Abstract
The production of reactive oxygen species (ROS) exerts an additional tier of control over tyrosine phosphorylation-dependent signal transduction by transiently inhibiting the catalytic activity of specific protein tyrosine phosphatases (PTPs). Hence, the ability to detect reversible oxidation of PTPs in vivo is critical to understanding the complex biological role of ROS in the control of cellular signaling. Here, we describe an assay for identifying those PTPs that are reversibly oxidized in vivo, which utilizes the unique chemistry of the invariant catalytic Cys residue in labeling the active site with biotinylated small molecules under mildly acidic conditions. We have applied this cysteinyl-labeling assay to the study of platelet-derived growth factor (PDGF) receptor signaling in an angiomyolipoma cell model. Doing so has allowed us to detect reversible oxidation of several proteins in response to sustained PDGF stimulation. As in other cell systems, we have observed the reversible oxidation of the classical PTP SHP2 and the tumor suppressor phosphatase PTEN in response to PDGF stimulation. Furthermore, we detected reversible oxidation of members of two other subclasses of PTPs, the receptor PTP LAR and the dual-specificity phosphatase MKP1. These data demonstrate the broad selectivity of the assay, allowing us to detect representatives of all of the major subgroups of the PTP superfamily. We anticipate that this cysteinyl-labeling enrichment strategy can be applied broadly to study reversible oxidation as a mechanism of harnessing PTP catalytic activity in a variety of signaling pathways.
Keywords: reactive oxygen species, signal transduction, tyrosine phosphorylation, dual specificity phosphatases, cancer
Regulated and localized generation of reactive oxygen species (ROS) creates an oxidative microenvironment important for optimal tyrosine phosphorylation-dependent signal transduction. Previous studies have shown that ROS-mediated transient inhibition of the catalytic activity of members of the protein tyrosine phosphatase (PTP) superfamily plays a crucial role in facilitating signal transduction (1–3). The PTP superfamily consists of ≈100 genes encoding structurally diverse enzymes, which are characterized by a conserved catalytic domain and which all perform phosphoryl hydrolysis by using a cysteinyl-based nucleophilic core within a conserved signature motif. The PTP signature motif, HC(X)5R(S/T), creates a unique environment for the catalytic Cys residue. In this setting, the pKa of the Cys residue, located at the base of a cleft formed by the extremity of β-sheet 12 and α-helix 4 [features named for the structure of PTP1B (4)], is lowered by the presence of the conserved Arg residue, in addition to the helix dipole of α-4 and microdipoles generated by backbone atoms in the signature motif (4, 5). Hence, the Cys residue of the signature motif displays an unusually low pKa, making it both a good nucleophile at neutral pH in addition to being highly susceptible to oxidation (6). The sensitivity of PTPs to inactivation by oxidation or alkylation of the active-site Cys was recognized early in the characterization of these enzymes (7).
A substantial body of literature reports the regulated production of ROS in response to various cellular stimuli, including peptide growth factors, cytokines, G protein-coupled receptor agonists, and stress (8). In such cellular contexts, ROS serve as second-messenger molecules and cause oxidation of specific residues in proteins leading to a change in the activity or function of the oxidized protein. In the case of the PTPs, oxidation impairs catalysis by abrogating the nucleophilic function of the active-site Cys residue. In this context, it is important to note that a recent study, in which the oxidation state of PTP1B in cancer cells was characterized by mass spectrometry, revealed that this modification was specific to the catalytic Cys residue (9), highlighting the importance of the architecture of the active site in determining the sensitivity of the catalytic Cys residue in PTPs to this regulatory modification. For classical PTPs, such as PTP1B and receptor-like tyrosine phosphatase α (RPTPα), oxidation of the catalytic cysteine to sulfenic acid is followed by a rapid condensation reaction to yield a cyclic sulfenamide (10, 11); this modification favors reversible inhibition over the irreversible oxidation to sulfinic and sulfonic acid. There have been reports of glutathionylation of PTP1B (12), which may also contribute to promoting reactivation of the enzyme after oxidation. Other members of the PTP superfamily, such as the dual-specificity phosphatases (DSPs) PTEN and cdc25C, and the low-molecular-weight PTP (6), are protected from irreversible oxidation by forming a disulfide bond with a vicinal cysteine within the active site. It appears that reversible oxidation has the potential to be a general mechanism of harnessing PTP activity, ultimately conferring an additional layer of regulation over signaling networks.
