Abstract
Adjuvants are substances that enhance immune responses and thus improve the efficacy of vaccination. Few adjuvants are available for use in humans, and the one that is most commonly used (alum) often induces suboptimal immunity for protection against many pathogens. There is thus an obvious need to develop new and improved adjuvants. We have therefore taken an approach to adjuvant discovery that uses in silico modeling and structure-based drug-design. As proof-of-principle we chose to target the interaction of the chemokines CCL22 and CCL17 with their receptor CCR4. CCR4 was posited as an adjuvant target based on its expression on CD4+CD25+ regulatory T cells (Tregs), which negatively regulate immune responses induced by dendritic cells (DC), whereas CCL17 and CCL22 are chemotactic agents produced by DC, which are crucial in promoting contact between DC and CCR4+ T cells. Molecules identified by virtual screening and molecular docking as CCR4 antagonists were able to block CCL22- and CCL17-mediated recruitment of human Tregs and Th2 cells. Furthermore, CCR4 antagonists enhanced DC-mediated human CD4+ T cell proliferation in an in vitro immune response model and amplified cellular and humoral immune responses in vivo in experimental models when injected in combination with either Modified Vaccinia Ankara expressing Ag85A from Mycobacterium tuberculosis (MVA85A) or recombinant hepatitis B virus surface antigen (rHBsAg) vaccines. The significant adjuvant activity observed provides good evidence supporting our hypothesis that CCR4 is a viable target for rational adjuvant design.
Keywords: CD4+CD25+ regulatory T cells, dendritic cells, immune response, vaccine
The efficacy of vaccines can be greatly improved by adjuvants that enhance and modify the magnitude and duration of protective immunity. A variety of adjuvants including alum, water-in-oil adjuvants, and complete Freund's adjuvant (CFA) with differing modes of action have been used experimentally (1, 2). Alum, the most commonly used adjuvant in human vaccines, is a relatively weak adjuvant and induces responses of Th2-type that may not confer protection against many pathogens. Conversely, although CFA stimulates potent Th1-type immune responses in experimental animals, toxic effects due to excessive inflammation make CFA unsuitable for humans. Thus development of new and better adjuvants for human vaccines remains a challenging prerequisite of vaccine research.
Past approaches to adjuvant identification have been largely empirical. However, new vaccine targets may require novel adjuvants that can induce well defined cellular and humoral immune responses. With increasing knowledge of the molecular basis of immunoregulatory mechanisms coupled with bioinformatics, rational approaches to the design of molecular adjuvants directed toward specific immune cell receptors are now possible (3). One class of molecules that may serve as a useful adjuvant target is the chemokine receptor family and in particular, CCR4, based on its expression on CD4+CD25+ regulatory T cells (Tregs) (4). Tregs play a crucial role in down-modulating immune responses, contributing both to the maintenance of self-tolerance and to the prevention of excessive responses against infection (5). CCR4 is the receptor for two chemokines: CCL17 and CCL22. They are produced by dendritic cells (DC) the sentinels of the immune system, are chemotactic for Tregs, and are crucial in promoting contact between DC and CCR4+ T cells (4, 6–8).
Recent reports suggest Tregs can suppress DC-mediated immune responses (9) by inhibiting DC maturation and the expression of costimulatory molecules and hence their ability to activate T cells (10, 11). Blocking CCR4 function, and thus inhibiting the interaction of Tregs with DC at the time of vaccination, would be predicted to enhance vaccine-induced immune responses. Such a strategy would require transient inhibition of CCR4 and Treg function and, to avoid damaging autoimmunity, an absence of Treg depletion (12–14). This strategy can be realized by using small molecule CCR4 antagonists that would act as adjuvants by transiently blocking Treg functions.
Here, we used homology modeling and molecular docking to CCR4 to identify potential lead compounds that display antagonistic properties. We identified CCR4 antagonists that enhance DC-mediated human T cell proliferation in an in vitro immune response model and which induce cellular and humoral responses in vivo. Our results provide a “proof of principle” for the utility and efficiency of in silico modeling as an aid to the rational design of molecular adjuvants targeting specific receptors.
Results
Development of a Homology Model for CCR4.
Chemokine receptors belong to the rhodopsin family of heterotrimeric guanine nucleotide-binding protein (G protein)-coupled receptors (GPCR). GPCR share a conserved structure: seven transmembrane α-helices connected by six loops of varying lengths (15). As is the case for all GPCR, the structure of CCR4 comprises seven α-helices forming a flattened two-layer structure joined by three intracellular and extracellular loops. The transmembrane region is composed of seven segments of 20–30 consecutive residues with high overall hydrophobicity.
