Abstract
Cell membranes are not randomly organized, but rather are populated by fluctuating nanoassemblies of increased translational order termed lipid rafts. This lateral heterogeneity can be biophysically extended because cooling formaldehyde-isolated plasma membrane preparations results in separation into phases similar to the liquid-ordered (Lo) and liquid-disordered (Ld) states seen in model membrane systems [Baumgart T, et al. (2007) Proc Natl Acad Sci USA 104:3165–3170]. In this work we demonstrate that raft clustering, i.e., amplifying underlying raft-based connectivity to a larger scale, makes an analogous capacity accessible at 37°C. In plasma membranes at this temperature, cholera toxin-mediated cross-linking of the raft ganglioside GM1 induced the sterol-dependent emergence of a slower diffusing micrometer-scale phase that was enriched in cholesterol and selectively reorganized the lateral distribution of membrane proteins. Although parallels can be drawn, we argue that this raft coalescence in a complex biological matrix cannot be explained by only those interactions that define Lo formation in model membranes. Under this light, our induction of raft-phase separation suggests that plasma membrane composition is poised for selective and functional raft clustering at physiologically relevant temperature.
Keywords: cholera toxin, clustering, ganglioside, lateral sorting
Heterogeneity is a fundamental feature of biological membranes, especially those of eukaryotes. It has been calculated that the mammalian bilayer could possess up to 9,600 species of glycerophospholipid, >100,000 species of sphingolipid, thousands of mono/di/triacylglycerol variants, and not to mention numerous fatty acid- and sterol-based structures (1). Combined with an abundance of protein types, this constitutes an enormous compositional complexity that in itself is difficult to conceptualize as a homogeneous milieu. The most compelling evidence for lateral diversity in the bilayer is the presence of lipid rafts, currently defined as dynamic, nano-sized, sterol–sphingolipid-enriched assemblies in which protein and lipid content fluctuates on a subsecond time scale (2, 3). It is clear that in model membrane systems, sphingolipid and cholesterol self-associate into liquid-ordered (Lo) phases wherein hydrocarbon chains are longer and more saturated, leading to a thicker, more condensed assemblage that segregates away from liquid-disordered (Ld) unsaturated glycerophospholipids (4–13). The challenge of the raft concept relates to how we rationalize these biophysical principles in the context of the compositional complexity of cell membranes.
Evaluation of raft-based organization in biological membranes has been difficult because of the lack of available tools that can both be successfully applied to living cells and used to measure fluctuating nanoscale heterogeneities reliably (3). Nevertheless, recent advances in a number of biophysical techniques have identified cholesterol-dependent nanoassemblies consistent with the notion of metastable raft entities in a number of cell types (14–17). Furthermore, work by Baumgart et al. (18) has shown that these lateral organizing properties may be revealed at micrometer scales as by induction of phase separation into Lo-like and Ld-like states in cooled formaldehyde-isolated plasma membrane vesicles. Our work concerns the activation of an analogous capacity at 37°C.
Raft-based heterogeneity can be visualized by analysis of the activated condition, a situation in which the metastable raft resting state is stimulated to coalesce into larger, more stable raft domains by specific lipid–lipid, protein–lipid, and protein–protein interactions (19). When clustered, bilayer components are thought to be laterally stabilized according to their underlying affinity for preexisting raft domains; i.e., clustering enhances the inclusion of proteins partitioning both strongly and weakly to rafts and further excludes those that segregate away (13, 19, 20). Although the lipid basis for raft clustering has been verified in model membrane systems (21), the organizing principle with respect to the compositional capacity of biological membranes has been less clear. Can this principle evoke phase separation in cell membranes with their complex lipid and protein composition?
In a similar approach to the formaldehyde-based plasma membrane-blebbing methodology introduced by Baumgart et al. (18), we used a cell-swelling procedure to separate plasma membrane spheres (PMS) from the cytoskeletal/endocytic/exocytic influence in A431 cells, an epidermoid carcinoma cell line expressing the raft ganglioside GM1 (22). We found that at 37°C, clustering of GM1 by its fluorescently labeled ligand, the pentavalent cholera toxin B subunit (CTB), induced cholesterol-dependent micrometer-scale coalescence of GM1 domains on the surface of previously uniform-phased PMS. The GM1 phase exhibited slower translational diffusion, was enriched in cholesterol, and recruited both transmembrane and exoplasmic/cytoplasmic lipid-anchored raft proteins but not the transferrin receptor, a classical nonraft marker. Our data indicate that at physiological temperature, biological membranes possess the compositional capacity to access underlying biophysical/biochemical raft-based connectivity and amplify it to the level of a distinct membrane phase.
Results
PMS Induction.