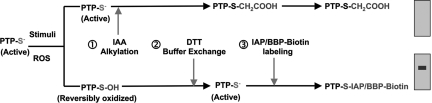
To devise a sensitive method to enrich and identify oxidized PTPs that can be applied across the PTP superfamily as a whole, we have used the low pKa of the invariant catalytic Cys residue as the basis for a labeling strategy. A three-step method has been developed in which the reversibly oxidized catalytic Cys residues are specifically biotinylated (Fig. 1). After a physiological stimulus, cells were lysed under anaerobic conditions in the presence of iodoacetic acid (IAA), an alkylating agent that was used to modify covalently the thiolate anion of the active-site Cys in those PTPs that remained in a reduced state. In contrast, any PTPs that were oxidized by the localized, stimulus-induced production of ROS were protected from this irreversible alkylation. Lysates were then subjected to buffer exchange on a size-exclusion chromatography column. In this key step, IAA was removed, and the addition of a reducing buffer allowed the oxidized PTPs to be reduced back to the active state, in which they may react with biotinylated active-site-directed compounds. The two biotinylated compounds used were a sulfhydryl-reactive iodoacetylpolyethylene oxide (IAP) probe or an α-bromobenzylphosphonate (BBP) activity-based probe. Those PTPs that were oxidized in the original sample can then be identified after purification by streptavidin pull-down and visualization with streptavidin–horseradish peroxidase (HRP). Because the composition of the PTP superfamily has been defined, the apparent molecular mass of the reactive proteins can be used to predict identity, which can subsequently be tested by immunoblotting for candidate PTPs.
Fig. 1.
Schematic outline of the cysteinyl-labeling assay. An active PTP is indicated with the active-site cysteinyl residue as a thiolate anion, representing the cell at a resting state. (1) After a physiological stimulus, the cells were lysed in a degassed buffer at pH 5.5 containing IAA. The low-pKa Cys residue at the active site of those PTPs that remained in a reduced state are alkylated, terminally inactivating this pool of PTPs. Conversely, the Cys residues that were oxidized by second-messenger ROS molecules were protected from irreversible alkylation. (2) IAA was then removed from the lysate by buffer exchange using size-exclusion spin columns, and the oxidized Cys residues were reduced back to the thiolate ion with DTT. (3) The oxidized PTPs were maintained in pH 5.5 buffers and incubated with either of two biotinylated, active-site-directed compounds, a sulfhydryl-reactive IAP probe or a BBP activity-based probe. Purification by streptavidin pull-down and immunoblotting permits identification of ROS-targeted PTPs.
We have applied this assay to the study of PDGF receptor-induced signaling in angiomyolipoma cells. We detected PDGF-induced reversible oxidation of members of both classes of classical PTPs (receptor-like and nontransmembrane PTPs) and of two different classes of DSPs (VH1-like and PTEN-like), which demonstrates the broader sensitivity of this improved assay compared with preexisting techniques. This three-step cysteinyl-labeling method for detecting reversible oxidation of the entire PTP superfamily will help delineate new signaling cascades and improve our understanding of the complex biology of ROS signaling in vivo.
Results
Measurement of PTP Oxidation by Using a Sulfhydryl-Reactive Probe in PDGF-Transformed Angiomyolipoma Cells.
Because ROS have been shown to be important in the regulation of signaling in response to PDGF (13, 14), we chose to test this assay in a PDGF-transformed cell system. Angiomyolipomas are benign tumors of the kidney that express functional PDGF receptors (PDGFRs). A cell line that was derived from a human angiomyolipoma, by immortalization after sequential introduction of SV40 Large T and telomerase (SV7tert), was observed to express active PDGFRβ but did not form tumors in nude mice (15). However, upon constitutive retroviral expression of PDGF-BB, the cells became tumorigenic (15), which was associated with increased ROS production and enhanced levels of tyrosine phosphorylation, in particular on the PDGFR (data not shown).
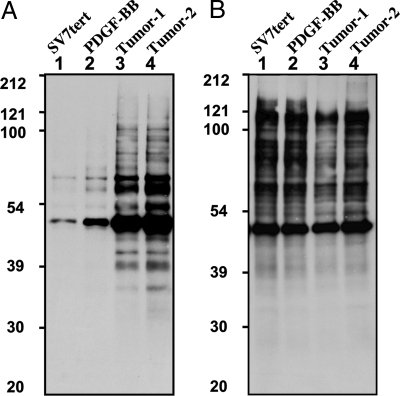
Considering that members of the PTP superfamily are highly susceptible to oxidation by the second-messenger molecule hydrogen peroxide (H2O2), we examined their oxidation in these angiomyolipoma cell populations by using this modified cysteinyl-labeling assay. First, we tested the biotinylated IAP probe, which reacts via a nucleophilic substitution of the halide group by the PTP-reactive thiol group, resulting in a stable thioether bond. As shown in Fig. 2A, in the immortalized cells, in which the levels of ROS are low, there was minimal labeling of proteins; however, in the PDGF-BB-transformed cells and tumor-derived cell populations, several reactive proteins were detected.
Fig. 2.
