Abstract
Chemokines and their receptors direct leukocyte migration among blood, lymph and tissues. Evidence has recently accumulated that, besides their chemotactic functions, chemokine receptors are highly versatile players that fine tune immune responses. During human T cell activation by antigen-presenting cells, the chemokine receptors CCR5 and CXCR4 are recruited into the immunological synapse, where they deliver costimulatory signals. However, the molecular mechanisms allowing signaling versatility of chemokine receptors are unknown. Here, we describe the functional interaction between CXCR4 and CCR5 to exert specific biological functions and modulate T lymphocyte responses. We demonstrate that simultaneous expression and cooperation between CCR5 and CXCR4 are required for chemokine-induced T cell costimulation at the immunological synapse. In addition, we provide evidence for a physical association of the two receptors in a signaling complex that activates distinct T cell functions. We suggest that cooperation between receptors represents one key strategy for the functional plasticity of chemokines.
Keywords: chemokine receptors, heterodimerization, T cell costimulation
The immune system is able to mount an immune response against antigens present in the body at very low concentrations and, at the same time, to discriminate precisely between an infectious stimulus and a noninfectious one. During T cell activation, this sensitivity and specificity are achieved by mechanisms of sustained interactions with antigen-presenting cells (APCs) as well as by tunable activation thresholds and signal modulation (1). Thus, in addition to the interaction between the T cell receptor (TCR) and its ligand, T cell activation depends on accessory signals delivered by costimulatory molecules. CD28 is one of the most important costimulatory receptors for T cell priming in lymph nodes, but the costimulatory signals for effector T cells in the inflammatory microenvironment are less defined.
Chemokines are small cytokines with selective chemoattractant properties coordinating tissue homeostasis and inflammation. Besides their chemotactic functions, chemokines are involved in several biological and physiopathological processes. Thus, deregulated expression of chemokines and their receptors is involved in the development of autoimmunity, chronic inflammation, immunodeficiency and cancer (2, 3). The broad range of activities displayed by chemokines is the consequence of multiple signaling pathways induced by chemokine receptors—seven-transmembrane molecules coupled to heterotrimeric G proteins (4).
In T lymphocytes, the chemokine receptor CXCR4 is constitutively expressed and regulates T cell migration along gradients of the chemokine CXCL12. In contrast, CCR5 is expressed in activated T cells only and directs their migration along CCL3, CCL4 and CCL5 gradients. CXCR4 and CCR5 receptors are involved in several pathological processes, including autoimmunity, cancer, and HIV infection (3). We recently demonstrated that during T cell stimulation CCR5 and CXCR4 are recruited to and accumulate at the immunological synapse (IS) by a mechanism requiring chemokine secretion by APCs and chemokine-receptor signaling through a Gαi-independent pathway (5). Recruitment of chemokine receptors into the IS results in stronger T cell–APC attraction, reduction of T cell responsiveness to chemotactic gradients, and in higher levels of T cell proliferation and IFN-γ production (5).
To understand the basis of the signaling and functional versatility of CXCR4 and CCR5, we performed a study aimed at identifying the requirements for chemokine-induced costimulation. Here, we show that CXCR4 and CCR5 are co-recruited into the T cell IS and that cooperation between the two receptors is required for chemokine-mediated T cell costimulation. Our data suggest that CXCR4 and CCR5 may hetero-oligomerize to allow signaling versatility in T lymphocytes.
Results
CXCR4 and CCR5 Are Co-Recruited into the IS.
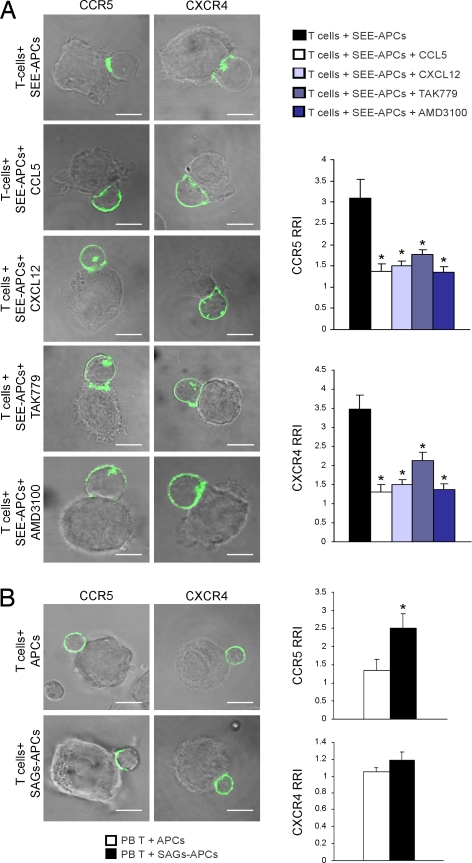
We had previously shown that CCR5- and CXCR4-specific antagonists inhibited recruitment of the specific receptor into the T cell IS (5). In Jurkat T cells that endogenously express both CXCR4 and CCR5, in which either GFP-CCR5 or CXCR4-GFP were transfected [see supporting information (SI) Fig. S1 for expression levels], we found that preincubation of cells with either the chemokine CXCL12 (CXCR4-ligand) or CCL5 (CCR5-ligand), or with the antagonist AMD3100 (CXCR4-specific) or TAK779 (CCR5-specific) inhibited recruitment of both chemokine receptors into the IS (Fig. 1A). These data suggest that CXCR4 and CCR5 are likely co-recruited into the synapse because the recruitment of each receptor is inhibited by the ligand/antagonist of the other receptor. In support of the data showing that CXCR4 needs CCR5 to be recruited into the IS, we found that CXCR4 did not accumulate at the IS of peripheral blood (PB) resting T cells (Fig. 1B), which do not express CCR5 (3). In contrast, pretreatment of T cells with TAK779 or AMD3100 did not affect T cell migration toward CXCL12 or CCL5, respectively (Fig. S2). Collectively, our data suggest that CXCR4 and CCR5 must cooperate to be recruited to the IS but not for their chemotactic functions.