PMS appeared after 8- to 12-h exposure to PMS buffer. Membrane dye (Bodipy) staining revealed that PMS appeared as a spherical cell surface membrane outgrowth, 25- to 30-μm diameter, which was attached to the remaining cell body (Fig. 1); costaining with TRITC-phalloidin revealed that actin was only present at the point of cellular attachment, indicating that the plasma membrane had been separated from the previously underlying cytoskeleton. Additionally, PMS were not contaminated with endosomal (Rab5 GFP), Golgi (Rab6 GFP), and endoplasmic reticulum (Sec61 GFP) markers [supporting information (SI) Fig. S1]. However, PMS bound exogenous annexin V-Cy5, indicating that phosphatidylserine asymmetry was not maintained (data not shown). In general, PMS morphology was consistent with electroformation of red blood cell ghosts (23) and plasma membranes separated by organic solvent (18).
Fig. 1.
PMS costained with Bodipy and TRITC-phalloidin. PMS (arrow A) retain attachment to remaining cell body (arrow B) (Lower) but could be imaged at a higher hemisphere where this attachment is no longer in focus (Upper). A431 cells were incubated with Bodipy and TRITC-phalloidin before PMS formation. TRITC-phalloidin only colocalized with the remaining cell body, indicating that actin cytoskeleton was separated from plasma membrane material during formation of PMS. (Scale bars, 5 μm.)
Induction of Phase Separation and Selectivity in Lateral Distribution of Membrane Proteins.
Clustering of GM1 by CTB was first analyzed with respect to its influence on the lateral distribution of a number of fluorescently linked raft protein analogues (Fig. 2A). LAT-mRFP and the proteolipid variant VIP17-mRFP served as transmembrane raft proteins (single-spanning and multispanning, respectively), whereas endoplasmic Pal-Pal-mCFP and exoplasmic GL-GPI-mRFP represented internal and external lipid-anchored raft targets. In unclustered PMS, these raft protein analogues (Fig. 2A) did not show optically resolvable domain formation at 37°C (Fig. 2B) or at 25°C (data not shown). However, after cross-linking by CTB, the lateral distributions of all were dramatically reorganized into micrometer-sized phases enriched in GM1 (Fig. 2C). Furthermore, this emergent GM1 phase excluded the transferrin receptor, a nonraft marker (transferrin-Alexa Fluor 647 staining, Fig. 3), indicating that at the level of protein organization, this membrane-phase induction exhibited raft-based compositional selectivity. At 1 μg/ml CTB phase separation was seen in ≈50–60% of PMS; induction of biphasic PMS depended on CTB concentration (Fig. S2A).
Fig. 2.
Induction of phase separation in PMS 37°C. Fluorescent raft protein analogues representing single spanning transmembrane (LAT), multispanning transmembrane (VIP17), and exo/cytoplasmic lipid-anchored (GL-GPI and Pal-Pal linkages, respectively) membrane-targeting motifs (A) did not show large-scale separation of phase at this temperature (B) or at 25°C (data not shown). (C) However, after cross-linking by fluorescent CTB (Alexa Fluor 488 or Alexa Fluor 647 conjugates), the lateral distribution of these proteins was reorganized into a micrometer-scaled emergent GM1 phase. (Scale bars, 5 μm.)
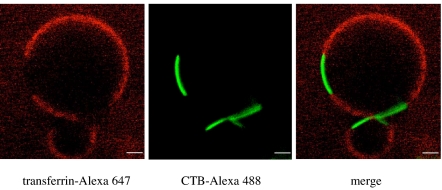
Fig. 3.
The emergent GM1 phase excludes the transferrin receptor. After clustering by CTB-Alexa Fluor 488, transferrin-Alexa Fluor 647 was added to PMS for 1 h at 37°C. Resultant LSM imaging clearly showed that the membrane-bound transferrin was not recruited to the coalesced GM1 phase, indicating that this lateral reorganization of membrane structure was selective in composition. (Scale bars, 5 μm.)
Cholesterol-Dependent Coalescence of the GM1 Phase.
In addition to recruitment of raft proteins, the induced GM1 phase was also enriched in cholesterol (filipin III staining; Fig. 4A), an important lipid in mammalian raft-based heterogeneity. To assess the sterol dependence of the process, PMS cholesterol was depleted with methyl-β-cyclodextrin, and GM1 was then clustered with CTB-Alexa Fluor 488. After sterol extraction, CTB-Alexa Fluor 488 induced a patchy distribution of GM1 binding, a phenotype in sharp contrast to the induction of global GM1 domain coalescence observed in the control condition (Fig. 4B). These data stress an importance for cholesterol in establishing uninterrupted connectivity between cross-linked GM1 (clustered up to a pentameric state) during coalescence of the GM1 phase.
Fig. 4.
Cholesterol dependence of the GM1 phase. (A) After cross-linking by CTB at 37°C, PMS were stained for nonesterified cholesterol via incubation with filipin III. The emergent GM1 phase was colabeled, indicating an enrichment of cholesterol. (B) To assess the sterol dependence of the process, PMS were treated with methyl-β-cyclodextrin (MβCD) to extract cholesterol and then clustered by CTB-Alexa Fluor 488. Compared with the control nonextracted condition, MβCD-treated PMS failed to exhibit global/uninterrupted coalescence of a GM1 phase, rather displaying a much smaller and patchy domain distribution. This indicates that cholesterol was important in establishing the larger-scale connectivity between cross-linked GM1 (CTB clusters up to pentameric state) as seen by micromolar-scale phase separation in the control condition. (Scale bars, 5 μm.)