Detection of reversible PTP oxidation by using a sulfhydryl-reactive IAP probe. Serum-deprived (16 h) angiomyolipoma cell lines (lanes: 1, SV7tert; 2, SV7tert-PDGF; 3, SV7tertPDGF-Tumor1; 4, SV7tertPDGF-Tumor2) were subjected to the cysteinyl-labeling assay by using biotinylated IAP at pH 5.5 (A) or pH 8 (B). Biotinylated proteins were purified on streptavidin–Sepharose beads, resolved by SDS/PAGE, and visualized by using streptavidin–HRP.
Thiols are alkylated in their anionic form. Therefore, these assays were performed at pH 5.5 to favor the low pKa-reactive Cys residue that is a characteristic of the active site of members of the PTP superfamily. When the assay was performed at pH values closer to the pKa of noncatalytic Cys residues (i.e., 8.5), the extent of protein labeling overall was increased, but the apparent specificity observed at pH 5.5 was lost (Fig. 3B).
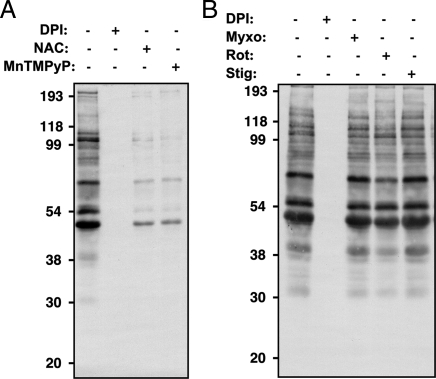
Fig. 3.
Reversible PTP oxidation in PDGF-BB-derived tumor cells. Tumor-derived PDGF-transformed SV7tert cells were serum-deprived (48 h) and treated for a 48-h period with (A) 25 μM DPI, 100 μM NAC, or 5 μM MnTMPyP; or (B) 25 μM DPI, 10 μM myxothiazol, 100 μM rotenone, or 10 μM stigmatellin, then subjected to the cysteinyl-labeling assay with the biotinylated IAP probe. Biotinylated proteins were purified on streptavidin–Sepharose beads, resolved by SDS/PAGE, and visualized by using streptavidin–HRP.
Characterization of the Source of ROS-Induced PTP Oxidation in PDGF-BB-Transformed Angiomyolipoma Cells.
We tested the effects of various ROS antagonists, both to ascertain whether the signal we detected using the cysteinyl-labeling assay in the PDGF-transformed angiomyolipoma tumor cells occurred as a result of PDGF-induced oxidation and to identify the potential sources of cellular ROS. The signal was decreased by N-acetylcysteine (NAC) and Mn(III)tetrakis(1-methyl-4-pyridyl)porphyrin (MnTMPyP), scavengers of H2O2 and of superoxide, respectively, and was abolished by diphenylene iodonium (DPI) (Fig. 3A). Conversely, a specific inhibitor of complex I (rotenone), and two specific inhibitors of complex III (myxothiazol and stigmatellin) of the mitochondrial electron transfer chain were without effect (Fig. 3B). Thus, signal detection in the assay requires ROS production, attesting to specificity. Furthermore, even though the NADPH oxidase (NOX) inhibitor DPI influences both NOX and the dehydrogenase from complex I, the lack of effect observed from the other mitochondrial electron transport chain inhibitors suggests that PDGF-induced oxidation may occur via a NOX-mediated H2O2 production.
Determination of the PTP-Labeling Specificity by Using a Phosphonate-Based Probe.
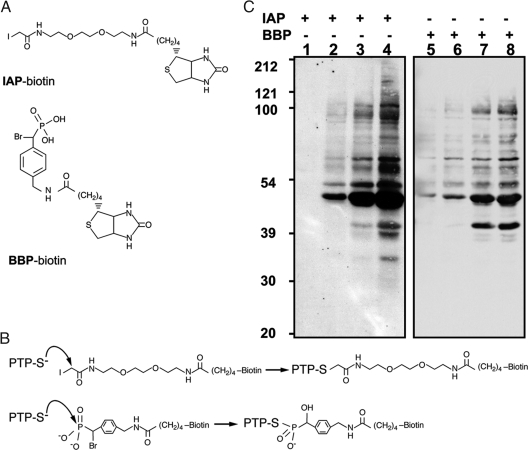
To explore further the specificity of the assay toward PTPs, we tested a second active-site-directed probe, BBP, in this model (Fig. 4A). Although both IAP and BBP modify a Cys residue, they do so by distinct mechanisms (Fig. 4B). The BBP probe is a phosphonate-based molecule that has been shown to display specificity toward PTPs (16). Unlike the IAP probe, which forms a stable thioether bond with the reactive thiol group, BBP interacts with the PTP active site as would a phosphorylated tyrosyl residue in a substrate and forms a nonhydrolyzable cysteinyl-phosphate bond (16). Therefore, the specificity of the probes was assessed in each of the cell populations. Interestingly, a similar pattern of reactive proteins was observed in both IAP and BBP labeling assays, suggesting that labeling with two probes possessing different biochemical properties leads to the detection of oxidized PTPs when the assay is performed at pH 5.5 (Fig. 4C).