The structure of only one member of the GPCR superfamily—bovine rhodopsin—had been determined by x-ray crystallography when our study was undertaken (16). Despite the low sequence identity between rhodopsin and CCR4, this structure can be used as a scaffold for the transmembrane regions. Initially, a homology model of CCR4 was created (Fig. 1). Sequences corresponding to the transmembrane, intracellular and extracellular regions of CCR4 were predicted and transposed onto the rhodopsin structure, with the transmembrane regions modeled as α-helices and the termini and loops added in an extended conformation. The final model was produced after solvation and optimization in a lipid bilayer.
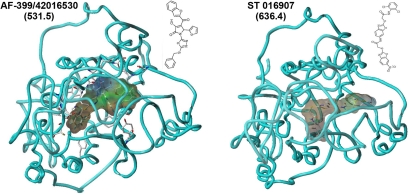
Fig. 1.
In silico modeling of CCR4 antagonists. Representative illustrations of two small molecule CCR4 antagonists AF-399/42016530 and ST 016907 docked by GOLD into the homology model of CCR4. The diagram depicts a view looking down on the protein in the membrane from outside the cell. Residues making principal van der Waals contacts are shown in full; the remainder of CCR4 is shown as a single ribbon after the amino acid backbone. Antagonists are visualized with a surrounding hydrophobic Connolly surface. Pictures generated by using Sybyl7.3. Values in parentheses denote molecular weight.
Identification of Potential Lead Molecules Through Structure-Based Virtual Screening and Molecular Docking to CCR4.
Unlike chemokines and other large peptide ligands, small molecules occupy a cavity within the transmembrane region of the receptor that corresponds to a typical ligand-binding site (17). To identify potential lead compounds that display CCR4 antagonistic properties, a database containing structures from a variety of suppliers was constructed within UNITY (SYBYL 7.0, Tripos Inc., USA) and screened for potentially reactive and undesirable molecules (18). The resulting “clean” database consisting of ≈450 000 molecules was prescreened by using a pseudopharmacophore derived from properties of known chemokine antagonists: Compounds must have a MW >500; contain two or more five- or six-membered aromatic rings; and one or more nitrogen atoms [supporting information (SI) Fig. S1]. The 3D structures of the 13 000 compounds thus selected were built using CORINA (19). These structures were tested for interaction with CCR4 by using the GOLD docking program and the GoldScore fitness function (20). The ligands docked within a predicted cavity in the transmembrane region of CCR4. Models of two docked CCR4 antagonists are shown in Fig. 1.
Assessment of CCR4 Antagonism and Specificity Through Chemotaxis Assay.
The 116 top ranked molecules were tested for their ability to inhibit CCL22-mediated chemotaxis of a CCR4+ human Caucasian acute T lymphoblastoid leukaemia cell line CCRF-CEM (Fig. 2A). Sixteen of the compounds (≈13.7%) inhibited CCR4-mediated migration of CCRF-CEM cells with IC50 values in the range of 179 × 10−11 to 229 × 10−14 M.
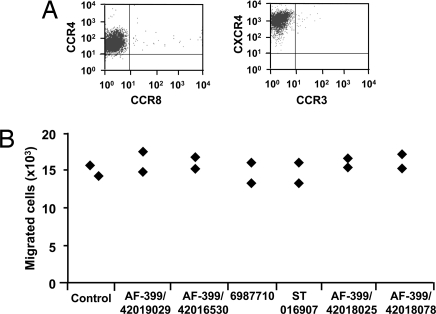
Fig. 2.
Assessment of the specificity of CCR4 antagonists. (A) Expression of chemokine receptors CCR4 and CXCR4 by CCRF-CEM cells. (B) CCR4 antagonists do not inhibit CXCL12-mediated chemotaxis of CCRF-CEM cells. Data (n = 2) show the number of migrated cells in response to 3 nM CXCL12 in the absence (Control) or presence of 2 μM CCR4 antagonists AF-399/42019029, AF-399/42016530, 6987710, ST 016907, AF-399/42018025, and AF-399/420018078.
CCRF-CEM also expresses another chemokine receptor, CXCR4 (Fig. 2A), which allowed us to test the specificity of the CCR4 antagonists. With the exception of one antagonist, the compounds had no effect on either CXCR4-mediated migration (Fig. 2B) or cell viability (data not shown), even at concentrations 1000 times higher than their IC50 values (≈2 μM).
Interference with CCL22- and CCL17-Mediated Recruitment of Human Tregs by CCR4 Antagonists.
Tregs negatively regulate immune responses induced by professional antigen presenting cells. Therefore inhibition of CCL22- and CCL17-mediated CCR4-dependent recruitment of Tregs represents a potential target for boosting immune responses. Tregs, which are enriched among CD4+CD45RO+ T cells expressing high levels of CD25, were isolated from the peripheral blood mononuclear cells (PBMC) of healthy donors. These CD4+CD25high cells expressed FoxP3 and CCR4 (Fig. 3A). Moreover, they failed to proliferate and to secrete T cell cytokines after in vitro stimulation and also suppressed the proliferation of cocultured conventional T cells (data not shown), thus confirming that isolated CD4+CD25high cells are bona fide Tregs.