Fig. 1.
CXCR4 and CCR5 are co-recruited into the IS. (A) Jurkat cells expressing GFP-tagged CCR5 or CXCR4 were incubated with SEE-loaded B cells for 10 min in the presence of AMD3100, TAK779, CXCL12 or CCL5. (B) PB T cells expressing GFP-tagged CCR5 or CXCR4 were incubated with superantigens (SAGs)-loaded B cells for 10 min. (A and B) Cells were then fixed and analyzed by confocal microscopy. Bar, 10 μm. Quantitative analyses of CCR5 and CXCR4 accumulation at the IS are shown. The relative recruitment index (RRI) (5) represents mean (± SE) of 30 cells out of four independent experiments. *, P < 0.05 compared with control T cells.
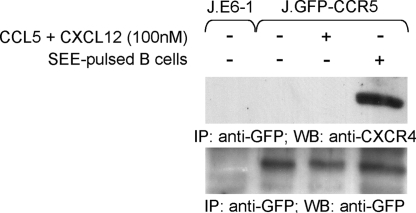
To understand if there is a physical interaction between CXCR4 and CCR5 at the IS, we performed immunoprecipitation experiments in Jurkat T cells stably expressing GFP-CCR5 (5). In these experimental conditions, the endogenous expression of CXCR4 and the low expression of CCR5 (Fig. S1) limit possible artifacts because of receptor over-expression. Association of the two chemokine receptors was evident in T cells stimulated with APCs, whereas CCR5 was unable to co-precipitate with CXCR4 in both unstimulated T cells and in cells stimulated by soluble chemokines, either used alone (not shown) or in combination (Fig. 2). Densitometric analyses indicated that 50% (± 13% SD; n = 3) of CXCR4 was co-immunoprecipitated with GFP-CCR5 in activated T cells. The co-precipitation of the two receptors was not because of the association of these molecules with unsolubilized membrane domains from either T cells or APCs because no proteins enriched in the IS were detected in the immunoprecipitates (Fig. S3).
Fig. 2.
Physical association between CXCR4 and CCR5. GFP-CCR5 Jurkat T cells were stimulated as indicated, and CCR5 was immunoprecipitated with anti-GFP. Immunoprecipitated proteins were blotted with anti-CXCR4. Blotting with anti-GFP resolved in the same gel is shown, and Jurkat cells lacking GFP-CCR5 were included as control. Results are representative of at least three experiments.
CXCR4 and CCR5 Cooperate for T Cell Costimulation.
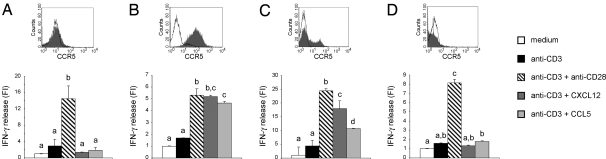
We had previously shown that recruitment of CCR5 and CXCR4 into the T cell IS results in enhanced T cell activation, as measured by proliferation assays and IFN-γ-production (5). CXCR4 is constitutively expressed in both resting and activated T cells, whereas CCR5 is expressed in activated T cells only (3). To evaluate the functional significance of CXCR4-CCR5 co-recruitment into the T cell IS, we asked whether CXCR4 requires CCR5 expression to enhance T cell responses. Thus, we analyzed the capacity of CXCL12 to induce costimulation in resting (CXCR4+ CCR5−) versus activated (CXCR4+ CCR5+) human T lymphocytes. Toward this aim, we stimulated T cells by using beads coated with an anti-CD3 monoclonal antibody (mAb), which binds and triggers the CD3 signaling complex associated with the TCR, in the presence or absence of either CXCL12 or CCL5 (5). Moreover, beads coated with anti-CD3 plus anti-CD28 mAb were used as a positive control for costimulation (5). CXCL12-induced costimulation was observed only in activated cells, which express both CXCR4 and CCR5 (Fig. 3 A and B). We therefore speculated that expression of CCR5 in resting T cells would confer costimulation competency to the CXCR4. Thus, PB T cells were transfected with GFP-CCR5 and stimulated as described above. Co-expression of both receptors in resting cells recovered CXCL12- and CCL5-induced costimulation, indicating that the difference observed between resting and activated T cells was not related to the different activation status of lymphocytes (Fig. 3C). Finally, we analyzed the capacity of CXCR4 to deliver costimulatory signals in T cells in which the plasma membrane expression of CCR5 is genetically impeded by a naturally occurring loss-of-function mutation (6–8). In activated T cells from a homozygous CCR5Δ32 healthy donor, both CCL5 and CXCL12 did not enhance T cell activation, thus demonstrating that CXCR4 needs CCR5 expression to induce costimulation (Fig. 3D).
Fig. 3.