Fig. 4.
Comparison of IAP- and BBP- based probes for detection of reversibly oxidized PTPs in PDGF-BB-transformed angiomyolipoma cells. (A) Structures of biotinylated IAP and BBP probes. (B) Mechanisms of PTP labeling by the IAP and BBP probes. (C) Serum-deprived (16 h) angiomyolipoma cell lines (lanes: 1 and 5, SV7tert; 2 and 6, SV7tert-PDGF; 3 and 7, SV7tertPDGF-Tumor1; 4 and 8, SV7tertPDGF-Tumor2) were subjected to the cysteinyl-labeling assay using either biotinylated IAP (lanes 1–4) or BBP probes (lanes 5–8). Biotinylated proteins were purified on streptavidin–Sepharose beads, resolved by SDS/PAGE and visualized by using streptavidin–HRP.
Identification of Reversibly Oxidized PTPs in Angiomyolipoma Cells.
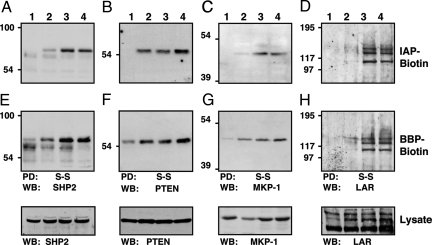
Transduction of angiomyolipoma cells with recombinant retrovirus expressing PDGF-BB triggered a variety of changes in cell signaling that culminated in a transformed phenotype, some of which were reversible by PTK inhibitors (15). As a first step to deciphering these changes in signaling, we undertook the identification of PTPs that were targeted by PDGF-induced H2O2 by performing cysteinyl-labeling assays in parallel on SV7tert and PDGF-transformed cell populations by using IAP or BBP probes. There are reports in the literature of oxidation of both SHP-2 and PTEN upon PDGFR signaling (14, 17). The enrichment of oxidized PTPs via indirect cysteinyl biotinylation allowed us to confirm SHP-2 and PTEN as targets of PDGF-induced ROS in these cells (Fig. 5A B, E, and F). In addition, previously unrecognized targets of PDGF-induced oxidation were identified, including the DSP mitogen-activated protein kinase phosphatase-1 (MKP-1) (Fig. 5 C and G). Furthermore, we detected the reversible oxidation of a receptor PTP LAR (leukocyte common antigen-related), a member of the R2A subtype of receptor-like PTPs (1), in the PDGF-transformed angiomyolipoma cells (Fig. 5 D and G). These data highlight the broad selectivity of the assay, which provides the means to detect reversible oxidation of representatives of all of the major subgroups of the PTP superfamily.
Fig. 5.
Identification of reversibly oxidized PTPs in PDGF-BB-transformed angiomyolipoma cells by using IAP- and BBP-based-probes. Serum-deprived (16 h) angiomyolipoma cell lines (lanes: 1, SV7tert; 2, SV7tert-PDGF; 3, SV7tertPDGF-Tumor1; 4, SV7tertPDGF-Tumor2) were subjected to the cysteinyl-labeling assay using either biotinylated IAP (A–D) or BBP probes (E and F). Biotinylated proteins were purified on streptavidin–Sepharose beads, resolved by SDS/PAGE, and immunoblotted against SHP2 (A and E), PTEN (B and F), MKP-1 (C and G), or LAR (D and H). Reversible PTP oxidation was restricted to PDGF-BB-transformed angiomyolipoma cells using both probes. WB, Western blotting.
Oxidation of Receptor-Like PTP-α upon Treatment of Angiomyolipoma Cells with H2O2.
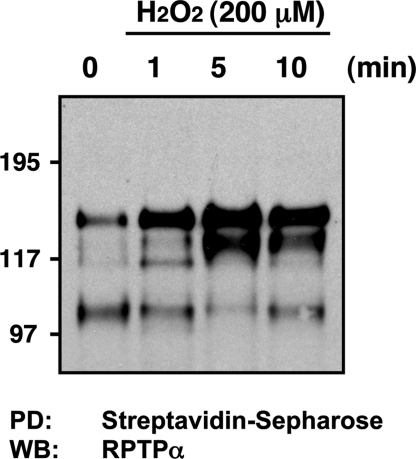
Because the oxidation of receptor-like PTPs is not registered in broad-spectrum assay formats such as the in-gel assay (18), which depends on renaturation of a catalytically functional enzyme, we tested further the effectiveness of this cysteinyl-labeling assay in detecting reversible oxidation of RPTPs in cells. After exposure of SV7tert cells to H2O2, we observed a time-dependent enrichment of RPTPα, with a peak of cysteinyl-biotinylation at 5 min (Fig. 6). The fact that oxidation of RPTPα was only observed after treatment with H2O2 and not in the PDGF-transformed cells also attests to the specificity of the assay in its ability to distinguish oxidation of different RPTPs in response to distinct conditions.