Diffusion in Phase-Separated PMS.
To assess membrane ordering in biphasic PMS we performed two-focus scanning fluorescence correlation spectroscopy (FCS) (24) to measure diffusion of pentavalent CTB and the tetraspanning proteolipid VIP17/MAL analogue VIP17-mRFP. This technique measures calibration-free diffusion coefficients with a high accuracy and is robust against instabilities. CTB-Alexa Fluor 488 was found to diffuse ≈10-fold slower in the GM1 phase compared with the surrounding membrane (Fig. 5). No high-order CTB aggregates were detected in either phase with this method, suggesting that the diffusive changes were related to differences in lateral packing interactions between cross-linked GM1 (clustered up to a pentameric state) and other lipids and/or proteins. CTB diffusion below the concentration required to induce phase separation was similar to faster diffusing phase of biphasic PMS (Fig. S2B). In biphasic PMS, the diffusivity of VIP17-mRFP was also retarded ≈10-fold in the GM1 phase compared with the remaining phase (D = 0.10 ± 0.03 μm2/s versus D = 1.27 ± 0.25 μm2/s). Additionally, in unclustered PMS, VIP17-mRFP diffusion was comparable with the faster diffusing phase of biphasic PMS (D = 1.34 ± 0.72 μm2/s).
Fig. 5.
SFCS measurements on PMS. (a) LSM image of plasma membrane sphere with induced phase separation by CTB cross-linking and schematic of scan paths for two-focus scanning FCS. (b and c) Auto- (○, ◇) and cross-correlation curves (▾) measured in the bright (GM1 phase) and dark phase (surrounding membrane), respectively, and global fit to elliptical Gaussian model (24). (d) Diffusion coefficients measured with two-focus scanning FCS. Error bars denote the geometric mean and geometric 1σ confidence intervals of the measured diffusion coefficients. Ddark = 1.3−0.2+0.4 μm2/s, Dbright = 0.13−0.06+0.11 μm2/s.
Discussion
Selective recruitment of membrane components to clustered GM1 domains is a common theme in plasma membrane cross-linking studies. As in our study, antibody-cross-linked raft proteins (both GPI-anchored proteins and transmembrane proteins) copatch both with each other and with clustered GM1, but not with nonraft proteins (typically the transferrin receptor) (25–28). Additionally, in results similar to our observation of exoplasmic GM1 phase-mediated redistribution of endoplasmic Pal-Pal-mCFP, membrane patching has identified outer to inner leaflet communication via clustering of raft surface antigens (28). However, there are important differences between our system and the membrane-patching technique. First, patching is typically performed at 4°C. Although this is necessary to prevent endocytosis of clustered antigens, it introduces a temperature bias toward the effect of interest, i.e., membrane ordering. Second, patching experiments typically employ not only primary cross-linking (up to pentameric clustering in the case of CTB) but also additive clustering by secondary IgG to visualize domain selectivity. In PMS, we achieve similar specificity in lateral reorganization, but at much larger scales (i.e., global phase separations). Furthermore, we observed these effects under more “compromised” conditions: primary CTB clustering only [i.e., no secondary antibody clustering; CTB does not self-aggregate on membranes (29)] and at much warmer/physiological temperature. These findings highlight the inherent capability of the plasma membrane to phase separate while stressing that in the living cell this capacity is more strictly controlled.
Increases in plasma membrane tension during cell swelling at 37°C can induce raft and nonraft components to segregate into domains comparable with the phenotypes seen with antibody cross-linking (30). Although tension-based forces cannot be excluded from the current study, our unclustered PMS did not show optically resolvable domains at either 25°C or 37°C, a phenotype in keeping with the observations of Baumgart et al. (18). Also, the size order of the segregation that we observe after clustering is beyond the patchy domain formation that Ayuan and Cohen (30) report, but rather a cohesive phase separation. More importantly, this GM1 phase separation was cholesterol-dependent. In model membranes, induction of phase separation by clustering depends on GM1 concentration (analogous to CTB concentration dependence that we note in PMS; Fig. S2) and on the levels of surrounding cholesterol and sphingomyelin (21). This indicates that these lipids contribute to a selective underlying connective potential that can be activated upon cross-linking. The cholesterol dependence of GM1 coalescence into a distinct phase in our PMS argues for a similar underlying connective potential in the composition of biological membranes (Fig. 6). Although the patchy CTB-binding profile seen after sterol extraction may be more reflective of tension-based heterogeneity, connectivity through cholesterol serves to explain how the creation of ganglioside oligomers leads to the emergence of a micrometer-scale GM1 phase. Although we cannot exclude the possibility that cholesterol removal can induce solid/gel phases (13), we assume that global domain coalescence is only possible when the clustered components possess a pre-cross-linked level of connective potential, i.e., GM1–cholesterol/raft interaction, that when activated, translates to large-scale amplification well beyond the valency of the initial clustering stimulation. Therefore, induction of cholesterol-dependent raft phase separation by GM1 oligomerization is best explained by preexisting raft-based membrane heterogeneity.