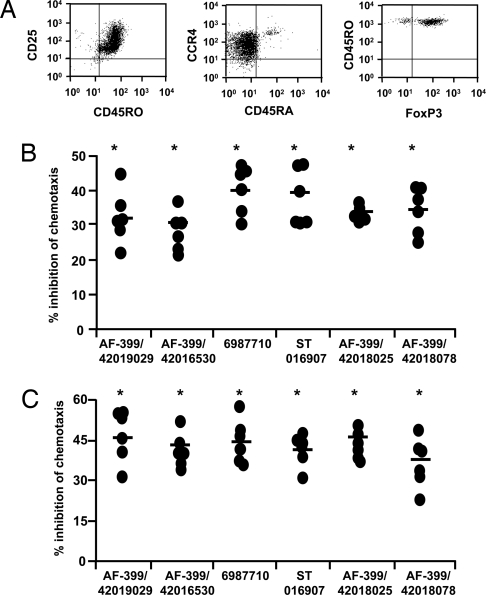
Fig. 3.
CCR4 antagonists block CCL22- and CCL17-mediated migration of human peripheral blood CD4+CD25+ regulatory T cells. (A) Expression of CCR4, CD25, CD45RO, CD45RA, and FoxP3 on Tregs. (B and C) Inhibition by CCR4 antagonists of CCL22 (1.2 nM)-mediated (B) and CCL17 (1.2 nM)-mediated (C) chemotaxis of Tregs. Data show the percent inhibition of chemotaxis by the indicated CCR4 antagonists (10 nM) for six donors. Percent inhibition of chemotaxis by CCR4 antagonists was calculated as follows: [(no. cells migrated in the presence of DMSO − no. cells migrated in the presence of antagonist)/no. cells migrated in the presence of DMSO] × 100. Mean values are indicated with a horizontal bar. *, P < 0.05 compared with DMSO controls.
We examined six compounds (AF-399/42019029, AF-399/42016530, 6987710, ST 016907, AF-399/42018025, AF-399/420018078) for their ability to block CCR4-mediated migration of Tregs. All six antagonists inhibited CCL22-mediated Treg migration (Fig. 3B) significantly: Inhibition was in the range of 29.6–40.1% (n = 6 donors). None of the compounds affected cell viability. In addition, all 6 compounds inhibited Treg migration in response to another CCR4 ligand, CCL17 (35.9–46.4%, Fig. 3C). Interestingly, inhibition was slightly greater for CCL17 than for CCL22, possibly reflecting the higher affinity of CCL22 for CCR4 (21). Our results thus indicate that in silico identified CCR4 antagonists can interfere with the recruitment of Tregs mediated by two CCR4 ligands.
Inhibition of CCL22- and CCL17-Mediated Chemotaxis of Human Th2 Cells by CCR4 Antagonists.
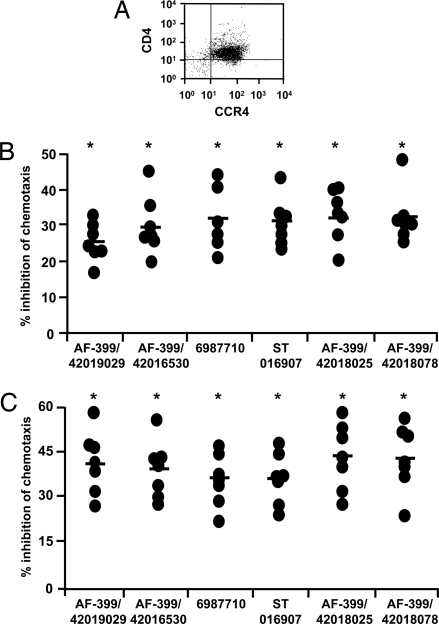
It is known that polarized effector T cells can influence the development of immune responses. Th2-based responses can inhibit Th1-biased cellular immune responses, which are thought to be more protective against intracellular pathogens (22). In addition to Tregs, polarized human Th2 cells express CCR4, and migrate in response to CCR4 ligands (23). Therefore it was important to determine whether novel adjuvants could inhibit migration of polarized Th2 cells, because this might be deleterious or useful, depending on the target pathogen. Fig. 4A confirms that in vitro generated polarized Th2 cells express CCR4. Furthermore, as observed with Tregs, all 6 CCR4 antagonists significantly inhibited both CCL22- and CCL17-directed migration of Th2 cells (Fig. 4 B and C), and the effects were comparatively greater for CCL17 than CCL22.
Fig. 4.
CCR4 antagonists inhibit CCL22- and CCL17-mediated migration of human Th2 cells. (A) The expression of CD4 and CCR4 on in vitro-generated Th2 cells. (B and C) Percent inhibition by CCR4 antagonists (10 nM) of CCL22 (1.2 nM)-mediated (B) and CCL17 (1.2 nM)- mediated (C) migration of Th2 cells (n = 7 donors). Mean values are indicated with a horizontal bar. *, P < 0.05 compared with DMSO controls.