CXCL12-induced costimulation requires CCR5 expression. IFN-γ production in resting (A), activated (B), CCR5-transfected resting (C), or CCR5Δ32 activated (D) human PB T cells stimulated with beads coated with anti-CD3 mAb in the presence or absence of anti-CD28 mAb, CXCL12, or CCL5. For each cell type, the expression of CCR5, as analyzed by flow cytometry, is indicated (the empty histograms represent the isotype control). Results are representative of at least three experiments. Bars with different letters are significantly different from each other (Student-Newman-Keuls test, P < 0.05). Mean amounts of IFN-γ produced by single living cells upon anti-CD3 stimulation were: 1.2 fg/ml (A), 18 fg/ml (B), 1.1 fg/ml (C) and 15 fg/ml (D). The percentage of living cells after 48 h of stimulation was: ≈100% in A and B, 10% in C, and 20% in D. FI = Fold of induction over unstimulated cells.
In our experiments, we did not observe enhanced T cell costimulation when beads were coated with both CCL5 and CXCL12, as compared with beads coated with CXCL12 alone (Fig. S4), suggesting that, in our specific experimental conditions, no significant synergistic signaling occurred between the two chemokines.
CXCR4 and CCR5 Are Expressed as a Multimeric Complex at the Plasma Membrane.
G protein–coupled receptors (GPCRs) have traditionally been thought to act as monomers, but the emerging view in the field is that the signaling unit is composed of dimers or even oligomers (9). Homo- or heterodimerization is an essential requirement for expression and signaling of several GPCRs. In addition, some GPCRs that do not require heterodimerization for their expression, trafficking, or signaling may specifically associate with other GPCR subtypes. This “nonobligatory” heterodimerization has important effects on receptor signaling, trafficking, and pharmacology (10).
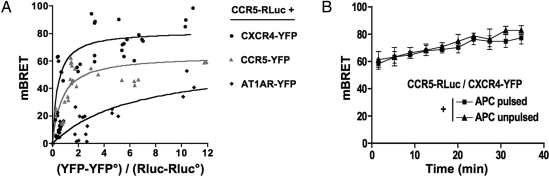
The association of CCR5 with CXCR4 was thus investigated by bioluminescence resonance energy transfer (BRET) (11) in intact Jurkat cells expressing receptors fused to either Renilla luciferase (Rluc), the BRET donor, or the yellow variant of EGFP (YFP), the BRET acceptor (Fig. 4A). In saturation experiments, conducted with CCR5-Rluc as the BRET donor, increasing concentration of both CCR5-YFP and CXCR4-YFP resulted in hyperbolic curves, characterized by similar BRET50 values (0.71 ± 0.18 and 0.39 ± 0.11). These data are consistent with a similar propensity of CCR5 to constitutively self-associate or interact with CXCR4 in Jurkat cells. Similar results were also observed in HEK-293 cells (not shown). Control experiments were conducted by using the angiotensin receptor AT1AR fused to YFP, as the BRET acceptor. Weaker BRET signals were observed with a much higher BRET50 value (6.45 ± 1.04), indicative of a significantly lower propensity of CCR5 to associate with this receptor. BRET experiments between CCR5-Rluc and CCR5-YFP or CCR5-Rluc and CXCR4-YFP, conducted in parallel in Jurkat cells preincubated with Staphylococcal Enterotoxin E (SEE)-pulsed or control APCs, yielded similar BRET values (Fig. 4B), indicating that the constitutive proximity of BRET partners was not enhanced further during their translocation to the IS.
Fig. 4.
Constitutive association between CXCR4 and CCR5. (A) BRET saturation curves obtained by measuring BRET in Jurkat T cells expressing fixed quantities of BRET donor (CCR5-Rluc) and increasing amounts of BRET acceptors (indicated C-terminally YFP-tagged GPCR constructs). Relative amounts of BRET acceptor are expressed as the ratio between the fluorescence of the acceptor over the luciferase activity of the donor. YFP° corresponds to background fluorescence in cells expressing the BRET donor alone. BRET-ratio values were from 18 individual transfections grouped as a function of the amount of BRET acceptor. (B) The transfer of energy between CCR5-Rluc and CXCR4-YFP was initiated by the addition of coelenterazine h, and the BRET ratio was monitored in real time in live Jurkat cells incubated with SEE-pulsed APCs (closed squares) or unpulsed APCs (closed triangles). The data shown represent the mean ± SD. of triplicates in an experiment representative of two independent experiments at constant (YFP-YFP°)/(Rluc-RLuc°) values (between 0.5 and 2).
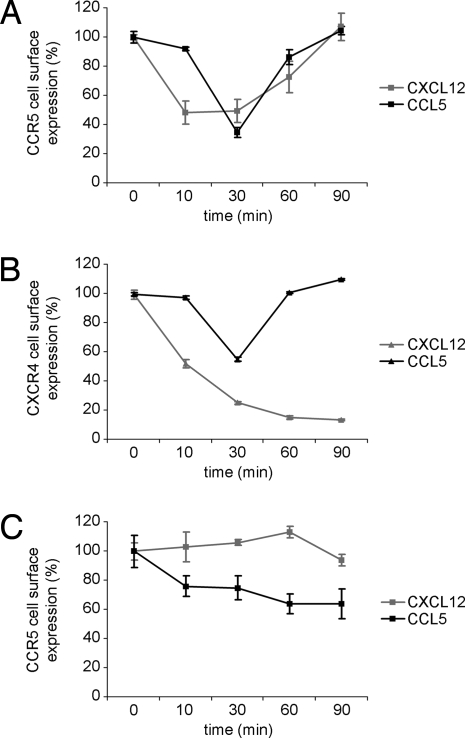
We speculated that if CXCR4 and CCR5 formed a signaling complex in T cells, both CXCR4 and CCR5 expression would be down-regulated in T cells stimulated with either CCL5 or CXCL12. CCR5+CXCR4+ Jurkat T cells were stimulated with CCL5 or CXCL12 for 90 min, and plasma membrane expression of CCR5 (Fig. 5A) and CXCR4 (Fig. 5B) was analyzed at different time points. Both stimuli induced down-modulation of CCR5 and CXCR4 expression at the plasma membrane. As expected, CXCL12 did not induce down-regulation of CCR5 in a CXCR4− cell line (Fig. S5) used as control (Fig. 5C).