Fig. 6.
RPTPα reversible oxidation is detected by using the cysteinyl-labeling assay. Serum-deprived (16 h) angiomyolipoma SV7tert cells were exposed to 200 μM H2O2 for 1, 5, or 10 min and subjected to the cysteinyl-labeling assay using a biotinylated IAP probe. Biotinylated proteins were purified on streptavidin–Sepharose beads, resolved by SDS/PAGE, and immunoblotted by using anti-RPTPα antiserum.
Discussion
The redox machinery that controls the level, half-life, and subcellular location of ROS is rapidly emerging as a critical determinant of the fine-tuning of tyrosine phosphorylation-dependent signal transduction (1, 8). The reversible oxidation/inactivation of members of the PTP superfamily regulates the balance between kinase and phosphatase activity and is now known to contribute directly to an elevation in tyrosine phosphorylation in response to a wide variety of physiological stimuli. At this point, PTPs appear to be the primary targets of ROS when acting on signal transduction cascades, and we describe here a cysteinyl-labeling assay that enabled us to enrich and detect the reversible oxidation of members of the PTP superfamily in vivo. Using cells derived from a human angiomyolipoma tumor (15), we have shown that sustained PDGF-BB stimulation led to the reversible oxidation of at least four members of the PTP superfamily: LAR, SHP-2, PTEN, and MKP-1.
A variety of approaches have been taken to the measurement of PTP oxidation, each with its own challenges and technical limitations. These approaches include monitoring changes in PTP activity in response to ROS (19, 20), which faces the technical difficulty of maintaining the PTP in a catalytically active state while leaving its redox status unperturbed after lysis. In addition, although it is possible to examine changes in activity of specific PTPs in immunoprecipitates, it is hard to predict which members of the PTP family to test. There have been several reports in which alkylating agents have been used to determine the oxidation status of specific members of the PTP family. In their pioneering study (21), Rhee and colleagues demonstrated the EGF-induced oxidation of PTP1B by following the decrease in covalent modification of PTP1B by radioactively labeled IAA in cell lysates after growth factor stimulation. The assay is based on the fact that upon oxidation the active-site Cys residue is no longer susceptible to alkylation. Similar approaches have been applied in other systems (17, 22). A disadvantage of such a strategy is the fact that it measures oxidation by following the decrease in labeling of the PTP, which limits the dynamic range of the assay. Although these approaches can be applied to specific PTPs through immunoprecipitation, small changes in oxidation would still be hard to detect. Oxidation of some members of the PTP superfamily, in particular DSPs, such as PTEN (23) and MKP3 (24), has been followed by monitoring changes in electrophoretic mobility; however, this approach is also limited by the requirement for prior knowledge of the identity of the oxidized PTP. For screening purposes, an assay format that can be applied across the PTP family as a whole and measures the appearance of the oxidized form, rather than a decrease in the reduced form, is required. To date, this has been addressed with two different approaches.
The modified in-gel phosphatase assay is a strategy for enriching those PTPs that have encountered ROS and become oxidized, while eliminating the background of those PTPs that remain in the reduced state (18). This task is achieved by harvesting cell lysates under anaerobic conditions, to prevent oxidation after lysis, in a buffer supplemented with an alkylating agent, which irreversibly inactivates any PTPs in a reduced state. In contrast, the invariant Cys of oxidized PTPs is protected from alkylation. The activity of this latter pool of PTPs can be regenerated and visualized in an in-gel phosphatase assay. It is this aspect of the approach that presents technical challenges. An aliquot of cell lysate is subjected to SDS/PAGE in a gel that has been cast containing a radioactively labeled substrate. Proteins in the gel are sequentially denatured and then renatured in the presence of reducing reagents, which restores the activity of those PTPs in which the active-site Cys had been subjected to stimulus-dependent oxidation. In contrast, those PTPs that were not oxidized in response to the initial stimulus and were irreversibly alkylated in the lysis step remain inactive and are not registered in the assay. During the renaturation step, dephosphorylation of substrate occurs in the region of the gel immediately surrounding the active PTP proteins. The reaction is then terminated by staining and destaining of the gel, which is finally dried and exposed to film. The presence of a PTP is visualized by substrate dephosphorylation and the appearance of a clear, white area on the black background of labeled substrate in the autoradiogram. The success of this approach depends on the ability of the PTP family members to renature in an enzymatically active form in an SDS/polyacrylamide gel, but the extent to which they do so varies. In particular, the receptor-like PTPs are unable to renature in the SDS/polyacrylamide gel, and so oxidation of this large subgroup of the PTPs cannot be measured by this approach.