Enhancement of DC-Mediated Human CD4+ T Cell Proliferation by CCR4 Antagonists in an in Vitro Immune Response Model.
The utility of a CCR4 antagonist as an adjuvant is predicated on the hypothesis that this molecule would inhibit recruitment of Tregs to DC, resulting in an enhanced immune response. To test this hypothesis, we modeled the early stages of a human immune response in vitro. Six-day old immature DC (0.2 × 106 per milliliter) were placed in the lower chambers of transwell plates. The cells were stimulated with a TLR ligand (LPS, 100 ng/ml) to induce activation and secretion of DC-chemokines. After 24 h, we added to the upper chambers 0.5 × 106 CD4+ T cells that contain a mixture of total CD4+ T cells and Tregs from an allogeneic donor (8:1 ratio). The ratio 8:1 of total CD4+ T cells and Tregs was chosen based on our previous experiments demonstrating that Tregs inhibit in a dose-dependent manner the proliferation of non-Treg T cells and expression of costimulatory molecules CD80 and CD86 on DC when Tregs and non-Treg T cells are present at various ratios (11).
The T cells were added to the upper chambers in medium alone or medium containing DMSO or CCR4 antagonists (10 nM). In this setting, the T cells migrate to lower chambers of the transwells in response to chemokines secreted by TLR-stimulated DC. After 2 h incubation, the top chambers were removed. The lower chambers containing migrated CD4+ T cells and mature DC were incubated for a further 4 days. Because DC and T cells were from unrelated donors, presentation of alloantigens by TLR-stimulated DC serves as a stimulus for T cell activation. The non-Tregs were CFSE-labeled, so that their proliferation could be measured by the dilution of this fluorescent dye, which occurs upon cell division. Greater or lesser migration of Tregs toward DC would result in lower or higher proliferation respectively.
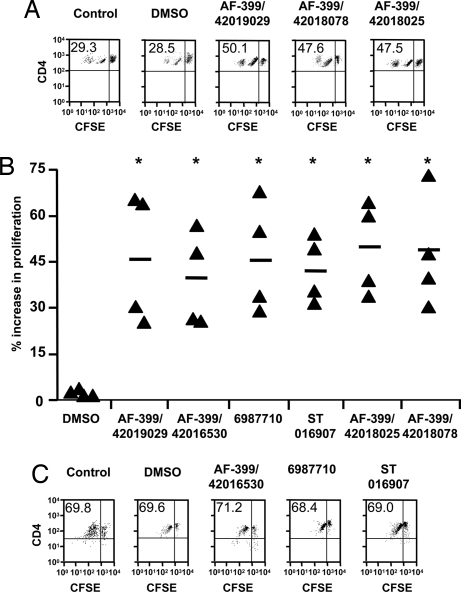
As shown in Fig. 5 A and B, DC-mediated T cell proliferation was significantly higher for T cells exposed to CCR4 antagonists than for controls (P < 0.05). The mean enhancement of proliferation of T cells was in the range of 39.1–49.2% as compared with controls. Further, the observed activities were due to bona fide CCR4 antagonism, because CCR4 antagonists did not modify human DC phenotype (data not shown) nor the mature DC-mediated chemotaxis and proliferation of CD4+CD45RA+ naïve T cells that lack Tregs in the population (Fig. 5C). Therefore, the results indicate that CCR4 antagonists enhance T cell responses by inhibiting recruitment of Tregs.
Fig. 5.
CCR4 antagonists boost DC-mediated human CD4+ T cell proliferation by blocking Treg recruitment. (A) CFSE profiles of DC-stimulated T cells treated with medium alone (Control), or with solvent (DMSO) or representative CCR4 antagonists (10 nM). The upper right quadrant represents undivided cells, whereas upper left quadrant represents cells that have divided and therefore diluted CFSE fluorescence. The values denote percent of cells that have undergone division. (B) The percent increase in DC-mediated T cell division upon exposure to CCR4 antagonists compared with controls. Percentage enhancement of T cell proliferation by CCR4 antagonists was calculated as follows: [(% divided cells in the presence of antagonist − % divided cells in control)/% divided cells in control] × 100. Statistical significance as analyzed by Mann–Whitney test is denoted by * (P < 0.05 compared with DMSO). (C) CCR4 antagonists do not modify mature DC-mediated proliferation of CD4+CD45RA+ naïve T cells lacking both Tregs in the population and expression of CCR4. The values denote percent of cells that have undergone division.
Amplification of the Immunogenicity of Vaccines by CCR4 Antagonists in Vivo.