Fig. 5.
Co-modulation of CXCR4 and CCR5. CCR5+CXCR4+ Jurkat T cells (A and B) or CCR5+CXCR4− A7 cells (C) were stimulated with CCL5 or CXCL12 for 90 min, and plasma membrane expression of CCR5 (A and C) and CXCR4 (B) was analyzed at different time points. The graphs show the mean fluorescence intensity (MFI) for treated cells as a proportion of the MFI for untreated cells at the indicated times. Data points represent mean (± SE) of triplicates.
Discussion
Our results are compatible with both a heterodimerization model (i.e., a dimer comprising one protomer each of CCR5 and CXCR4) or a hetero-oligomerization model (i.e., a dimer of CCR5 associated with a dimer of CXCR4) for the association of CXCR4 and CCR5. A formal distinction between the two hypotheses goes beyond the aim of our study and is very complicated because of several technical issues. For example, although computational models have identified residues involved in receptor heterodimerization, the same residues seem to be required for homodimerization of the receptors, thus excluding the possibility of using specific mutants to address this question. Nevertheless, some of our data fit better with the hetero-oligomerization model. Although previous studies failed to detect CXCR4-CCR5 heterodimers (12, 13), BRET saturation experiments, which allow a clear discrimination between specific and bystander BRET signals (14), indicated the same apparent propensity (15) of CCR5-YFP and CXCR4-YFP to associate with CCR5-Rluc in HEK-293 and Jurkat T cells, a result that is compatible with both models. However, in a previous study, we failed to displace the BRET signal between CCR5-Rluc and CCR5-YFP with unlabeled CXCR4, whereas unlabeled CCR5 did inhibit the association of two CCR5 protomers in a concentration-dependent manner (13). Interestingly, a previous study proposed a role for CD4 in promoting CXCR4-CCR5 interactions (16). Although we detected CXCR4-CCR5 complexes in CD4− HEK cells by using BRET, we cannot exclude the possibility that CD4 may modulate the interaction between the two receptors in lymphocytes. Further studies using CD8+ T cells will address this question.
Association between the two chemokine receptors was also confirmed by the co-modulation experiment, showing that CXCR4-specific stimuli induced down-modulation of CCR5 expression, and vice versa. When stimulated by their specific agonists, CXCR4 and CCR5 showed different kinetics of down-modulation, likely explained by different fates of the two chemokine receptors upon internalization, and suggesting that oligomeric complexes formed between the two receptors may be sensitive to endosomal pH. Indeed, upon stimulation CXCR4 is thought to be sorted to a degradative pathway (17), whereas CCR5 recycles at the plasma membrane (18). Interestingly, when the CCR5-CXCR4 heterodimer was internalized by a CCR5 ligand, CXCR4 showed kinetics of down-modulation similar to that of CCR5 upon its internalization, suggesting that when passively internalized by binding to CCR5, CXCR4 is not targeted to a degradative pathway.
The biological significance of the CXCR4-CCR5 interaction is provided by the functional data showing that CXCR4 requires CCR5 for its recruitment into the IS and costimulatory functions. These data indicate that a putative multimeric complex formed by the two receptors has distinctive signaling and biological properties, which argues against the possibility of mere aggregation of CXCR4 and CCR5 homodimers in the same membrane domains. The observation that BRET signals remained unchanged upon receptor translocation to the IS, whereas the association of CCR5 with CXCR4 appeared markedly enhanced in co-immunoprecipitation experiments conducted in Jurkat cells incubated with SEE-pulsed APCs, suggests that the two chemokine receptors are already in close proximity in resting cells and that, once in the IS, the complex is further stabilized by additional interactions. Thus, the highly sensitive BRET technique can detect CXCR4-CCR5 complexes even in resting cells, whereas the less sensitive biochemical approach (19) can only detect the chemokine receptor complexes stabilized at the IS.
It was recently suggested that soluble CXCL12 induces CXCR4 association with the TCR and that CXCL12 enhances expression of IL-2 and IL-10 in T cells stimulated with immobilized monoclonal anti-CD3 (20). In our experiments, CXCR4 was not recruited into the IS of CCR5− resting T cells with CXCL12-secreting APCs (Fig. 1B), suggesting that, in a physiological context of antigen and chemokine presentation, CXCR4 does not substantially associate with the TCR. Moreover, we could not detect costimulation induced by CXCL12 in resting CCR5− T cells (Fig. 3A). In addition, we found that, when compared to anti-CD3 stimulation, CXCL12 plus anti-CD3 mAb induced higher T cell responses in terms of IFN-γ, whereas IL-2 production was not affected (Fig. S6). These different results may be explained by the different stimulation protocol used. Indeed, plate-bound anti-CD3 mAb delivers a very strong signal to T cells, and thus soluble chemokines are probably working differently than membrane-bound ones. Interestingly, it was recently demonstrated that dendritic cells bind chemokines at their plasma membrane (21). The CXCR4-CCR5 in cis costimulation reported here may therefore be different from chemokine receptor in trans costimulation, as described for CCR7 (21). In agreement with these data, we have demonstrated that CCR7 is not recruited into the IS formed between T cells and APCs producing CCR7 ligands (5).