Fig. 6.
Model for emergence of a GM1 phase. Micrometer-scale (global) coalescence of CTB-cross-linked GM1 in PMS is only possible if there is preexisting (raft-based) connectivity between the ganglioside and other select elements of the plasma membrane (Right). Without these interactions, CTB cross-linked-GM1 (CTB only clusters up to pentameric state) could not be bridged into a continuous phase (Left). Our results indicate that cholesterol is an important component of these bridging interactions and that retarded diffusion in the GM1 phase is most consistent with condensation and therefore amplification of these forces.
Two-focus scanning FCS indicated that diffusion of CTB-Alexa Fluor 488 was retarded 10-fold in the GM1 phase. Because CTB does not self-aggregate on membranes (29) (additionally, we did not find evidence for high-order oligmerization of CTB subunits in either phase), we interpret this diffusional hindrance as evidence for tighter lateral association between cross-linked GM1 and lipids and/or proteins, a hypothesis supported by the fact that cross-linking by CTB also retarded the diffusivity of VIP17-mRFP in this phase. Similar changes for CTB-Alexa Fluor 488 diffusion in Lo versus Ld phases have been reported for supported bilayers [0.04 μm2/s versus 1.1 μm2/s, respectively (31)]; however, we do not equate our activated GM1 phase to the Lo state of model membranes. A Lo phase is defined quantitatively in artificial membrane systems where parameters relating to translational order (lateral diffusion) and conformational order (trans/gauche ratio in the acyl chains) can be accurately measured (13, 32, 33). In these systems, phase separation arises from interactions between different lipids that leads to a liquid–liquid immiscibility in the membrane plane, a concept valid for a number of lipid species and simple lipid/protein preparations (13). However, cell membranes encompass a multitude of specific biochemical structure–function relationships that generate a lateral interaction diversity not described by artificial systems. Here, we must consider all forms of lateral association/heterogeneity: oligomeric protein assemblies dominate over monomeric structures (34); variation in protein hydrophobic domain dimensionality creates lipid regions of varying thickness and composition (35); large protein ectodomains cover lipid and produce steric restrictions (34); and electrostatic interactions are not uniformly distributed (36).
Baumgart et al. (18) have clearly demonstrated that biological membrane constituents can be pushed to unify under physical principles; formaldehyde-isolated giant plasma membrane vesicles (GPMV) can be induced by low temperature to adopt Lo-like and Ld-like states (of course formaldehyde itself may also cause significant changes in the state of lipids and proteins). However, this biphasic system does not occur in GPMV or PMS at 37°C, indicating that the membrane basis for larger-scale raft heterogeneity at physiological temperature is different from function the lipid phase-based interactions that govern behavior in model membrane systems. Along these lines, it has become clear that Lo phases generally exclude transmembrane proteins, even those thought to have affinity for raft domains in cell membranes (37–39). Although this phenomenon has been confirmed for the cold-induced Lo-like phase of formaldehyde-isolated GPMVs (40), we found that our LAT and VIP17 analogues (representing single-spanning and tetra-spanning raft transmembrane proteins, respectively) were reorganized into the GM1 phase upon PMS activation by CTB. LAT is also critical for the in vivo membrane condensation that takes place during raft clustering and formation of the immunological synapse (41). We would therefore suggest that phase separation, as we understand it in model membranes, does not completely account for the specificity of raft-based organization of transmembrane proteins, at least in the activated/coalesced condition. Rather, the context for heterogeneity by rafts must be understood within the more complex chemical background of biological membranes, wherein lipid phase separation principles are likely coupled to specific lateral association (e.g., oligomerization, lipid–protein, protein–protein, and electrostatic interactions) such that when amplified during raft coalescence the level of inclusion and therefore compositional connectivity is enhanced (19).
Under this rationale, our data concerning transmembrane proteins are partially paralleled by the effects seen in model membranes. The concept that the oligomeric status of certain lipids is positively related to their affinity for the Lo phase was established for saturated phospholipid analogues in supported monolayers (42) and confirmed for CTB-clustered GM1 in giant unilamellar vesicles (GUVs) (43). Importantly, this Ld to Lo relocation of GM1 influences the lateral sorting of some transmembrane proteins because CTB cross-linking was found to increase the partitioning of the enzyme β-secretase to the Lo phase in GUVs (39). This suggests that although transmembrane proteins are physically excluded, an undefined specificity for GM1 cross-linking may overcome the energetic constraints of packing membrane-spanning α-helices into a Lo phase. Whether this speaks toward the direct coupling of an additional specificity to lipid phase-based lateral organization is not clear because in previously uniform GUV systems of DOPC:sphingomyelin:cholesterol, cross-linking of GM1 by CTB induces phase separation wherein the reconstituted LAT transmembrane domain is retained in the Ld phase (21). As such, we maintain that the interactions governing phase separation in artificial membranes do not encompass the effects we observe in PMS at 37°C.