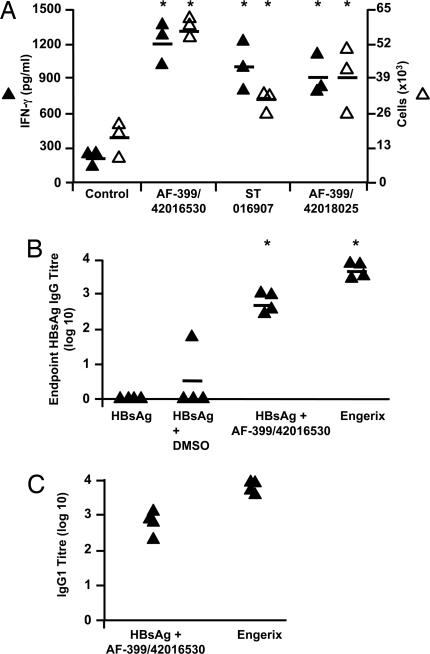
Our data demonstrating the efficacy of CCR4 antagonists in blocking the recruitment of Tregs and boosting DC-mediated T cell proliferation suggest that these compounds might exert adjuvant activity in vivo. Therefore, we examined the impact of our compounds on the quality of a primary immune response to vaccination in mice. Three compounds (AF-399/42018025, AF-399/42016530, ST 016907) were chosen, which inhibit the CCR4-mediated migration of mouse cells in vitro (data not shown). Simultaneous immunization of each of the compounds with Modified Vaccinia Ankara expressing antigen 85A of Mycobacterium tuberculosis (MVA85A) significantly enhanced the frequency of PPD-reactive IFN-γ-secreting cells (Fig. 6A). The increased cellular response by all three antagonists was also reflected in significantly greater production of IFN-γ in PPD-stimulated cultures (Fig. 6A).
Fig. 6.
CCR4 antagonists amplify immunogenicity of vaccines in vivo. (A) Assessment of T cell response in mice 6 days after MVA85A vaccination in the presence of ≈2.5 μM CCR4 antagonists (AF-399/42016530, ST 016907, and AF-399/42018025) or DMSO control. IFN-γ production by splenocytes in response to PPD was analyzed by measuring IFN-γ in the supernatants (filled triangles, pg/ml) and ELISPOT assay (open triangles, ×103 cells per spleen). Similar results were obtained in two or three independent experiments. (B) IgG responses against rHBsAg as measured by ELISA 14 days after the second vaccination of mice with rHBsAg, rHBsAg plus DMSO, rHBsAg plus AF-399/42016530 (≈2.5 μM), or Engerix-B. Four mice per group were tested individually. *, P < 0.05 compared with DMSO controls. (C) Anti-HBsAg IgG responses elicited by Engerix-B or rHBsAg plus AF-399/42016530 are of IgG1 subclass.
The potential for CCR4 antagonists to stimulate antibody responses was examined by using recombinant hepatitis B virus surface antigen (rHBsAg), ayw subtype. Immunization with HBsAg alone or HBsAg and DMSO induced minimal antibody responses (Fig. 6B). However, simultaneous administration of AF-399/42016530 with rHBsAg significantly enhanced the titer of HBsAg specific antibodies to a level similar to that of Engerix-B (Fig. 6B), a commercial alum-containing rHBsAg vaccine. Further, IgG subclass analysis revealed that the anti-HBsAg IgG response was predominantly of the IgG1 subtype in both AF-399/42016530 adjuvanted and Engerix-B immunized mice (Fig. 6C).
Discussion
Colocalization of Tregs with their target cells is important for their suppressive function. Tregs are found in a wide variety of lymphoid and nonlymphoid tissues. By altering the tissue and microenvironmental distribution of Tregs, it should be possible to modulate immune responses. The chemokine receptor CCR4 is associated with Treg migration and function (4, 24, 25). Upon maturation and activation, DC in both lymphoid and nonlymphoid tissues produce CCL22 and CCL17, and the binding of these ligands to CCR4 helps to guide Tregs toward DC (4, 7, 26). If CCR4+ Tregs fail to compete effectively with naive T cells for access to DC because CCL22 and CCL17-binding to CCR4 is blocked then this should result in increased activation and differentiation of vaccine antigen-specific effector T cells. Our results support this hypothesis, because we demonstrate in a model of the early immune response that CCR4 antagonists block Treg recruitment in response to DC-chemokines and thus enhance DC-mediated T cell proliferation. A similar mechanism may underlie the adjuvant activity of CCR4 antagonists observed in vivo.
Chemokines function by signaling through a complex system of receptors that guide the migration and interactions of immune cells and thus regulate immune responses. Several strategies have been proposed for intervening in the chemokine system to modulate immune responses (27, 28). However, designing chemokine receptor antagonists remains challenging because there is great redundancy and promiscuity in receptor-ligand interactions and a palpable lack of structural information. Previous attempts to develop CCR4 antagonists have revealed that racemic thiazolidinones display good receptor affinity but lack sufficient drug-like properties in vivo. Although switching the thiazolidinone core to lactam, pyrimidine, isoxazole, and pyrazole produced better drug-like properties, only molecules with isoxazole retained potency (29, 30).