An alternative strategy uses an antibody that recognizes terminally oxidized PTPs. This antibody was generated against a short peptide, VHCSAG, representing a consensus sequence for the conserved signature motif at the active site of members of the PTP superfamily, in which the Cys residue had been oxidized to sulfonic acid by treatment with performic acid. The strategy also involves cell lysis in the presence of IAA, to alkylate reduced Cys residues, and reduction of any reversibly oxidized PTPs back to the active state. However, in a separate step, these PTPs are then terminally oxidized to the sulfonic acid form by treatment with pervanadate and identified by immunoblotting with the oxPTP antibodies (25). This approach has been used successfully to distinguish the susceptibility of different PTPs to oxidation (26), in particular demonstrating that the second, membrane-distal PTP domain of RPTPα is more sensitive to oxidation than the membrane-proximal catalytic domain and may serve as a redox sensor (26). A problem with this approach is that it would measure not only those PTPs that were subject to regulation by reversible oxidation, but also register any PTPs that were already present in the cell in a terminally oxidized state. This issue could be significant in cancer cells (9). Other potential challenges include the specificity and sensitivity of the antibody. Most of the studies to date that have used this approach have examined the oxidation of ectopically expressed classical PTPs (25, 26). It remains to be established whether this antibody is able to recognize all members of the PTP superfamily, in particular the DSPs, and to what extent it has the sensitivity to measure oxidation of PTPs at physiological levels of expression.
The strategy described here overcomes several of the technical challenges that are encountered with existing methods. As in the modified in-gel phosphatase assay, lysis under anaerobic conditions in the presence of an alkylating agent simplifies the mixture by eliminating any PTPs that have not encountered ROS, thereby focusing attention on the pool of these enzymes that have been regulated by reversible oxidation. In replacing the in-gel assay by labeling with biotinylated probes, we have removed the requirement for denaturation/renaturation cycles and activity-based detection, and, consequently, we can now detect the reversible oxidation of receptor PTPs. Because the presence of the sulfhydryl group as a thiolate ion directly determines the susceptibility to such labeling, we conducted the assays at a pH close to the pKa of the PTP catalytic cysteine. By performing the assay under mildly acidic conditions, side reactions with noncatalytic cysteines and other amino acids, which have been shown to occur at higher pH, appeared to be minimized, thereby enhancing specificity. Furthermore, the use of biotinylated active-site-directed probes not only promotes enrichment of low abundance PTPs, but also permits detection on a blot with streptavidin–HRP, thereby providing information on the apparent Mr of the reactive proteins and facilitating identification by immunoblotting.
Recent progress in defining mechanisms for regulation of RPTP function has revealed the roles of oxidation of the membrane-distal (D2) PTP domain both in RPTPα dimerization and in sensing intracellular redox status (27). RPTP dimerization is thought to be inhibitory to catalytic function. Furthermore, the conformational change induced in the extracellular segment upon oxidation of the RPTPα D2 domain has the potential to affect ligand binding in an “inside-out” manner. Hence, it appears that oxidation is an important mechanism of regulating RPTP function. Using this cysteinyl-labeling assay, we have been able to detect the reversible oxidation of LAR in response to PDGF and RPTPα in response to treatment with H2O2 in the angiomyolipoma cells. To our knowledge, this assay is the first to display a broad sensitivity across all of the PTP superfamily, including the RPTPs, and should be useful in clarifying the roles of these enzymes and their oxidation in the regulation of signal transduction.
The design of specific protein-modifying reagents that can drive proteomic analyses is a cornerstone of modern functional studies of distinct enzyme families within a complex proteome. The pronounced similarity in biotin labeling observed between the alkylating agent IAP and the PTP active-site-directed probe BBP suggests that this assay is measuring PTP oxidation effectively in these cells. Furthermore, we have used this assay to detect PTP oxidation in response to acute stimulation by PDGF in other cell systems, for example Rat-1 fibroblasts (data not shown). In the future, the use of BPP as a suicide substrate probe to enrich PTPs that are reversibly oxidized in vivo may be integrated into proteomic-based approach to define the specific pool of reversibly oxidized PTPs in various signaling contexts. There are small differences in biotin labeling observed between IAP and BBP, which raises the possibility of other ROS targets possessing low pKa catalytic Cys residues that may be involved in signaling networks. It will be interesting to ascertain whether, and by what mechanism, other ROS-regulated proteins may have an impact on growth factor-induced signals.