Clearly, the critical questions are when CXCR4-CCR5 cooperation occurs and how CXCR4-CCR5 signaling influences T cell responses in vivo. Although CXCR4 is constitutively expressed in T cells, CCR5 expression is restricted to activated T cells. It would be therefore possible to conclude that chemokine-mediated costimulation occurs in the periphery, likely in inflamed tissues, and does not influence T cell priming in lymph nodes. It was recently demonstrated, however, that inflammation leads to CCR5 expression by naïve CD8+ T cells, permitting their recruitment to sites of CD4+ T cell interaction with dendritic cells, where CCL3 and CCL4 are produced (22). This chemokine-driven cell clustering appears to be fundamental for development of proper long-term CD8+ T cell memory, and thus it is tempting to speculate that the expression of CCR5 is not only important for CD8 cell recruitment but also for costimulatory signals required for efficient T cell priming. As an example, the expression of CCR5 is crucial for control of infection by West Nile virus, a re-emerging pathogen capable of causing human fatal encephalitis (23); hence, again we speculate that both CCR5-mediated recruitment and the key role of CCR5 in T cell costimulation (5) might explain the higher incidence of this disease in CCR5Δ32 individuals.
On one hand, CXCR4-CCR5 signaling may be beneficial for fighting pathogens, but on the other hand it may amplify T cell responses in chronic inflammation. The CCR5Δ32 polymorphism was found to be a genetic marker inversely related to the severity of rheumatoid arthritis (24), and several lines of evidence indicate that both receptors play key roles in autoimmunity (3). Although chemokines and their receptors represent ideal therapeutic targets in autoimmunity, they are also implicated in homeostatic cell trafficking (3). Our data suggest the possibility of uncoupling migration and in situ activation by designing specific antagonists that would not impair immune system homeostasis but would inhibit activation of autoreactive T cells.
CXCR4 and CCR5 are coreceptors for HIV entry in human cells. Almost all cases of HIV-1 transmission involve strains that use CCR5 for entry (R5 viruses); however, in up to 50% of infected people after five years, on average, viruses that are able to use CXCR4 become predominant (R5X4 or X4 viruses). Our study may explain recent “paradoxical findings”, as defined by the authors of the study, showing that CCR5 ligands protect neurons from HIV/gp120 and CXCL12 toxicity (25), because CCR5 ligands might indeed cross-compete with CXCR4 ligands and prevent their neurotoxic effects (9).
In conclusion, we have demonstrated a previously undescribed and functional cooperation between CXCR4 and CCR5 to modulate T lymphocyte responses. These results identify a molecular mechanism pivotal to chemokine-receptor signaling versatility, and a chemokine–receptor couple that may represent a good target for pharmacological research.
Materials and Methods
Cell Culture, Constructs, and Transfections.
The Jurkat T cell line J.E6–1, Jurkat E6–1 cells expressing GFP-CCR5 (5) and EBV-B 221 cell lines were cultured in RPMI medium 1640 (Gibco) supplemented with 10% FCS, 2 mM l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin. The melanoma cell line A7 expressing GFP-CCR5 (26) was cultured in D-MEM medium (Gibco) supplemented with 10% FCS, 2 mM l-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, and 0.5 mg/ml G418. Human PB CD4+ T cells were sorted by negative selection by using RosetteSep kit (StemCell Technologies). Blood from a CCR5Δ32 donor was kindly provided by Christophe Combadiere (Universite Pierre et Marie Curie Paris 6, Hopital Pitie-Salpetriere, Paris, France).
The GFP-CCR5 and CXCR4-GFP (5) and the CCR5-Rluc, CCR5-YFP, CXCR4-YFP and AT1AR-YFP (13) constructs already have been described.
Human PB T cells were transiently transfected with GFP-CCR5 or with CXCR4-GFP by using an electroporation system (Amaxa Biosystems) according to manufacturer's guidelines and were used for experiments 24 h later. Jurkat cells were transiently transfected with CXCR4-GFP by using a Bio-Rad electroporation system as described (27).
Flow Cytometry.
The expression of CCR5 and CXCR4 on Jurkat, A7 and PB T cells was assessed by flow cytometry analysis (FACSCalibur or FACS Canto; Becton Dickinson) by using the commercial anti-human CCR5 mAb (R&D, clone: 45531; or R&D, clone: CTC5) and the commercial anti-CXCR4 mAb (BD PharMingen, clone: 12G5; or R&D, clone: 44717). Data were processed by using CELLQUEST (Becton Dickinson).
Immunofluorescence Confocal Microscopy.
For experiments with human PB T cells, B cells were suspended at 107 per milliliter and incubated alone or with 1 μg/ml of bacterial superantigens [Staphylococcal Enterotoxin A, Staphylococcal Enterotoxin B, Staphylococcal Enterotoxin E (SEE); Toxin Technology] at 37°C for 2 h, mixing every 20 min. For experiments with Jurkat cells, B cells were loaded with 1 μg/ml SEE.