Drawing on such work, we used a pharmacophore as the starting point for structure-based virtual screening. By docking compounds to our modeled CCR4 in silico, we identified compounds able to bind within the transmembrane region. A significant proportion of compounds exhibited CCR4-specific antagonism in vitro, validating the virtual screening process. Driven by computational compound selection, a significant enrichment over random screening is observed. This enrichment compares favorably to the outcome of robotic high-throughput screening of hundreds of thousands of compounds over weeks or months, in unsupervised assays of uncertain reliability, where signal-to-noise issues remain important. Virtual screening should prove equally valuable in identifying molecules that mediate adjuvant activity targeting other receptors, such as Toll-like receptors.
Ideally, an adjuvant should be able to induce well defined cellular and humoral immune responses to vaccine antigens. Whereas cellular immune responses reduce pathogen burden, humoral responses prevent infection. When CCR4 antagonists were tested for their adjuvant function in vivo with MVA85A and rHBsAg vaccines, enhanced immunogenicity was observed for both cellular and humoral immune responses. The cellular immune responses induced by immunization of MVA85A with CCR4 antagonists are particularly striking, given that the vaccine vector used is known to be highly immunogenic (31, 32). However, because this viral vector has inherent adjuvant activity, it was important to show that CCR4 antagonists could also enhance the response to a poorly immunogenic protein antigen, such as rHBsAg. In this model, responses nearly as great as those obtained with the commercial alum-adjuvanted vaccine, Engerix-B, were seen. Further, our in vitro results suggest that CCR4 antagonists could function similarly in humans.
Although, CCR4 antagonists block the migration of Th2 cells in response to CCL22 and CCL17, this activity may not greatly influence a primary humoral immune response, i.e., in a setting that lacks prepolarized antigen-specific Th2 cells. Indeed, the antibodies elicited using a CCR4 antagonist as an adjuvant were predominantly IgG1, which is regarded as a Th2 antibody subclass in the mouse. Whether the blocking of antigen-specific memory Th2 cells during booster immunization reduced the secondary humoral immune responses observed is not known. Nevertheless, the magnitude of the antibody response induced by the CCR4 antagonist was impressive and promising when compared with that stimulated by a marketed vaccine (Engerix-B), especially considering that we have not yet optimized the dose of CCR4 antagonists for in vivo use. In this context, the significant adjuvant activity observed provides good evidence supporting our hypothesis that CCR4 is a viable target for rational adjuvant design. Moreover, unlike alum-containing vaccines, which are good inducers of antibody responses but poor inducers of cellular immune responses (33), CCR4 antagonists were able to induce both well defined cellular and humoral immune responses.
Inhibition of Treg activity results in markedly superior immune responses to foreign antigens, pathogens and vaccines (34–37). CCR4 antagonists may have advantages over other methods of inhibiting Treg activity, such as depletion of Tregs by anti-CD25 MAbs, which has been associated with adverse consequences. Thus, injection of anti-CD25 MAbs alone or in combination with anti-CTLA-4 antibodies was shown to induce localized autoimmune disease (12, 13). Because the half-life of small molecule antagonists is generally very much shorter (≈24 h) than therapeutic MAbs (≈10–21 days) (38), transient inhibition of Treg recruitment at the time of vaccination by using CCR4 antagonists might avoid the complications caused by longer-term Treg depletion by MAbs. Although we focused our studies on prophylactic vaccination against pathogens, CCR4 antagonists might also be used as adjuvants in therapeutic vaccination against cancer (37, 39) and chronic infectious diseases such as tuberculosis (40).
Methods
In Silico Modeling.
For the creation of a homology model of CCR4, transmembrane, intracellular, and extracellular regions of CCR4 were predicted from GPCR multisequence alignments. Transmembrane regions were docked together by using bovine rhodopsin as a scaffold. WHATIF was used to generate the helical transmembrane domains (41). Termini and loops were added in an extended conformation. The complete structure was inserted into an optimized lipid bilayer (42). Hydrogen atoms were added to the structure. The system was fully solvated with TIP3 water molecules (43) using the program leap (Case DA and coresearchers), part of the AMBER suite (AMBER 6, Univ. of California). Known disulphide bonds between Cys-29-Cys-276 and Cys-110-Cys-187 were incorporated into the structure. The energy of the protein-lipid system was minimized by using the program sander.
In Vitro Experiments.
A detailed description of structure-based virtual screening and molecular docking; isolation and generation of human DC, Tregs, and Th2 cells; chemotaxis assay; in vitro immune response model; and measurement of IFN-γ and IgG are presented in SI Text.
In Vivo Experiments.
CCR4 antagonists were tested for their adjuvant function in vivo with MVA85A and rHBsAg vaccines. The experiments were performed in 6- to 8-week-old female BALB/c mice as described in SI Text.
Statistical Analysis.
Statistical analysis was performed by nonparametric Mann–Whitney test. A detailed methods section is provided in SI Text.