Experimental Procedures
Materials.
SHP-2 (sc424) and PTP-LAR (sc25434) antibodies were purchased from Santa Cruz Biotechnology. Anti-PTEN (9552) was from Cell Signaling Technology. The MKP-1 antibody was described in ref. 28, and the PTPα antibody was a kind gift from Jeroen Den Hertog (Hubrecht Institute). Zeba desalt spin columns (89889), IAA (35603), and EZ-link iodoacetyl-PEO (21334) biotin probes were from Pierce Biotechnology. Biotinylated BBP was prepared as described in ref. 16.
Preparation of Lysates.
Cells were routinely maintained in low-glucose DMEM supplemented with 10% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin (15). Cells were grown to confluence and serum-starved before lysis as indicated. Cells were lysed essentially as described in ref. 18. To prevent the spontaneous oxidation of PTPs through the air oxygen dissolved in solution, the freshly prepared lysis buffer [25 mM sodium acetate (pH 5.5), 1% Nonidet P-40, 150 mM NaCl, 10% (vol/vol) glycerol, 25 μg/ml aprotinin, 25 μg/ml leupeptin] was degassed at 30–35 in-Hg on a vacuum line for 1 h and placed on ice. Under these conditions, the pO2 level of the lysis buffer was 0.35–0.50 ppm, and no lysis-induced oxidation was observed. The degassed buffer was supplemented with freshly prepared 10 mM IAA, 100 μg/ml catalase, and superoxide dismutase (219261 and 574594; Calbiochem) before use. Cell plates were moved inside an anaerobic work station, purged, and constantly supplied with ultrapure argon gas for further handling. After removing the DMEM, cells were rapidly lysed with ice-cold IAA-supplemented degassed buffer and transferred to brown-colored tubes.
Cysteinyl-Labeling Assay.
Lysates were placed on a shaker for 1 h at room temperature to allow complete alkylation of free thiols. Cell debris were then cleared by centrifugation at 12,000 × g for 3 min at room temperature. Protein concentrations were determined by the method of Bradford, and 1 mg of cell lysate was slowly applied to desalting columns that had been equilibrated with IAA-free lysis buffer. Buffer exchange was performed by centrifuging at 2,000 × g for 2 min at 4°C. IAA-cleared lysates were then supplemented with 1 mM DTT and allowed to incubate for 30 min on a shaker at room temperature. During this phase, cyclic sulfenamide and sulfenic acid forms of the active-site Cys residues, which were protected from alkylation in the previous step, were reduced back to their thiolate states. After this crucial step, the lysates were incubated with biotinylated BBP (16) or IAP probes (1 mM, 5 mM) for 1 h on a shaker at room temperature. Biotinylated proteins were enriched by using streptavidin–Sepharose beads for 16 h at 4°C on a rotating wheel, with sequential rounds of centrifugation (12,000 × g, 1 min, 4°C) using PBS to wash the beads. The beads were resuspended in 20 μl of 4× Laemmli sample buffer and heated at 90°C for 1 min.
Acknowledgments.
This work was supported by National Institutes of Health Grant R01-GM55989 (to N.K.T.), Cold Spring Harbor Laboratory Cancer Center Support Grant P30-CA45508 and R01-CA69202 (to Z.Y.Z.). J.L.A. was supported by a Veterans Administration Merit Award, National Institutes of Health Grant 5R01AR050727, and grants from the Jamie Rabinowitch-Davis Foundation and the Minsk Foundation. B.B. was the recipient of a postdoctoral fellowship from the Heart and Stroke Foundation of Canada and was supported by the Cold Spring Harbor Laboratory Association.
Footnotes
The authors declare no conflict of interest.