To summarize, we have demonstrated that when removed from underlying cytoskeletal influence and endo/endocytic turnover, plasma membranes can be induced to produce large-scale raft-based phase separation by CTB-mediated clustering of GM1 at 37°C. Coalescence of the slower diffusing GM1 phase was cholesterol-dependent and selectively reorganized proteins and lipids according to their affinity for raft domains. Being compositionally poised for raft activation at physiological temperature underscores a capacity to be selectively stimulated to evoke a larger-scale lateral reorganization of membrane structure and function. Although direct parallels can be drawn, the capacity for lipids to phase separate both in model membrane systems and in cooled/chemically blebbed plasma membrane preparations has different characteristics from the process we observe in PMS at 37°C. This emphasizes the need to rationalize how all forms of lateral interaction unite to produce functional heterogeneity in cell membranes.
Materials and Methods
Reagents.
All general reagents in addition to filipin III and tetramethylrhodamine B isothiocyanate (TRITC)-phalloidin were obtained from Sigma. CTB-Alexa Fluor 488/647 conjugates were from Invitrogen. Rh-DOPE was from Avanti. Transferrin-Alexa Fluor 647 and Bodipy were gifts from Marino Zerial and Christoph Thiele, respectively (Max Planck Institute for Molecular Cell Biology and Genetics, Dresden). The American Type Culture Collection (ATCC) supplied the epidermoid carcinoma A431 cell line. DMEM and Opti-MEM were from Gibco.
DNA Constructs.
An amplified AgeI-mRFP-BsrGI fragment (44) was ligated to AgeI–BsrGI-digested pSS-LAT-GFP (45) generating pSS-LAT-mRFP (LAT, linker of activated T cells; SS, rabbit lyzophorin hydrolase signal sequence). The sequence of the tetraspanning raft proteolipid VIP17/MAL (vesicular integral membrane protein 17/myelin and lymphocyte protein) from pBAT-VIP17 (46) was amplified with SalI-VIP17-BamHI overhangs and inserted into the SalI–BamHI site of pSS-LAT-mRFP yielding pSS-VIP17-mRFP. Double-palmitoylated monomeric CFP (Pal-Pal-mCFP) was from Meder et al. (45). Rab5 GFP and Rab 6 GFP were provided by Marino Zerial, and Sec61 GFP was a gift from Nica Borgese (National Research Council Institute for Neuroscience and Department of Medical Pharmacology, University of Milan). Plasmids were transfected with Lipofectamine reagent according to the manufacturer's instructions. An adenovirus encoding glycosylated-glycosylphosphatidylinositol-mRFP (GL-GPI-mRFP) was generated using the AdEasy adenoviral system (Stratagene) according to the manufacturer's instructions. For infection, 10 μl of virus was added to 500 μl of Opti-MEM and incubated with 80% confluent A431 cells for 2 h. Opti-MEM was then replaced with DMEM (10% fetal calf serum, 2 mM glutamine, 100 units/ml penicillin/streptomycin); plasma membrane localization of construct was observed within 8 h.
PMS Formation.
A431 cells were grown to 60–80% confluence in DMEM in 35-mm glass-bottom live-cell imaging dishes (P35G-1.5-10-C; MatTek). Cells were then washed and incubated PMS buffer [1.5 mM CaCl2, 1.5 mM MgCl2, 5 mM Hepes, 1 mg/ml glucose in 1× PBS (pH 7.4); a solution originally intended for live-cell imaging but an inducer of swelling and PMS formation after prolonged exposure] for a period of 8–12 h at 37°C. For visualization of resultant effects, cells were preincubated with Bodipy [1/1,000 dilution of 2.5 mg/ml (in ethanol) stock] as a membrane stain, and 1 μM TRITC-phalloidin to mark actin cytoskeleton [to ensure TRITC-phalloidin internalization, cells were prepermeabilized for 5 min with 0.1% (wt/vol) Triton X-100 or 0.1% (wt/vol) saponin in PMS buffer].
GM1 Clustering, Membrane Protein Distribution, and Cholesterol Extraction.
Upon formation of PMS, CTB fluorescent analogues were added to the live-cell imaging dishes at 1 μg/ml at 37°C for at least 2 h. For cells transfected/infected with the fluorescent raft proteins LAT-mRFP, VIP17-mRFP, or GL-GPI-mRFP, CTB-Alexa Fluor 488 was used; CTB-Alexa Fluor 647 was used in the case of Pal-Pal-mCFP. To assess the lateral organization of cholesterol, PMS clustered with CTB-Alexa Fluor 647 were also stained with filipin III (20 min, 37°C, 50 μg/ml). The distribution of transferrin receptor was evaluated by staining PMS with transferrin-Alexa Fluor 647 (1 h, 37°C at 10 μg/ml) after the GM1 cross-linking by CTB-Alexa Fluor 488. To assess cholesterol dependence of GM1 phase formation, sterol was extracted as described in ref. 47. Briefly, PMS were incubated with 10 mM methyl-β-cyclodextrin for 30 min at 37°C, after which time CTB-Alexa Fluor 488 was added (1 μg/ml) as described above.