Supplementary Material
Acknowledgments.
This is publication number 125 from the Edward Jenner Institute for Vaccine Research (EJIVR). The work was funded by EJIVR (J.B., M.N.D., P.C.L.B., D.R.F., D.F.T.), European Commission (E.Z.T.), Wellcome Trust (S.J.D., A.V.S.H.), Jenner Vaccine Foundation (D.R.F., P.C.L.B.), Institut National de la Santé et de la Recherche Médicale and Centre National de la Recherche Scientifique (J.B., S.V.K., M.D.K.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803453105/DCSupplemental.
References
- 1.Guy B. The perfect mix: Recent progress in adjuvant research. Nat Rev Microbiol. 2007;5:505–517. doi: 10.1038/nrmicro1681. [DOI] [PubMed] [Google Scholar]
- 2.Schijns VE, Degen WG. Vaccine immunopotentiators of the future. Clin Pharmacol Ther. 2007;82:750–755. doi: 10.1038/sj.clpt.6100394. [DOI] [PubMed] [Google Scholar]
- 3.Davies MN, Flower DR. Harnessing bioinformatics to discover new vaccines. Drug Discov Today. 2007;12:389–395. doi: 10.1016/j.drudis.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Iellem A, et al. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2001;194:847–853. doi: 10.1084/jem.194.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyara M, Sakaguchi S. Natural regulatory T cells: Mechanisms of suppression. Trends Mol Med. 2007;13:108–116. doi: 10.1016/j.molmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Tang HL, Cyster JG. Chemokine up-regulation and activated T cell attraction by maturing dendritic cells. Science. 1999;284:819–822. doi: 10.1126/science.284.5415.819. [DOI] [PubMed] [Google Scholar]
- 7.Katou F, et al. Macrophage-derived chemokine (MDC/CCL22) and CCR4 are involved in the formation of T lymphocyte-dendritic cell clusters in human inflamed skin and secondary lymphoid tissues. Am J Pathol. 2001;158:1263–1270. doi: 10.1016/S0002-9440(10)64077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vulcano M, et al. Dendritic cells as a major source of macrophage-derived chemokine/CCL22 in vitro and in vivo. Eur J Immunol. 2001;31:812–822. doi: 10.1002/1521-4141(200103)31:3<812::aid-immu812>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 9.Tang Q, et al. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Houot R, Perrot I, Garcia E, Durand I, Lebecque S. Human CD4+CD25high regulatory T cells modulate myeloid but not plasmacytoid dendritic cells activation. J Immunol. 2006;176:5293–5298. doi: 10.4049/jimmunol.176.9.5293. [DOI] [PubMed] [Google Scholar]
- 11.Bayry J, Triebel F, Kaveri SV, Tough DF. Human dendritic cells acquire a semimature phenotype and lymph node homing potential through interaction with CD4+CD25+ regulatory T cells. J Immunol. 2007;178:4184–4193. doi: 10.4049/jimmunol.178.7.4184. [DOI] [PubMed] [Google Scholar]
- 12.Taguchi O, Takahashi T. Administration of anti-interleukin-2 receptor alpha antibody in vivo induces localized autoimmune disease. Eur J Immunol. 1996;26:1608–1612. doi: 10.1002/eji.1830260730. [DOI] [PubMed] [Google Scholar]
- 13.Sutmuller RP, et al. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194:823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bayry J, Flower DR, Tough DF, Kaveri SV. From ‘perfect mix’ to ‘potion magique’- regulatory T cells and anti-inflammatory cytokines as adjuvant targets. Nat Rev Microbiol. 2008;6:C1. doi: 10.1038/nrmicro1681-c1. [DOI] [PubMed] [Google Scholar]
- 15.Baldwin JM, Schertler GF, Unger VM. An alpha-carbon template for the transmembrane helices in the rhodopsin family of G-protein-coupled receptors. J Mol Biol. 1997;272:144–164. doi: 10.1006/jmbi.1997.1240. [DOI] [PubMed] [Google Scholar]
- 16.Palczewski K, et al. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 17.Dragic T, et al. A binding pocket for a small molecule inhibitor of HIV-1 entry within the transmembrane helices of CCR5. Proc Natl Acad Sci USA. 2000;97:5639–5644. doi: 10.1073/pnas.090576697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flower DR. DISSIM: A program for the analysis of chemical diversity. J Mol Graphics Model. 1998;16:239–253. doi: 10.1016/s1093-3263(98)80008-9. [DOI] [PubMed] [Google Scholar]
- 19.Sadowski J, Gasteiger J. From atoms and bonds to three-dimensional atomic coordinates: Automatic model builders. Chem Rev. 1993;93:2567–2581. [Google Scholar]
- 20.Verdonk ML, Cole JC, Hartshorn MJ, Murray CW, Taylor RD. Improved protein-ligand docking using GOLD. Proteins. 2003;52:609–623. doi: 10.1002/prot.10465. [DOI] [PubMed] [Google Scholar]