In some experiments, Jurkat cells expressing GPF-CCR5 or CXCR4-GFP were treated with 10 μg/ml AMD3100 (Sigma) at room temperature for 15 min or with 5 μM TAK-779 (NIH AIDS Research and Reference Reagent Program) at 37°C for 15 min or with 100 nM CXCL12 or CCL5 (Peprotech) at 37°C for 15 min. After the Treatment T Cells Were Incubated with Equal Numbers of B Cells (37°C, 15 min). Conjugates were fixed with 4% paraformaldehyde, adhered to microscope slides coated with 0.05 mg/ml poly-l-lysine, washed and mounted in 2.5% 1,4-diazobicyclo[2.2.2]octane (DABCO, Fluka) in 90% glycerol/10% PBS. Confocal microscopy was performed with a Leica confocal microscope TCS SP5 (Leica) using laser excitation at 488 nm. Images were analyzed by using Adobe Photoshop 7.0 and NIH-Image J programs. A minimum of 20 cells (or 20 conjugates) was examined quantitatively for each experiment. The fluorescence patterns reported in all of the figures are representative of at least 90% of the cells.
Fluorescence Quantification.
To quantify GFP-CCR5 or CXCR4-GFP recruitment at the IS, boxes were drawn at the immune synapse, at the cell membrane not in contact with the APC, and at a background area outside the cell. Conjugates in which the plasma membrane and Golgi fluorescence could not be clearly distinguished were not included in the analysis. The relative recruitment index was calculated as indicated: [mean fluorescence intensity (MFI) at synapse − background] / [MFI at regions not in contact with APC − background]. Quantitative analysis of MFI was performed with the Image J program.
Immunoprecipitation and Western Blotting.
Jurkat cells (107 cells) expressing GFP-CCR5 were stimulated (or not) for 15 min at 37°C with 107 SEE-pulsed B cells. Cells were then lysed in 1% Brij 96 V (Fluka) buffer (20 mM Tris-HCl pH 7.5, 150 mM NaCl, 1 mM MgCl2, 1 mM EGTA), containing 10 μg/ml aprotinin, 10 μg/ml leupeptin, 20 mM NaF, 1 mM Pefabloc-SC, 1 mM Na3VO4 and 10 mM Na4P2O7. Postnuclear lysates were precleared for 30 min at 4°C with protein G-Sepharose (Amersham Pharmacia Biotech Inc.) and then incubated for 2 h with anti-GFP rabbit polyclonal antibodies (Clontech) preadsorbed to protein G. Immunoprecipitates were washed twice in 1% Brij 96 V, twice in 0.05% Brij 96 V lysis buffer, and boiled in SDS-PAGE sample buffer before electrophoresis on 10% SDS-polyacrylamide gels. After protein transfer, nitrocellulose membranes were blotted with anti-CXCR4 antibody (28), anti-GFP polyclonal antibodies (Clontech), anti-B7.1 (R&D) and anti-LAT (Upstate biotechnology).
In some experiments GFP-CCR5-expressing Jurkat cells were serum-starved for 4 h and then stimulated for 15 min with soluble chemokines, and GFP-CCR5 was immunoprecipitated as above. For control experiments, GFP was immunoprecipitated as above from 107 Jurkat cells not expressing GFP-CCR5.
Densitometric analyses were performed on a Image Master VDS-CL densitometer by using volume analysis of Image MasterTM Total Lab software (Amersham Biosciences). All densitometric values obtained were calculated from nonsaturated signals. The percentage of CXCR4 coimmunoprecipitated with GFP-CCR5 was calculated as: [volume of immunoprecipitated CXCR4 band / volume of cell total lysate CXCR4 band] / [volume of immunoprecipitated GFP band / volume of cell total lysate GFP band] %.
T Cell Activation and ELISA.
PB CD4+ T cells were stimulated with 2 μg/ml phytohemagglutinin (PHA) (Sigma) in the presence of 400 units/ml IL-2 (Chemicon) and feeders for 10 days. Beads (Polybead carboxylate 4.5-μm microspheres, Polysciences, Inc.) were coated with 1 μg/ml anti-CD3 (OKT3 clone) alone or in combination with 10 nM recombinant human CCL5 (PeproTech), or with 10 nM recombinant human CXCL12 (PeproTech) or 1 μg/ml anti-CD28 (clone CD28.1, PharMingen).
Activated PB CD4+ T cells (either from normal or from homozygous CCR5Δ32 donors) or resting PB CD4+ T cells (either CCR5− or expressing GFP-CCR5) were plated with beads at a 1:2 ratio, in a 96-well, U-bottom culture plate (FALCON). After 48 h, supernatants were collected and IFN-γ concentrations were measured by using standard commercially available ELISA kits (Pierce Endogen) according to the manufacturer's instructions. Because transfected T cells have a high mortality and CCR5Δ32 T cells were frozen and shipped before the experiment, for each cell type we calculated the percentage of living cells by flow cytometry before plating, and every 24 h by Trypan Blue exclusion.
Supernatants from three different experiments were also analyzed by SearchLight human TH1/TH2 cytokine array 1 (Pierce Biotechnology). For the multiplex arrays, in each separate experiment, duplicates of three dilutions of each sample were used.
BRET Saturation Assays.