Imaging.
Microscopy was performed at 37°C with a Zeiss LSM (Carl Zeiss); fillipin III, mCFP, GFP–Alexa Fluor 488, mRFP, Alexa Fluor 643 were excited with 360-, 405-, 488-, 594-, and 633-nm laser lines, respectively. Temperature at 37°C was maintained by a Bachhoffer chamber (tempcontrol 37-2 digital; H. Sauer Laborbedarf).
Two-Focus Scanning FCS.
Two-focus scanning FCS was performed as described in ref. 24. The optical setup consisted of a LSM Meta 510 system (Carl Zeiss) with a 40× NA 1.2 UV-VIS-IR C-Apochromat water-immersion objective and a home-built detection unit at the fiber output channel. A bandpass filter HQ525/60 (AHF Analyze Technik) was used behind a collimating achromat to reject the residual laser and background light. Another achromat (LINOS Photonics) with a shorter focal length was used to image the internal pinhole onto the aperture of the fiber connected to the avalanche photo diode (PerkinElmer). The photon arrival times were recorded in the photon mode of the hardware correlator Flex 02-01D (correlator.com). The movement of the detection volume was controlled directly with the Zeiss LSM operation software. For two-focus scanning FCS, two parallel lines with a distance d were scanned alternately in a perpendicular way through the vertical part of the membrane. Instabilities were corrected for, and intensity traces were extracted from each intersection of the scan path with the membrane. Auto- and cross-correlation curves, calculated from the two intensity traces, were fitted globally to a 2D elliptical Gaussian model (24) with software written in MATLAB (Math-Works). The distance d was determined by repeatedly scanning over a film of dried fluorophores and measuring the distance between the bleached traces in a high resolution LSM-image. All FCS measurements were made at 25°C.
Supplementary Material
Acknowledgments.
We thank J. Howard (Max Planck Institute for Molecular Cell Biology and Genetics) and C. A. Lingwood (Hospital for Sick Children, Toronto) for critical reading of the manuscript; and Hermann-Josef Kaiser, Lawrence Rajendran, and the Simons laboratory for helpful suggestions and discussion. We also thank Aki Manninen (University of Oulu, Finland) for raising the GL-GPI-mRFP adenovirus. This work was supported by EUFP6 PRISM, and SP1175 grants from Deutsche Forschungsgemeinschaft (to K.S.). D.L. was the recipient of an international Natural Sciences and Engineering Research Council Postgraduate Scholarship.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804374105/DCSupplemental.
References
- 1.Yetukuri L, Ekroos K, Vidal-Puig A, Oresuc M. Informatics and computational strategies for the study of lipids. Mol BioSyst. 2008;4:121–127. doi: 10.1039/b715468b. [DOI] [PubMed] [Google Scholar]
- 2.Pike LJ. Rafts defined: A report on the Keystone Symposium on Lipid Rafts and Cell Function. J Lipid Res. 2006;46:1597–1598. doi: 10.1194/jlr.E600002-JLR200. [DOI] [PubMed] [Google Scholar]
- 3.Hancock JF. Lipid rafts: Contentious only from simplistic standpoints. Nat Rev Mol Cell Biol. 2006;7:456–462. doi: 10.1038/nrm1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson TE, Tillack TW. Organization of glycosphingolipids in bilayers and plasma membrane of mammalian cells. Annu Rev Biophys Biophys Chem. 1985;14:361–386. doi: 10.1146/annurev.bb.14.060185.002045. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed SN, Brown DA, London E. On the origin of sphingolipid-cholesterol-rich detergent insoluble domains in cell membranes: Physiological concentrations of cholesterol and sphingolipid induce formations of a detergent-insoluble, liquid ordered phase in model membranes. Biochemistry. 1997;39:10944–10953. doi: 10.1021/bi971167g. [DOI] [PubMed] [Google Scholar]
- 6.Brown RE. Sphingolipid organization in biomembranes: What physical studies of model membranes reveal. J Cell Sci. 1998;111:1–9. doi: 10.1242/jcs.111.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown DA, London E. Structure and origin of ordered lipid domains in biological membranes. J Membr Biol. 1998;15:103–114. doi: 10.1007/s002329900397. [DOI] [PubMed] [Google Scholar]
- 8.Patra SK, Alonso A, Arrondo J, Goñi F. Liposomes containing sphingomyelin and cholesterol: Detergent solubilization and infrared spectroscopic studies. J Liposome Res. 1999;9:247–260. [Google Scholar]
- 9.London E, Brown DA. Insolubility of lipids in Triton X-100: Physical origin and relationship to sphingolipid/cholesterol membrane domains (rafts) Biochim Biophys Acta. 2000;1508:182–195. doi: 10.1016/s0304-4157(00)00007-1. [DOI] [PubMed] [Google Scholar]
