33–39. doi: 10.1016/0014-4827(91)90453-2. [DOI] [PubMed] [Google Scholar]
- 7.Vulliamy T, Dokal I. Dyskeratosis congenita. Semin Hematol. 2006;43:157–166. doi: 10.1053/j.seminhematol.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Heiss NS, et al. X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions. Nat Genet. 1998;19:32–38. doi: 10.1038/ng0598-32. [DOI] [PubMed] [Google Scholar]
- 9.Meier UT. The many facets of H/ACA ribonucleoproteins. Chromosoma. 2005;114:1–14. doi: 10.1007/s00412-005-0333-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell JR, Cheng J, Collins K. A box H/ACA small nucleolar RNA-like domain at the human telomerase RNA 3′ end. Mol Cell Biol. 1999;19:567–576. doi: 10.1128/mcb.19.1.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell JR, Wood E, Collins K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature. 1999;402:551–555. doi: 10.1038/990141. [DOI] [PubMed] [Google Scholar]
- 12.Pogacic V, Dragon F, Filipowicz W. Human H/ACA small nucleolar RNPs and telomerase share evolutionarily conserved proteins NHP2 and NOP10. Mol Cell Biol. 2000;20:9028–9040. doi: 10.1128/mcb.20.23.9028-9040.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He J, et al. Targeted disruption of Dkc1, the gene mutated in X-linked dyskeratosis congenita, causes embryonic lethality in mice. Oncogene. 2002;21:7740–7744. doi: 10.1038/sj.onc.1205969. [DOI] [PubMed] [Google Scholar]
- 14.Ruggero D, et al. Dyskeratosis congenita and cancer in mice deficient in ribosomal RNA modification. Science. 2003;299:259–262. doi: 10.1126/science.1079447. [DOI] [PubMed] [Google Scholar]
- 15.Mochizuki Y, He J, Kulkarni S, Bessler M, Mason PJ. Mouse dyskerin mutations affect accumulation of telomerase RNA and small nucleolar RNA, telomerase activity, and ribosomal RNA processing. Proc Natl Acad Sci USA. 2004;101:10756–10761. doi: 10.1073/pnas.0402560101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong JM, Collins K. Telomerase RNA level limits telomere maintenance in X-linked dyskeratosis congenita. Genes Dev. 2006;20:2848–2858. doi: 10.1101/gad.1476206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vulliamy T, et al. The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature. 2001;413:432–435. doi: 10.1038/35096585. [DOI] [PubMed] [Google Scholar]
- 18.Yamaguchi H, et al. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N Engl J Med. 2005;352:1413–1424. doi: 10.1056/NEJMoa042980. [DOI] [PubMed] [Google Scholar]
- 19.Savage SA, et al. TINF2, a component of the shelterin telomere protection complex, is mutated in dyskeratosis congenita. Am J Hum Genet. 2008;82:501–509. doi: 10.1016/j.ajhg.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vulliamy TJ, Knight SW, Mason PJ, Dokal I. Very short telomeres in the peripheral blood of patients with X-linked and autosomal dyskeratosis congenita. Blood Cells Mol Dis. 2001;27:353–357. doi: 10.1006/bcmd.2001.0389. [DOI] [PubMed] [Google Scholar]
- 21.Vulliamy TJ, et al. Dyskeratosis congenita caused by a 3′ deletion: Germ line and somatic mosaicism in a female carrier. Blood. 1999;94:1254–1260. [PubMed] [Google Scholar]
- 22.Blasco MA, et al. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91:25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- 23.Herrera E, et al. Disease states associated with telomerase deficiency appear earlier in mice with short telomeres. EMBO J. 1999;18:2950–2960. doi: 10.1093/emboj/18.11.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y, Kha H, Ungrin M, Robinson MO, Harrington L. Preferential maintenance of critically short telomeres in mammalian cells heterozygous for mTert. Proc Natl Acad Sci USA. 2002;99:3597–3602. doi: 10.1073/pnas.062549199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takai H, Smogorzewska A, de Lange T. DNA damage foci at dysfunctional telomeres. Curr Biol. 2003;13:1549–1556. doi: 10.1016/s0960-9822(03)00542-6. [DOI] [PubMed] [Google Scholar]
- 26.d'Adda di Fagagna F, et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- 27.Hao LY, et al. Short telomeres, even in the presence of telomerase, limit tissue renewal capacity. Cell. 2005;123:1121–1131. doi: 10.1016/j.cell.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 28.Meier A, et al. Spreading of mammalian DNA-damage response factors studied by ChIP-chip at damaged telomeres. EMBO J. 2007;26:2707–2718. doi: 10.1038/sj.emboj.7601719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen SB, et al. Protein composition of catalytically active human telomerase from immortal cells. Science. 2007;315:1850–1853. doi: 10.1126/science.1138596. [DOI] [PubMed] [Google Scholar]
- 30.Samper E, et al. Long-term repopulating ability of telomerase-deficient murine hematopoietic stem cells. Blood. 2002;99:2767–2775. doi: 10.1182/blood.v99.8.2767. [DOI] [PubMed] [Google Scholar]
- 31.Zhu J, Wang H, Bishop JM, Blackburn EH. Telomerase extends the lifespan of virus-transformed human cells without net telomere lengthening. Proc Natl Acad Sci USA. 1999;96:3723–3728. doi: 10.1073/pnas.96.7.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung HK, Cheong C, Song J, Lee HW. Extratelomeric functions of telomerase. Curr Mol Med. 2005;5:233–241. doi: 10.2174/1566524053586635. [DOI] [PubMed] [Google Scholar]
- 33.Vulliamy TJ, Knight SW, Dokal I, Mason PJ. Skewed X-inactivation in carriers of X-linked dyskeratosis congenita. Blood. 1997;90:2213–2216. [PubMed] [Google Scholar]
- 34.Niedernhofer LJ. DNA repair is crucial for maintaining hematopoietic stem cell function. DNA Repair. 2008;7:523–529. doi: 10.1016/j.dnarep.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dionne I, Wellinger RJ. Cell cycle-regulated generation of single-stranded G-rich DNA in the absence of telomerase. Proc Natl Acad Sci USA. 1996;93:13902–13907. doi: 10.1073/pnas.93.24.13902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Celli GB, de Lange T. DNA processing is not required for ATM-mediated telomere damage response after TRF2 deletion. Nat Cell Biol. 2005;7:712–718. doi: 10.1038/ncb1275. [DOI] [PubMed] [Google Scholar]
- 37.Jacks T, et al. Tumor spectrum analysis in p53-mutant mice. Curr Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- 38.Lakso M, et al. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc Natl Acad Sci USA. 1996;93:5860–5865. doi: 10.1073/pnas.93.12.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.