Jurkat cells (5 × 106) expressing the SV40 T-antigen (JTAg cells) were electroporated with 0.5 μg of the DNA construct encoding BRET donor (CCR5-Rluc) and increasing amounts (0.5–10 μg) of the BRET acceptor plasmid (CCR5-YFP, CXCR4-YFP or AT1AR-YFP). Electroporation was performed in a Gene Pulser II electroporator at 950 μF and 250 V in a Gene-Pulse cuvette (Bio-Rad, Hercules, CA, USA). Total transfected DNA was maintained constant by using appropriate amounts of pcDNA3 (Invitrogen). At 24 h after transfection, the luciferase substrate, coelenterazine (Molecular Probes), was added at a final concentration of 5 μM to 1 × 105 cells. Luminescence and fluorescence were measured simultaneously by using the MithrasTM fluorescence-luminescence detector (Berthold). Cells expressing BRET donors alone were used to determine background. Filter sets were 485 ± 10 nm for luciferase emission and 530 ± 12.5 nm for YFP emission. BRET ratios were calculated as described (29).
CXCR4-CCR5 Co-Modulation.
Experiments were performed as described (30). In brief, Jurkat cells or A7 cells (2 × 107) stably expressing GFP-CCR5 by retroviral expression were incubated in basal medium containing 25 nM CXCL12 (Peprotech) or 50 nM CCL5 (Peprotech) at 37°C. Cells were analyzed by flow cytometry after staining with anti-CXCR4 (BD PharMingen, clone: 12G5) or anti-CCR5 (R&D, clone: CTC5) mAb.
Chemotaxis Assay.
Jurkat cells stably expressing GFP-CCR5 and endogenous CXCR4 were treated with 10 μM AMD3100 (Sigma) or 100 nM TAK779 (NIH AIDS Research and Reference Reagent Program) at 37°C for 30 min. Cells were then seeded in the upper chamber of a Transwell plate (CORNING) in serum-free medium. The lower chambers were filled with serum-free medium alone or serum-free medium containing CXCL12 (25 nM) or CCL5 (25 nM). After 2 h at 37°C, the number of T cells that migrated into the lower chamber was estimated by flow cytometry on a FACSCalibur.
Statistical Analysis.
All data are representative of at least three different experiments. Values are expressed as mean ± SE or SD. Statistical analysis was performed by using Student's t test (Microsoft Office) or, where indicated, by ANOVA followed by the nonparametric Student-Newman-Keuls test for multiple comparisons.
Supplementary Material
Acknowledgments.
We thank Anna Cabrelle, Denis Bison, Sonia Jimenez-Baranda, Felix Ortego and Achille Anselmo for technical help. We are grateful to Christophe Combadiere for providing CCR5Δ32 peripheral blood cells and to Sergio Pantano, Marc Parmentier, Frances Lund, Mario Mellado and Giorgio Trinchieri for critical discussions. This work was supported by grants from the Italian Association for Cancer Research (AIRC), the MIUR-PRIN, Telethon, Alleanza contro il cancro to A.V., the Sidaction and Fondation de France to S. Marullo, and the European Community (INNOCHEM, LSHB-CT-2005–518167) and MEC (SAF2005–00241) to S. Manes. The following reagent was obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: TAK-779, (cat. No. 4983).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804286105/DCSupplemental.
References
- 1.Lanzavecchia A, Lezzi G, Viola A. From TCR engagement to T cell activation: A kinetic view of T cell behavior. Cell. 1999;96:1–4. doi: 10.1016/s0092-8674(00)80952-6. [DOI] [PubMed] [Google Scholar]
- 2.Gerard C, Rollins BJ. Chemokines and disease. Nat Immunol. 2001;2:108–115. doi: 10.1038/84209. [DOI] [PubMed] [Google Scholar]
- 3.Viola A, Luster AD. Chemokines and Their Receptors: Drug Targets in Immunity and Inflammation. Annu Rev Pharmacol Toxicol. 2008;48:171–197. doi: 10.1146/annurev.pharmtox.48.121806.154841. [DOI] [PubMed] [Google Scholar]
- 4.Viola A, Contento RL, Molon B. T cells and their partners: The chemokine dating agency. Trends Immunol. 2006;27:421–427. doi: 10.1016/j.it.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Molon B, Gri G, Bettella M, Gomez-Mouton C, Lanzavecchia A, et al. T cell costimulation by chemokine receptors. Nat Immunol. 2005;6:465–471. doi: 10.1038/ni1191. [DOI] [PubMed] [Google Scholar]
- 6.Benkirane M, Jin DY, Chun RF, Koup RA, Jeang KT. Mechanism of transdominant inhibition of CCR5-mediated HIV-1 infection by ccr5delta32. J Biol Chem. 1997;272:30603–30606. doi: 10.1074/jbc.272.49.30603. [DOI] [PubMed] [Google Scholar]
- 7.Mellado M, Rodriguez-Frade JM, Vila-Coro AJ, Fernandez S, Martin de Ana A, et al. Chemokine receptor homo- or heterodimerization activates distinct signaling pathways. EMBO J. 2001;20:2497–2507. doi: 10.1093/emboj/20.10.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu R, Paxton WA, Choe S, Ceradini D, Martin SR, et al. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 9.Springael JY, Urizar E, Parmentier M. Dimerization of chemokine receptors and its functional consequences. Cytokine Growth Factor Rev. 2005;16:611–623. doi: 10.1016/j.cytogfr.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Bulenger S, Marullo S, Bouvier M. Emerging role of homo- and heterodimerization in G-protein-coupled receptor biosynthesis and maturation. Trends Pharmacol Sci. 2005;26:131–137. doi: 10.1016/j.tips.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Boute N, Jockers R, Issad T. The use of resonance energy transfer in high-throughput screening: BRET versus FRET. Trends Pharmacol Sci. 2002;23:351–354. doi: 10.1016/s0165-6147(02)02062-x. [DOI] [PubMed] [Google Scholar]