- 21.D'Ambrosio D, et al. Quantitative differences in chemokine receptor engagement generate diversity in integrin-dependent lymphocyte adhesion. J Immunol. 2002;169:2303–2312. doi: 10.4049/jimmunol.169.5.2303. [DOI] [PubMed] [Google Scholar]
- 22.Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating TH1 immune responses. Annu Rev Immunol. 2003;21:713–758. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- 23.Bonecchi R, et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee I, et al. Recruitment of Foxp3+ T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J Exp Med. 2005;201:1037–1044. doi: 10.1084/jem.20041709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sather BD, et al. Altering the distribution of Foxp3(+) regulatory T cells results in tissue-specific inflammatory disease. J Exp Med. 2007;204:1335–1347. doi: 10.1084/jem.20070081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curiel TJ, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 27.Wells TN, Power CA, Shaw JP, Proudfoot AE. Chemokine blockers-therapeutics in the making? Trends Pharmacol Sci. 2006;27:41–47. doi: 10.1016/j.tips.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Allen SJ, Crown SE, Handel TM. Chemokine: Receptor structure, interactions, and antagonism. Annu Rev Immunol. 2007;25:787–820. doi: 10.1146/annurev.immunol.24.021605.090529. [DOI] [PubMed] [Google Scholar]
- 29.Newhouse B, et al. Racemic and chiral lactams as potent, selective and functionally active CCR4 antagonists. Bioorg Med Chem Lett. 2004;14:5537–5542. doi: 10.1016/j.bmcl.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, et al. Optimization of 2-aminothiazole derivatives as CCR4 antagonists. Bioorg Med Chem Lett. 2006;16:2800–2803. doi: 10.1016/j.bmcl.2006.01.126. [DOI] [PubMed] [Google Scholar]
- 31.Goonetilleke NP, et al. Enhanced immunogenicity and protective efficacy against Mycobacterium tuberculosis of bacille Calmette-Guerin vaccine using mucosal administration and boosting with a recombinant modified vaccinia virus Ankara. J Immunol. 2003;171:1602–1609. doi: 10.4049/jimmunol.171.3.1602. [DOI] [PubMed] [Google Scholar]
- 32.McShane H, et al. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat Med. 2004;10:1240–1244. doi: 10.1038/nm1128. [DOI] [PubMed] [Google Scholar]
- 33.Hutchings CL, Gilbert SC, Hill AV, Moore AC. Novel protein and poxvirus-based vaccine combinations for simultaneous induction of humoral and cell-mediated immunity. J Immunol. 2005;175:599–606. doi: 10.4049/jimmunol.175.1.599. [DOI] [PubMed] [Google Scholar]
- 34.Suvas S, Kumaraguru U, Pack CD, Lee S, Rouse BT. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J Exp Med. 2003;198:889–901. doi: 10.1084/jem.20030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haribhai D, et al. Regulatory T cells dynamically control the primary immune response to foreign antigen. J Immunol. 2007;178:2961–2972. doi: 10.4049/jimmunol.178.5.2961. [DOI] [PubMed] [Google Scholar]
- 36.Easterbrook JD, Zink MC, Klein SL. Regulatory T cells enhance persistence of the zoonotic pathogen Seoul virus in its reservoir host. Proc Natl Acad Sci USA. 2007;104:15502–15507. doi: 10.1073/pnas.0707453104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beyer M, Schultze JL. Regulatory T cells in cancer. Blood. 2006;108:804–811. doi: 10.1182/blood-2006-02-002774. [DOI] [PubMed] [Google Scholar]
- 38.Tabrizi MA, Tseng CM, Roskos LK. Elimination mechanisms of therapeutic monoclonal antibodies. Drug Discov Today. 2006;11:81–88. doi: 10.1016/S1359-6446(05)03638-X. [DOI] [PubMed] [Google Scholar]
- 39.Vence L, et al. Circulating tumor antigen-specific regulatory T cells in patients with metastatic melanoma. Proc Natl Acad Sci USA. 2007;104:20884–20889. doi: 10.1073/pnas.0710557105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scott-Browne JP, et al. Expansion and function of Foxp3-expressing T regulatory cells during tuberculosis. J Exp Med. 2007;204:2159–2169. doi: 10.1084/jem.20062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vriend G. WHATIF: A molecular modeling and drug design program. J Mol Graphics. 1990;8:52–56. doi: 10.1016/0263-7855(90)80070-v. [DOI] [PubMed] [Google Scholar]
- 42.Pandit SA, Bostick D, Berkowitz ML. Molecular dynamics simulation of a dipalmitoylphosphatidylcholine bilayer with NaCl. Biophys J. 2003;84:3743–3750. doi: 10.1016/S0006-3495(03)75102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML. Comparison of simple potential functions for simulating liquid water. J Chem Phys. 1983;79:926–935. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.