- 10.Veiga MP, Arrondo JL, Goñi FM, Alonso A, Marsh D. Interaction of cholesterol and sphingomyelin in mixed membranes containing phosphatidylcholine, studied by spin-label ESR and IR spectroscopies: A possible stabilization of gel-phase sphingolipid domains by cholesterol. Biochemistry. 2001;40:2614–2622. doi: 10.1021/bi0019803. [DOI] [PubMed] [Google Scholar]
- 11.Xu X, et al. Effect of the structure of natural sterols and sphingolipids on the formation of ordered sphingolipid/sterol domains (rafts): Comparison of cholesterol to plant, fungal, and disease-associated sterols and comparison of sphingomyelin, cerebrosides, and ceramide. J Biol Chem. 2001;276:33540–33546. doi: 10.1074/jbc.M104776200. [DOI] [PubMed] [Google Scholar]
- 12.Edidin M. The state of lipid rafts: From model membranes to cells. Annu Rev Biophys Biomol Struct. 2003;32:257–283. doi: 10.1146/annurev.biophys.32.110601.142439. [DOI] [PubMed] [Google Scholar]
- 13.Simons K, Vaz WL. Model systems, lipid rafts, and cell membranes. Annu Rev Biophys Biomol Struct. 2004;33:269–295. doi: 10.1146/annurev.biophys.32.110601.141803. [DOI] [PubMed] [Google Scholar]
- 14.Lenne P-F, et al. Dynamic molecular confinement in the plasma membrane by microdomains and the cytoskeleton meshwork. EMBO J. 2006;25:3245–3256. doi: 10.1038/sj.emboj.7601214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chadda R, et al. Cholesterol-sensitive Cdc42 activation regulates actin polymerization for endocytosis via the GEEC pathway. Traffic. 2007;8:702–717. doi: 10.1111/j.1600-0854.2007.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki KG, et al. GPI-anchored receptor clusters transiently recruit Lyn and Gα for temporary cluster immobilization and Lyn activation: Single-molecule tracking study 1. J Cell Biol. 2007;177:717–730. doi: 10.1083/jcb.200609174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki KG, Fujiwara TK, Edidin M, Kusumi A. Dynamic recruitment of phospholipase Cγ at transiently immobilized GPI-anchored receptor clusters induces IP3-Ca2+ signaling: Single molecule tracking study 2. J Cell Biol. 2007;177:731–742. doi: 10.1083/jcb.200609175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baumgart T, et al. Large-scale fluid/fluid phase separation of proteins and lipids in giant plasma membrane vesicles. Proc Natl Acad Sci USA. 2007;104:3165–3170. doi: 10.1073/pnas.0611357104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meder D, Simons K. In: Lipid Rafts and Caveolae: From Membrane Biophysics to Cell Biology. Fielding CJ, editor. New York: Wiley; 2006. pp. 1–23. [Google Scholar]
- 20.Schuck S, Simons K. Polarized sorting in epithelial cells: Raft clustering and the biogenesis of the apical membrane. J Cell Sci. 2004;117:5955–5964. doi: 10.1242/jcs.01596. [DOI] [PubMed] [Google Scholar]
- 21.Hammond AT, et al. Cross-linking a lipid raft component triggers liquid ordered–liquid disordered phase separation in model plasma membranes. Proc Natl Acad Sci USA. 2005;102:6320–6325. doi: 10.1073/pnas.0405654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parton R. Ultrastructural localization of gangliosides: GM1 is concentrated in caveolae. J Histochem Cytochem. 1994;42:155–166. doi: 10.1177/42.2.8288861. [DOI] [PubMed] [Google Scholar]
- 23.Montes L-R, Alonso A, Goñi FM, Bagatolli A. Giant unilamellar vesicles electroformed from native membranes and organic lipid mixtures under physiological conditions. Biophys J. 2007;93:3548–3554. doi: 10.1529/biophysj.107.116228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ries J, Schwille P. Studying slow membrane dynamics with continuous wave scanning fluorescence correlation spectroscopy. Biophys J. 2006;91:1915–1924. doi: 10.1529/biophysj.106.082297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harder T, Scheiffele P, Verkade P, Simons K. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J Cell Biol. 1998;141:929–942. doi: 10.1083/jcb.141.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harder T, Simons K. Clusters of glycolipid and glycosylphosphatidylinositol-anchored proteins in lymphoid cells: Accumulation of actin regulated by local tyrosine phosphorylation. Eur J Immunol. 1999;29:556–562. doi: 10.1002/(SICI)1521-4141(199902)29:02<556::AID-IMMU556>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 27.Janes PW, Ley SC, Magee AI. Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. J Cell Biol. 1999;147:447–461. doi: 10.1083/jcb.147.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gri G, Molon B, Manes S, Pozzan T, Viola A. The inner side of T cell lipid rafts. Immunol Lett. 2004;94:247–252. doi: 10.1016/j.imlet.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 29.Cai XE, Yang J. The binding potential between the cholera toxin B oligomer and its receptor. Biochemistry. 2003;42:4028–4034. doi: 10.1021/bi027016h. [DOI] [PubMed] [Google Scholar]