- 12.Babcock GJ, Farzan M, Sodroski J. Ligand-independent dimerization of CXCR4, a principal HIV-1 coreceptor. J Biol Chem. 2003;278:3378–3385. doi: 10.1074/jbc.M210140200. [DOI] [PubMed] [Google Scholar]
- 13.Issafras H, Angers S, Bulenger S, Blanpain C, Parmentier M, et al. Constitutive agonist-independent CCR5 oligomerization and antibody-mediated clustering occurring at physiological levels of receptors. J Biol Chem. 2002;277:34666–34673. doi: 10.1074/jbc.M202386200. [DOI] [PubMed] [Google Scholar]
- 14.Marullo S, Bouvier M. Resonance energy transfer approaches in molecular pharmacology and beyond. Trends Pharmacol Sci. 2007;28:362–365. doi: 10.1016/j.tips.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Issad T, Jockers R. Bioluminescence resonance energy transfer to monitor protein-protein interactions. Methods Mol Biol. 2006;332:195–209. doi: 10.1385/1-59745-048-0:193. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Alvarez R, Roderiquez G, Guan E, Norcross MA. Constitutive association of cell surface CCR5 and CXCR4 in the presence of CD4. J Cell Biochem. 2004;93:753–760. doi: 10.1002/jcb.20161. [DOI] [PubMed] [Google Scholar]
- 17.Marchese A, Benovic JL. Agonist-promoted ubiquitination of the G protein-coupled receptor CXCR4 mediates lysosomal sorting. J Biol Chem. 2001;276:45509–45512. doi: 10.1074/jbc.C100527200. [DOI] [PubMed] [Google Scholar]
- 18.Signoret N, Pelchen-Matthews A, Mack M, Proudfoot AE, Marsh M. Endocytosis and recycling of the HIV coreceptor CCR5. J Cell Biol. 2000;151:1281–1294. doi: 10.1083/jcb.151.6.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernanz-Falcon P, Rodriguez-Frade JM, Serrano A, Juan D, del Sol A, et al. Identification of amino acid residues crucial for chemokine receptor dimerization. Nat Immunol. 2004;5:216–223. doi: 10.1038/ni1027. [DOI] [PubMed] [Google Scholar]
- 20.Kumar A, Humphreys TD, Kremer KN, Bramati PS, Bradfield L, et al. CXCR4 physically associates with the T cell receptor to signal in T cells. Immunity. 2006;25:213–224. doi: 10.1016/j.immuni.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 21.Friedman RS, Jacobelli J, Krummel MF. Surface-bound chemokines capture and prime T cells for synapse formation. Nat Immunol. 2006;7:1101–1108. doi: 10.1038/ni1384. [DOI] [PubMed] [Google Scholar]
- 22.Castellino F, Huang AY, Altan-Bonnet G, Stoll S, Scheinecker C, et al. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. 2006;440:890–895. doi: 10.1038/nature04651. [DOI] [PubMed] [Google Scholar]
- 23.Glass WG, Lim JK, Cholera R, Pletnev AG, Gao JL, et al. Chemokine receptor CCR5 promotes leukocyte trafficking to the brain and survival in West Nile virus infection. J Exp Med. 2005;202:1087–1098. doi: 10.1084/jem.20042530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zapico I, Coto E, Rodriguez A, Alvarez C, Torre JC, et al. CCR5 (chemokine receptor-5) DNA-polymorphism influences the severity of rheumatoid arthritis. Genes Immun. 2000;1:288–289. doi: 10.1038/sj.gene.6363673. [DOI] [PubMed] [Google Scholar]
- 25.Kaul M, Ma Q, Medders KE, Desai MK, Lipton SA. HIV-1 coreceptors CCR5 and CXCR4 both mediate neuronal cell death but CCR5 paradoxically can also contribute to protection. Cell Death Differ. 2007;14:296–305. doi: 10.1038/sj.cdd.4402006. [DOI] [PubMed] [Google Scholar]
- 26.Jimenez-Baranda S, Gomez-Mouton C, Rojas A, Martinez-Prats L, Mira E, et al. Filamin-A regulates actin-dependent clustering of HIV receptors. Nat Cell Biol. 2007;9:838–846. doi: 10.1038/ncb1610. [DOI] [PubMed] [Google Scholar]
- 27.Gri G, Molon B, Manes S, Pozzan T, Viola A. The inner side of T cell lipid rafts. Immunol Lett. 2004;94:247–252. doi: 10.1016/j.imlet.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 28.Vila-Coro AJ, Rodriguez-Frade JM, Martin De Ana A, Moreno-Ortiz MC, Martinez AC, et al. The chemokine SDF-1alpha triggers CXCR4 receptor dimerization and activates the JAK/STAT pathway. Faseb J. 1999;13:1699–1710. [PubMed] [Google Scholar]
- 29.Storez H, Scott MG, Issafras H, Burtey A, Benmerah A, et al. Homo- and hetero-oligomerization of beta-arrestins in living cells. J Biol Chem. 2005;280:40210–40215. doi: 10.1074/jbc.M508001200. [DOI] [PubMed] [Google Scholar]
- 30.Signoret N, Rosenkilde MM, Klasse PJ, Schwartz TW, Malim MH, et al. Differential regulation of CXCR4 and CCR5 endocytosis. J Cell Sci. 1998;111(Pt 18):2819–2830. doi: 10.1242/jcs.111.18.2819. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.