References
- 1.Tonks NK. Protein tyrosine phosphatases: From genes, to function, to disease. Nat Rev Mol Cell Biol. 2006;7:833–846. doi: 10.1038/nrm2039. [DOI] [PubMed] [Google Scholar]
- 2.Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15:247–254. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 3.Rhee SG, et al. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr Opin Cell Biol. 2005;17:183–189. doi: 10.1016/j.ceb.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Barford D, Flint AJ, Tonks NK. Crystal structure of human protein tyrosine phosphatase 1B. Science. 1994;263:1397–1404. [PubMed] [Google Scholar]
- 5.Denu JM, Dixon JE. Protein tyrosine phosphatases: Mechanisms of catalysis and regulation. Curr Opin Chem Biol. 1998;2:633–641. doi: 10.1016/s1367-5931(98)80095-1. [DOI] [PubMed] [Google Scholar]
- 6.Salmeen A, Barford D. Functions and mechanisms of redox regulation of cysteinyl-based phosphatases. Antioxid Redox Signal. 2005;7:560–577. doi: 10.1089/ars.2005.7.560. [DOI] [PubMed] [Google Scholar]
- 7.Denu JM, Tanner KG. Specific and reversible inactivation of protein tyrosine phosphatases by hydrogen peroxide: Evidence for a sulfenic acid intermediate and implications for redox regulation. Biochemistry. 1998;37:5633–5642. doi: 10.1021/bi973035t. [DOI] [PubMed] [Google Scholar]
- 8.Rhee SG. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 9.Lou YW, et al. Redox regulation of the protein tyrosine phosphatase PTP1B in cancer cells. FEBS J. 2008;275:69–88. doi: 10.1111/j.1742-4658.2007.06173.x. [DOI] [PubMed] [Google Scholar]
- 10.Salmeen A, et al. Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature. 2003;423:769–773. doi: 10.1038/nature01680. [DOI] [PubMed] [Google Scholar]
- 11.Yang J, et al. Reversible oxidation of the membrane distal domain of receptor PTPα is mediated by a cyclic sulfenamide. Biochemistry. 2007;46:709–719. doi: 10.1021/bi061546m. [DOI] [PubMed] [Google Scholar]
- 12.Barrett WC, et al. Roles of superoxide radical anion in signal transduction mediated by reversible regulation of protein-tyrosine phosphatase 1B. J Biol Chem. 1999;274:34543–34546. doi: 10.1074/jbc.274.49.34543. [DOI] [PubMed] [Google Scholar]
- 13.Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 14.Meng TC, Fukada T, Tonks NK. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell. 2002;9:387–399. doi: 10.1016/s1097-2765(02)00445-8. [DOI] [PubMed] [Google Scholar]
- 15.Govindarajan B, et al. Malignant transformation of human cells by constitutive expression of platelet-derived growth factor-BB. J Biol Chem. 2005;280:13936–13943. doi: 10.1074/jbc.M500411200. [DOI] [PubMed] [Google Scholar]
- 16.Kumar S, et al. Activity-based probes for protein tyrosine phosphatases. Proc Natl Acad Sci USA. 2004;101:7943–7948. doi: 10.1073/pnas.0402323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwon J, et al. Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc Natl Acad Sci USA. 2004;101:16419–16424. doi: 10.1073/pnas.0407396101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meng TC, Tonks NK. Analysis of the regulation of protein tyrosine phosphatases in vivo by reversible oxidation. Methods Enzymol. 2003;366:304–318. doi: 10.1016/s0076-6879(03)66023-4. [DOI] [PubMed] [Google Scholar]
- 19.Mahadev K, Zilbering A, Zhu L, Goldstein BJ. Insulin-stimulated hydrogen peroxide reversibly inhibits protein-tyrosine phosphatase 1b in vivo and enhances the early insulin action cascade. J Biol Chem. 2001;276:21938–21942. doi: 10.1074/jbc.C100109200. [DOI] [PubMed] [Google Scholar]
- 20.Singh DK, et al. The strength of receptor signaling is centrally controlled through a cooperative loop between Ca2+ and an oxidant signal. Cell. 2005;121:281–293. doi: 10.1016/j.cell.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 21.Lee SR, Kwon KS, Kim SR, Rhee SG. Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. J Biol Chem. 1998;273:15366–15372. doi: 10.1074/jbc.273.25.15366. [DOI] [PubMed] [Google Scholar]
- 22.Kwon J, et al. Receptor-stimulated oxidation of SHP-2 promotes T cell adhesion through SLP-76-ADAP. EMBO J. 2005;24:2331–2341. doi: 10.1038/sj.emboj.7600706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee SR, et al. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem. 2002;277:20336–20342. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- 24.Kamata H, et al. Reactive oxygen species promote TNFα-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 25.Persson C, et al. Preferential oxidation of the second phosphatase domain of receptor-like PTP-α revealed by an antibody against oxidized protein tyrosine phosphatases. Proc Natl Acad Sci USA. 2004;101:1886–1891. doi: 10.1073/pnas.0304403101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Groen A, et al. Differential oxidation of protein-tyrosine phosphatases. J Biol Chem. 2004;280:10298–10304. doi: 10.1074/jbc.M412424200. [DOI] [PubMed] [Google Scholar]
- 27.van der Wijk T, Overvoorde J, den Hertog J. H2O2-induced intermolecular disulfide bond formation between receptor protein-tyrosine phosphatases. J Biol Chem. 2004;279:44355–44361. doi: 10.1074/jbc.M407483200. [DOI] [PubMed] [Google Scholar]
- 28.Sun H, Charles CH, Lau LF, Tonks NK. MKP-1 (3CH134), an immediate-early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell. 1993;75:487–493. doi: 10.1016/0092-8674(93)90383-2. [DOI] [PubMed] [Google Scholar]