- 30.Ayuyan AG, Cohnen FS. Raft composition at physiological temperature and pH in the absence of detergents. Biophys J. 2008;94:2654–2666. doi: 10.1529/biophysj.107.118596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiantia S, Ries J, Kahya N, Schwille P. Combined AFM and two-focus SFCS study of raft-exhibiting model membranes. Chemphyschem. 2006;7:2409–2418. doi: 10.1002/cphc.200600464. [DOI] [PubMed] [Google Scholar]
- 32.Ipsen JH, et al. Phase equilibria in the phosphatidylcholine–cholesterol system. Biochim Biophys Acta. 1987;905:162–172. doi: 10.1016/0005-2736(87)90020-4. [DOI] [PubMed] [Google Scholar]
- 33.Ipsen JH, Mouritsen OG, Zuckermann MJ. Theory of thermal anomalies in the specific heat of lipid bilayers containing cholesterol. Biophys J. 1989;4:661–667. doi: 10.1016/S0006-3495(89)82713-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Engelman DM. Membranes are more mosaic than fluid. Nature. 2005;438:578–580. doi: 10.1038/nature04394. [DOI] [PubMed] [Google Scholar]
- 35.Mitra K, Ubarretxena-Belandia I, Taguchi T, Warren G, Engelman DM. Modulation of the bilayer thickness of exocytic pathway membranes by membrane proteins rather than cholesterol. Proc Natl Acad Sci USA. 2004;101:4083–4088. doi: 10.1073/pnas.0307332101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mulgrew-Nesbitt A, et al. The role of electrostatics in protein–membrane interactions. Biochim Biophys Acta. 2006;1761:812–826. doi: 10.1016/j.bbalip.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 37.de Planque MR, Killian JA. Protein–lipid interactions studied with designed transmembrane peptides: Role of hydrophobic matching and interfacial anchoring. Mol Membr Biol. 2003;20:271–284. doi: 10.1080/09687680310001605352. [DOI] [PubMed] [Google Scholar]
- 38.Shogomori H, et al. Palmitoylation and intracellular domain interactions both contribute to raft targeting of linker for activation of T cells. J Biol Chem. 2005;280:18931–18942. doi: 10.1074/jbc.M500247200. [DOI] [PubMed] [Google Scholar]
- 39.Kalvodova L, et al. Lipids as modulators of proteolytic activity of BACE: Involvement of cholesterol, glycosphingolipids and anionic phospholipids in vitro. J Biol Chem. 2005;280:36815–36823. doi: 10.1074/jbc.M504484200. [DOI] [PubMed] [Google Scholar]
- 40.Sengupta P, Hammond AT, Holowka D, Baird B. Structural determinants for partitioning of lipids and proteins between coexisting fluid phases in giant plasma membrane vesicles. Biochim Biophys Acta. 2008;1778:20–32. doi: 10.1016/j.bbamem.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gaus K, et al. Condensation of the plasma membrane at the site of T lymphocyte activation. J Cell Biol. 2005;171:121–131. doi: 10.1083/jcb.200505047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dietrich C, Volovyk ZN, Levi M, Thompson NL, Jacobson K. Partitioning of Thy-1, GM1, and cross-linked phospholipids analogues into lipid rafts reconstituted in supported model membrane monolayers. Proc Natl Acad Sci USA. 2001;98:10642–10647. doi: 10.1073/pnas.191168698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bacia K, Schwille P, Kurzchalia T. Sterol structure determines the phases and curvature of the liquid ordered phase of model membranes. Proc Natl Acad Sci USA. 2005;102:3272–3277. doi: 10.1073/pnas.0408215102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Campbell R, et al. A monomeric red fluorescent protein. Proc Natl Acad Sci USA. 2002;99:7877–7882. doi: 10.1073/pnas.082243699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meder D, Moreno MJ, Verkade P, Vaz WL, Simons K. Phase coexistence and connectivity in the apical membrane of polarized epithelial cells. Proc Natl Acad Sci USA. 2006;103:329–334. doi: 10.1073/pnas.0509885103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zacchetti D, Peranen J, Murata M, Fiedler K, Simons K. VIP17/MAL, a proteolipid in apical transport vesicles. FEBS Lett. 1995;375:465–469. doi: 10.1016/0014-5793(95)01396-2. [DOI] [PubMed] [Google Scholar]
- 47.Keller P, Simons K. Cholesterol is required for surface transport of influenza virus hemagglutinin. J Cell Biol. 1998;140:1357–1367. doi: 10.1083/jcb.140.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.